The Strong Position of Silver Fir (Abies alba Mill.) in Fertile Variants of Beech and Oak-Hornbeam Forests in the Light of Studies Conducted in the Sudetes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
| N [FL] with Different Share in the Stand | ||||||
|---|---|---|---|---|---|---|
| Name of a Group of Plant Communities | N [FL] | S [ha] | N/S [FL/ha] | Up to 10% | 11%–49% | 50% or More |
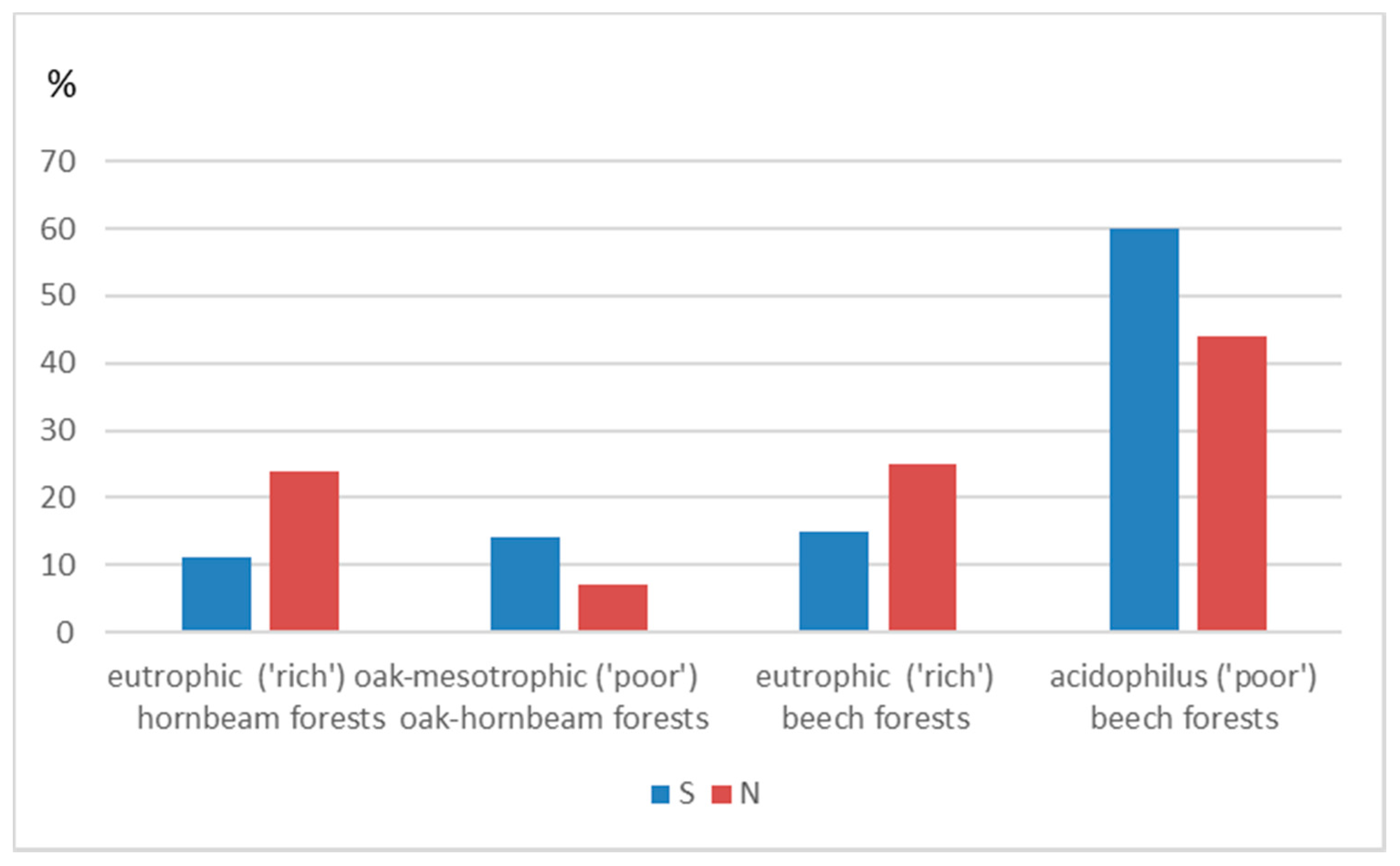
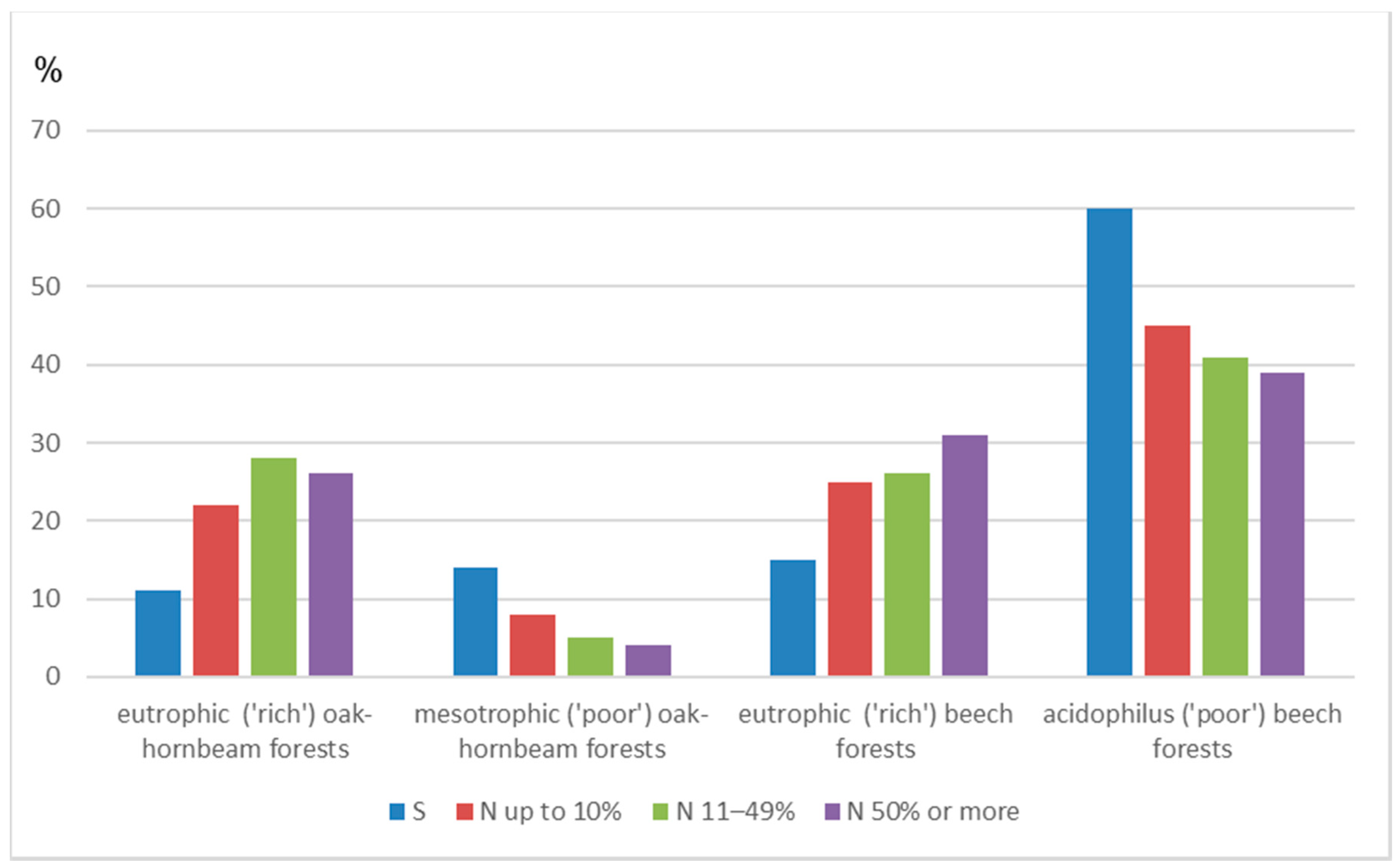
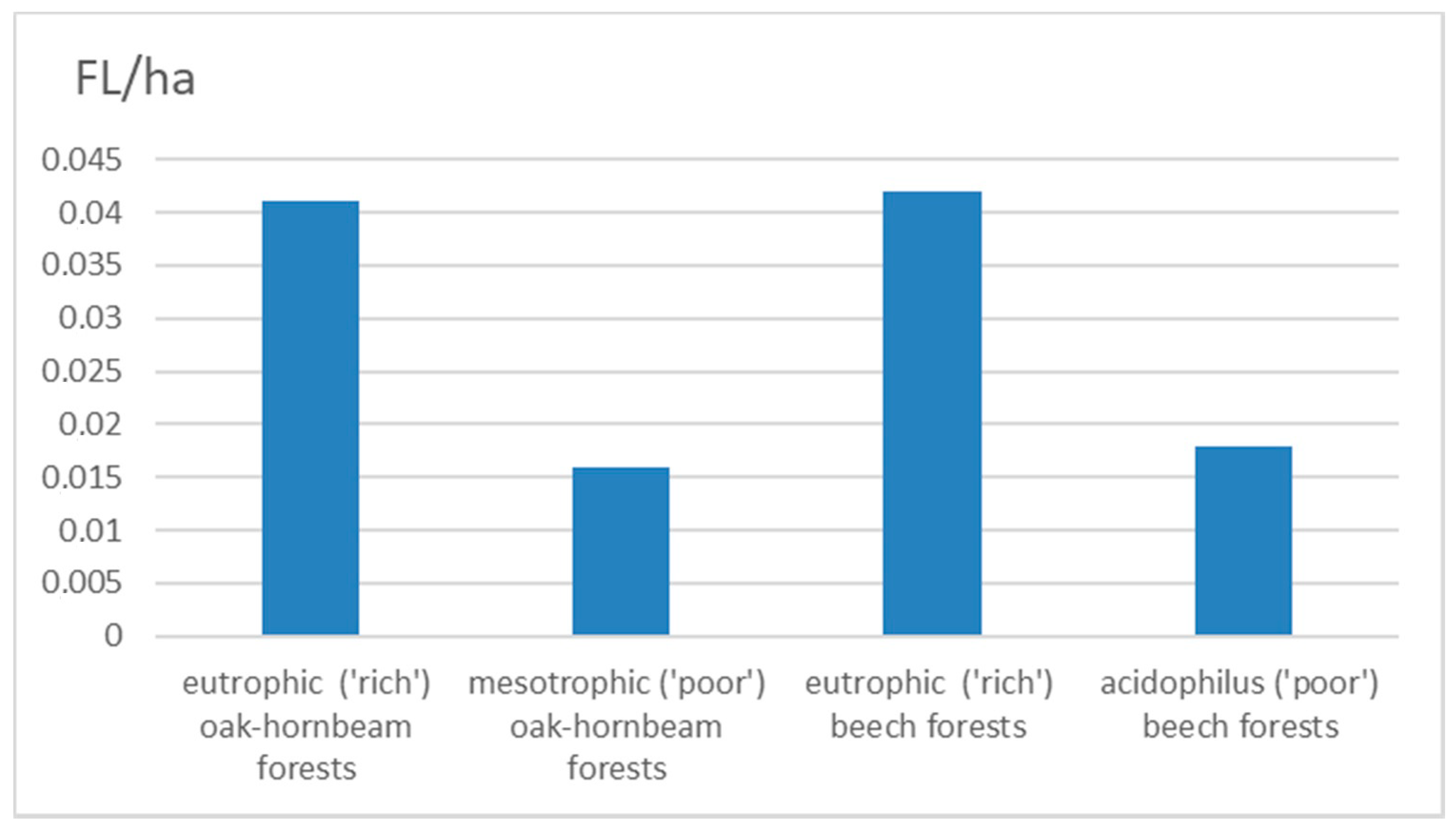
| Eutrophic (‘rich’) oak-hornbeam forests | 178 | 4332.30 | 0.04 | 109 | 56 | 13 |
| Mesotrophic (‘poor’) oak-hornbeam forests | 55 | 3455.31 | 0.02 | 43 | 10 | 2 |
| Eutrophic (‘rich’) beech forests | 192 | 4581.77 | 0.04 | 124 | 53 | 15 |
| Acidophilus (‘poor’) beech forests | 330 | 18,441.20 | 0.02 | 229 | 82 | 19 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellenberg, H.H. Vegetation Ecology of Central Europe, 4th ed.; Cambridge University Press: Cambridge, UK, 2009; p. 731. [Google Scholar]
- Farjon, A. A Handbook of the World’s Conifers; BRILL: Boston, MA, USA, 2010. [Google Scholar]
- Jaworski, A. Hodowla Lasu. Charakterystyka Hodowlana Drzew i Krzewów Leśnych [Silviculture, Characteristics of Forest Trees and Shrubs]; PWRiL: Warszawa, Poland, 2011. [Google Scholar]
- Mauri, A.; de Rigo, D.; Caudullo, G. Abies alba in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; The Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Praciak, A.; Pasiecznik, N.; Sheil, D.; van Heist, M.; Sassen, M.; Correia, C.S.; Dixon, C.; Fyson, G.; Rushford, K.; Teeling, C. The CABI Encyclopedia of Forest Trees; CABI: Oxfordshire, UK, 2013. [Google Scholar]
- Dobrowolska, D.; Bončina, A.; Klumpp, R. Ecology and silviculture of silver fir (Abies alba Mill.): A review. J. For. Res. 2017, 22, 326–335. [Google Scholar] [CrossRef]
