Physiological and Genetic Analysis of Leaves from the Resprouters of an Old Ginkgo biloba Tree
Abstract
:1. Introduction
2. Materials and Methods
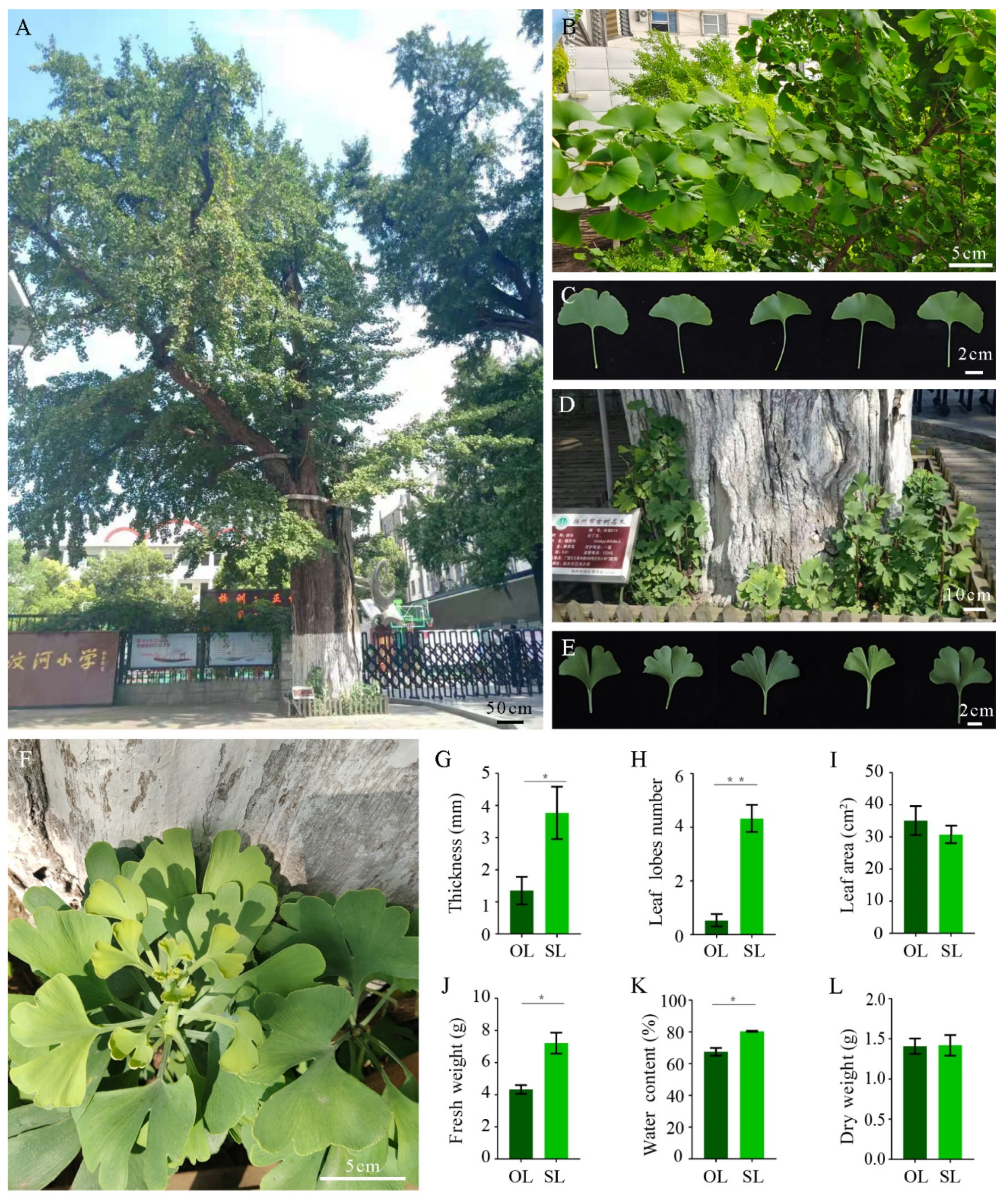
2.1. Plant Materials
2.2. Measurement of Leaf Morphology and Weight
2.3. Extraction and Quantification of Flavonol and Terpenoid Constituents
2.4. Transcriptome Sequencing
2.5. Quantitative Real-Time PCR
2.6. Statistical Analyses
3. Results
3.1. Differences in Morphology between Leaves from Resprouting Stems and Leaves from Old Branches
3.2. Transcriptome Analysis of Leaves from Resprouters and Old Branches
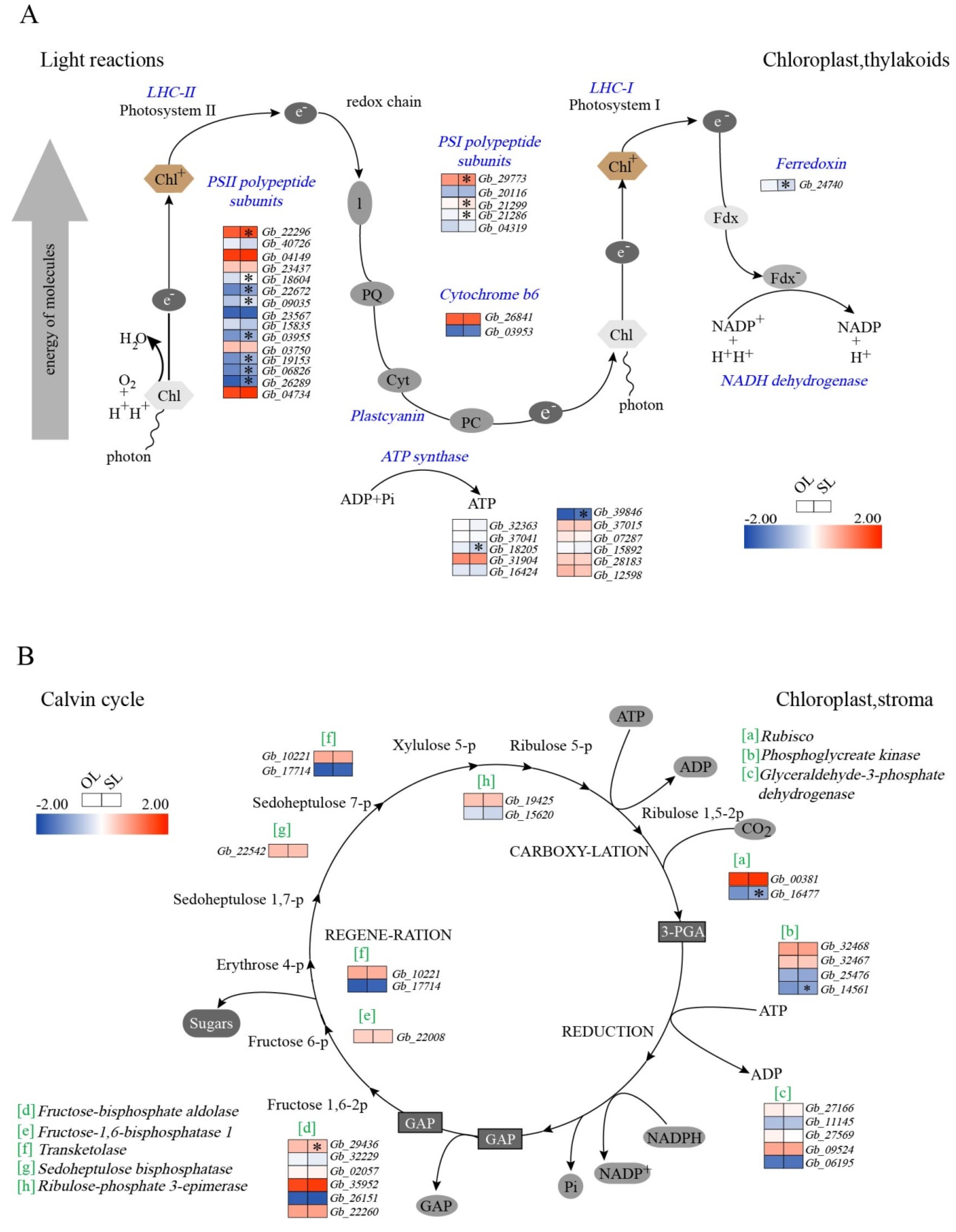
3.3. Changes in the Expression of Genes Associated with Photosynthesis
3.4. Changes in the Expression of Genes Associated with Plant Hormones
3.5. Expression of Flavonoid Biosynthesis-Related Genes in Leaves from Resprouters and Old Branches
3.6. Identification of Terpene Lactones in Leaves from Resprouters and Old Branches
4. Discussion
4.1. Resprouting and Rejuvenation in Old Ginkgo Trees
4.2. Photosynthesis Associated Gene Expression
4.3. Plant Hormone-Associated Gene Expression
4.4. Secondary Metabolite Accumulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef] [Green Version]
- Diggle, P.K. A developmental morphologist’s perspective on plasticity. Evol. Ecol. 2002, 16, 267–283. [Google Scholar] [CrossRef]
- Pei, D.; Gu, R.S. A Review on the rejuvenation of mature trees. Chin. Bull. Bot. 2005, 22, 753–760. [Google Scholar]
- Copes, D.L. Effects of annual crown pruning and serial propagation on rooting of stem cuttings from Douglas-fir. Can. J. For. Res. 1983, 13, 419–424. [Google Scholar] [CrossRef]
- Copes, D.L. Effects of long-term pruning, meristem origin, and branch order on the rooting of Douglas-fir stem cuttings. Can. J. For. Res. 1992, 22, 1888–1894. [Google Scholar] [CrossRef]
- Dang, C. Redundancy structure in phytocoenosiums as an explanation of ecosystem stability. Acta Ecol. Sin. 1998, 18, 665–672. [Google Scholar]
- Hamann, A. Adventitious root formation in cuttings of loblolly pine (Pinus taeda L.): Developmental sequence and effects of maturation. Trees 1998, 12, 175–180. [Google Scholar] [CrossRef]
- Rosier, C.L.; Frampton, J.; Goldfarb, B.; Wise, F.C.; Blazich, F.A. Stumping height, crown position, and age of parent tree influence rooting of stem cuttings of Fraser fir. HortScience 2005, 40, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.J.; Midgley, J.J. The evolutionary ecology of sprouting in woody plants. Int. J. Plant Sci. 2003, 164, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Zhu, J.; Wang, G.G. Resprouting of tree species: A research review. Chin. J. Ecol. 2020, 39, 4178–4184. [Google Scholar]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, S. The missing link in Ginkgo evolution. Nature 2003, 423, 821–822. [Google Scholar] [CrossRef]
- Li, W.; Ye, Y.; Cheng, F.; Lu, Y.; Jin, B.; Wang, L. Cytological and proteomic analysis of Ginkgo biloba pollen intine. Hortic. Plant J. 2020, 6, 257–266. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wang, G.; Cui, P.; Wu, S.; Ai, C.; Hu, N.; Li, A.; He, B.; Shao, X.; et al. The nearly complete genome of Ginkgo biloba illuminates gymnosperm evolution. Nat. Plants 2021, 7, 748–756. [Google Scholar] [CrossRef]
- Jia, Z.; Zhao, B.; Liu, S.; Lu, Z.; Chang, B.; Jiang, H.; Cui, H.; He, Q.; Li, W.; Jin, B.; et al. Embryo transcriptome and miRNA analyses reveal the regulatory network of seed dormancy in Ginkgo biloba. Tree Physiol. 2021, 41, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, J.; Jin, B.; Zhao, J.; Xu, H.; Lu, Z.; Li, W.; Li, X.; Li, L.; Liang, E.; et al. Multifeature analyses of vascular cambial cells reveal longevity mechanisms in old Ginkgo biloba trees. Proc. Natl. Acad. Sci. USA 2020, 117, 2201–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tredici, P.D. Sprouting in temperate trees: A morphological and ecological review. Bot. Rev. 2001, 67, 121–140. [Google Scholar] [CrossRef]
- Mostacedo, B.; Putz, F.E.; Fredericksen, T.S.; Villca, A.; Palacios, T. Contributions of root and stump sprouts to natural regeneration of a logged tropica dry forest in Bolivia. For. Ecol. Manag. 2009, 258, 978–985. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kaji, M.; Hamaya, T. Structure of primary Japanese beech (Fagus japonica Maxim.) forests in the Chichibu mountains, central Japan, with special reference to regeneration processes. Ecol. Res. 1988, 3, 101–116. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Ye, J.; Zhang, X.; Tan, J.; Xu, F.; Cheng, S.; Chen, Z.; Zhang, W.; Liao, Y. Global identification of Ginkgo biloba microRNAs and insight into their role in metabolism regulatory network of terpene trilactones by high-throughput sequencing and degradome analysis. Ind. Crops Prod. 2020, 148, 112289. [Google Scholar] [CrossRef]