- Tinner, W.; Colombaroli, D.; Heiri, O.; Henne, P.; Steinacher, M.; Untenecker, J.; Vescovi, E.; Allen, J.R.M.; Carraro, G.; Conedera, M.; et al. The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecol. Monogr. 2013, 83, 419. [Google Scholar] [CrossRef] [Green Version]
- Cheddadi, R.; Birks, H.J.B.; Tarroso, P.; Liepelt, S.; Gömöry, D.; Dullinger, S.; Meier, E.S.; Hülber, K.; Maiorano, L.; Laborde, H. Revisiting tree-migration rates: Abies alba (Mill.), a case study. Veg. Hist. Archaeobot. 2014, 23, 113–122. [Google Scholar] [CrossRef]
- Litkowiec, M.; Lewandowski, A.; Rączka, G. Spatial Pattern of the Mitochondrial and Chloroplast Genetic Variation in Poland as a Result of the Migration of Abies alba Mill. from Different Glacial Refugia. Forests 2016, 7, 284. [Google Scholar] [CrossRef]
- Filipiak, M.; Ufnalski, K. Growth reaction of silver-fir (Abies alba Mill.) asociated with air quality improvment in the Sudeten Mountains. Pol. J. Environ. Stud. 2004, 13, 267–273. [Google Scholar]
- Maiorano, L.; Cheddadi, R.; Zimmermann, N.E.; Pellissier, L.; Petitpierre, B.; Pottier, J.; Laborde, H.; Hurdu, B.I.; Pearman, P.B.; Psomas, A.; et al. Building the niche through time: Using 13,000 years of data to predict the effects of climate change on three tree species in Europe. Glob. Ecol. Biogeogr. 2013, 22, 302–317. [Google Scholar] [CrossRef]
- Cailleret, M.; Heurich, M.; Bugmann, H. Reduction in browsing intensity may not compensate climate change effects on tree species composition in the Bavarian Forest National Park. For. Ecol. Manag. 2014, 328, 179–192. [Google Scholar] [CrossRef]
- Bugmann, H.; Brang, P.; Elkin, C.; Henne, P.; Jakoby, O.; Lévesque, M.; Lischke, H.; Psomas, A.; Rigling, A.; Wermelinger, B.; et al. Climate change impacts on tree species, forest properties, and ecosystem services. In Scenarios of Climate Change Impacts in Switzerland; Raible, C.C., Strassmann, K.M., Eds.; OCCR FOEN: Bern, Switzerland, 2014. [Google Scholar]