- Yao, X.; Shang, E.; Zhou, G.; Tang, Y.; Guo, S.; Su, S.; Jin, C.; Qian, D.; Qin, Y.; Duan, J.A. Comparative characterization of total flavonol glycosides and terpene lactones at different ages, from different cultivation sources and genders of Ginkgo biloba leaves. Int. J. Mol. Sci. 2012, 13, 10305. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Pang, S.; Jia, Z.; Wang, L.; Li, W.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Vesk, P.A. Plant size and resprouting ability: Trading tolerance and avoidance of damage? J. Ecol. 2006, 94, 1027–1034. [Google Scholar] [CrossRef]
- Li, R.; Zhang, T.; Xie, J.; Wang, J.; Yan, Q. Research progress on the tradeoff between seed regeneration and sprout regeneration and the factors influencing the early process of forest regeneration. Chin. J. Ecol. 2021, 40, 2234–2242. [Google Scholar]
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef]
- Wending, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part I: Concepts, regulation and consequences of phase change. New For. 2014, 45, 449–471. [Google Scholar] [CrossRef]
- James, S.A.; Bell, D.T. Leaf morphological and anatomical characteristics of heteroblastic Eucalyptus globulus ssp. globulus (Myrtaceae). Aust. J. Bot. 2001, 49, 259–269. [Google Scholar] [CrossRef]
- Way, D.A.; Yamori, W. Thermal acclimation of photosynthesis: On the importance of adjusting our definitions and accounting for thermal acclimation of respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Xiong, Y.; Xu, D.; Fan, X.; Li, F. Responses of Caragana korshinskii Kom. to shoot removal: Mechanisms underlying regrowth. Ecol. Res. 2008, 23, 863–871. [Google Scholar] [CrossRef]
- Huang, L.C.; Weng, J.H.; Wang, C.H.; Kuo, C.I.; Shieh, Y.J. Photosynthetic potentials of in vitro-grown juvenile, adult, and rejuvenated Sequoia sempervirens (D. Don) endl. shoots. Bot. Bull. Acad. Sin. 2003, 44, 31–35. [Google Scholar]
- Eberhard, S.; Finazzi, G.; Wollman, F.A. The dynamics of photosynthesis. Annu. Rev. 2008, 42, 463–515. [Google Scholar] [CrossRef] [Green Version]
- Nickelsen, J.; Rengstl, B. Photosystem II assembly: From cyanobacteria to plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitnis, P.R. PHOTOSYSTEM I: Function and physiology. Annu. Rev. Plant Biol. 2001, 52, 593–626. [Google Scholar] [CrossRef]
- Schöttler, M.A.; Bock, R. Extranuclear inheritance: Plastid-nuclear cooperation in photosystem I assembly in photosynthetic eukaryotes. Prog. Bot. 2008, 69, 89–115. [Google Scholar]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Bari, R.; Jones, J.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Kaur, P.; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tian, Y.; He, F.; Zhou, H. Endophytes from Ginkgo biloba and their secondary metabolites. Chin. Med. 2019, 14, 51. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Rethinking Ginkgo biloba L.: Medicinal uses and conservation. Pharmacogn. Rev. 2015, 9, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, M.; Liao, Z.; Gong, Y. Advances in studies on biosynthetic pathway and biotechnology of ginkgolides. Chin. Tradit. Herb. Drugs 2007, 38, 941–945. [Google Scholar]
- Zhu, J.; Xu, F.; Liao, Y.; Wang, Y.; Cheng, S.Y. Progress in studys of ginkgolides contents regulating. Chin. Agric. Sci. Bull. 2007, 23, 301–305. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhang, S.; Tong, M.; Lu, J.; Wang, T.; Xu, Y.; Li, W.; Wang, L. Physiological and Genetic Analysis of Leaves from the Resprouters of an Old Ginkgo biloba Tree. Forests 2021, 12, 1255. https://doi.org/10.3390/f12091255
Yan J, Zhang S, Tong M, Lu J, Wang T, Xu Y, Li W, Wang L. Physiological and Genetic Analysis of Leaves from the Resprouters of an Old Ginkgo biloba Tree. Forests. 2021; 12(9):1255. https://doi.org/10.3390/f12091255
Chicago/Turabian StyleYan, Jiali, Sixuan Zhang, Miaomiao Tong, Jinkai Lu, Tongfei Wang, Yuan Xu, Weixing Li, and Li Wang. 2021. "Physiological and Genetic Analysis of Leaves from the Resprouters of an Old Ginkgo biloba Tree" Forests 12, no. 9: 1255. https://doi.org/10.3390/f12091255
APA StyleYan, J., Zhang, S., Tong, M., Lu, J., Wang, T., Xu, Y., Li, W., & Wang, L. (2021). Physiological and Genetic Analysis of Leaves from the Resprouters of an Old Ginkgo biloba Tree. Forests, 12(9), 1255. https://doi.org/10.3390/f12091255