- Ruosch, M.; Spahni, R.; Joos, F.; Henne, P.D.; van der Knaap, W.O.; Tinner, W. Past and future evolution of Abies alba forests in Europe—Comparison of a dynamic vegetation model with palaeo data and observations. Glob. Chang. Biol. 2016, 22, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Sidor, C.G.; Vlad, R.; Popa, I.; Semeniuc, A.; Apostol, E.; Badea, O. Impact of Industrial Pollution on Radial Growth of Conifers in a Former Mining Area in the Eastern Carpathians (Northern Romania). Forests 2021, 12, 640. [Google Scholar] [CrossRef]
- Mihai, G.; Alexandru, A.M.; Stoica, E.; Birsan, M.V. Intraspecific Growth Response to Drought of Abies alba in the Southeastern Carpathians. Forests 2021, 12, 387. [Google Scholar] [CrossRef]
- Larysch, E.; Stangler, D.F.; Nazari, M.; Seifert, T.; Kahle, H.P. Xylem Phenology and Growth Response of European Beech, Silver Fir and Scots Pine along an Elevational Gradient during the Extreme Drought Year 2018. Forests 2021, 12, 75. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Panis, B.; Swennen, R.; Salaj, J. Physiological and Structural Aspects of In Vitro Somatic Embryogenesis in Abies alba Mill. Forests 2020, 11, 1210. [Google Scholar] [CrossRef]
- Wrońska-Pilarek, D.; Dering, M.; Bocianowski, J.; Lechowicz, K.; Kowalkowski, W.; Barzdajn, W.; Hauke-Kowalska, M. Pollen Morphology and Variability of Abies alba Mill. Genotypes from South-Western Poland. Forests 2020, 11, 1125. [Google Scholar] [CrossRef]
- Matuszkiewicz, J.M. Zespoły Leśne Polski [Forest Plants Communities in Poland]; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2002. [Google Scholar]
- Matuszkiewicz, W.; Faliński, B.; Kostrowicki, A.S.; Matuszkiewicz, A.; Olaczek, R.; Wojterski, T. Potencjalna Roślinność Naturalna Polski. Mapa przeglądowa 1:300,000 [Potential Natural Vegetation of Poland. Outline Map 1: 300,000]; Instytut Geografii i Przestrzennego Zagospodarowania PAN: Warszawa, Poland, 1995. [Google Scholar]
- Falińska, K. Ekologia Roślin [Plant Ecology]; PWN: Warszawa, Poland, 2021. [Google Scholar]
- Matuszkiewicz, W.; Szwed, W.; Sikorski, P.; Wierzba, M. Zbiorowiska Roślinne Polski—Ilustrowany Przewodnik. Lasy i Zarośla [Plant Communities of Poland—An Illustrated Guide. Forests and Thickets]; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2013. [Google Scholar]
- Bohn, U.; Gollub, G.; Hettwer, C.; Neuhäuslová, Z.; Raus, T.; Schlüter, H.; Weber, H. Karte der Natürlichen Vegetation Europas Map of the Natural Vegetation of Europe Maßstab/Scale 1: 2,500,000; Bundesamt für Naturschutz (BfN)/Federal Agency for Nature Conservation: Bonn, Germany, 2004. [Google Scholar]
- Filipiak, M. Funkcjonowanie Abies alba (Pinaceae) w warunkach silnej antropopresji w Sudetach [Life of Abies alba (Pinaceae) under the conditions of intense anthropopressure in the Sudety Mountains]. Fragm. Florist. Geobot. Pol. 2006, 13, 113–138. [Google Scholar]
- Kondracki, J. Geografia Regionalna Polski [Regional Geography of Poland]; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2009. [Google Scholar]
- Barzdajn, W. Strategia restytucji jodły pospolitej (Abies alba Mill.) w Sudetach [Strategy for the restitution of silver fir (Abies alba Mill.) In the Sudetes]. Sylwan 2000, 144, 63–77. [Google Scholar]
- Filipiak, M.; Barzdajn, W. An assessment of natural resources of European silver-fir (Abies alba Mill.) in the Polish Sudeten Mts. Dendrobiology 2004, 51, 19–24. [Google Scholar]
- Lewandowski, A.; Filipiak, M.; Burczyk, J. Genetic Variation of Abies alba Mill. in polish part of Sudety Mts. Acta Soc. Bot. Pol. 2002, 70, 215–219. [Google Scholar] [CrossRef]
- Filipiak, M. Distribution of silver-fir (Abies alba Mill.) resources in the Sudety Mts. Biodivers. Res. Conserv. 2005, 1, 294–299. [Google Scholar]
- Filipiak, M.; Kosiński, P. Forest communities with European silver-fir (Abies alba Mill.) in the Sudety Mts. Dendrobiology 2002, 48, 15–22. [Google Scholar]
- Filipiak, M. Changes of silver fir (Abies alba Mill.) crown state and stand quality class in Sudety Mountains. Dendrobiology 2005, 54, 11–17. [Google Scholar]
- Filipiak, M.; Napierała-Filipiak, A. Effect of canopy density on the defoliation of the European silver fir (Abies alba Mill.) due to heavy industrial pollution. Dendrobiology 2009, 62, 17–21. [Google Scholar]
- Filipiak, M. Age structure of natural regeneration of European silver-fir (Abies alba Mill.) in the Sudety Mts. Dendrobiology 2002, 48, 9–14. [Google Scholar]
- Filipiak, M.; Iszkuło, G.; Korybo, J. Relation between photosynthetic photon flux density (PPFD) and growth of Silver fir (Abies alba Mill.) seedlings in a forest stand dominated by spruce in the Sudety Mts. (SW Poland). Pol. J. Ecol. 2005, 53, 177–184. [Google Scholar]
- Filipiak, M.; Komisarek, J. Regeneration of the European silver-fir (Abies alba Mill.) in the Sudety Moutains on soils with different physico-chemical properties. Dendrobiology 2005, 53, 17–25. [Google Scholar]
- Filipiak, M.; Komisarek, J.; Nowiński, M. Natural regeneration of the European silver fir in the Sudety Mountains on soils with different particle size distribution. Dendrobiology 2003, 50, 15–22. [Google Scholar]
- Bernadzki, E. Zamieranie jodły w granicach naturalnego zasięgu [Fir dieback within its natural range]. In Jodła Pospolita Abies alba Mill. Nasze Drzewa Leśne [European Silver Fir Abies alba Mill. Our Forest Trees]; Białobok, S., Ed.; PWN: Warszawa, Poland, 1983; Volume 4, pp. 483–501. [Google Scholar]
- Jaworski, A. Zmiany tendencji wzrostowych głównych lasotwórczych gatunków drzew w Europie i obszarach górskich Polski oraz ich przyczyny [Changes in the growth tendencies of the main forest-forming tree species in Europe and in the mountain areas of Poland and their causes]. Sylwan 2003, 147, 99–106. [Google Scholar]
- Jaworek-Jakubska, J.; Filipiak, M.; Napierała-Filipiak, A. Traditional Landscapes: Mapping Trajectories of Changes in Mountain Territories (1824–2016), on the Example of Jeleniogórska Basin, Poland. Forests 2020, 11, 867. [Google Scholar] [CrossRef]
- Jaworek-Jakubska, J.; Filipiak, M.; Napierała-Filipiak, A. Understanding of Forest Cover Dynamics. In Wspólny Raport o Jakości Powietrza w Obszarze Czarnego Trójkąta [Joint Report on Air Quality in the Black Triangle]; Abraham, J., Ciechanowicz-Kusztal, R., Drueke, M., Jodłowska-Opyd, G., Kallweit, D., Eds.; ČHMU, WIOŚ, FUG, UBA: Jelenia Góra, Poland, 1999; p. 117. [Google Scholar]
- Gomory, D.; Longauer, R.; Liepelt, S.; Ballian, D.; Brus, R.; Kraigher, H.; Parpan, V.I.; Parpan, T.V.; Paule, L.; Stupar, V.I.; et al. Variation patterns of mitochondrial DNA of Abies alba Mill. in suture zones of postglacial migration in Europe. Acta Soc. Bot. Pol. 2004, 73, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Zielony, R.; Kliczkowska, A. Regionalizacja Przyrodniczo-Leśna Polski 2010 [Natural-Forest Regionalization of Poland 2010]; CILP: Warszawa, Poland, 2012. [Google Scholar]
- Raster Versions of Potential Vegetation Maps of Poland. Available online: https://www.igipz.pan.pl/Roslinnosc-potencjalna-zgik.html (accessed on 5 November 2019).
- Map D1D2. Available online: https://www.igipz.pan.pl/tl_files/igipz/ZGiK/opracowania/roslinnosc_potencjalna/D1D2.png (accessed on 5 November 2019).
- Forest Data Bank. Available online: https://www.bdl.lasy.gov.pl/portal/ (accessed on 9 November 2019).
- Zając, A. Atlas of distribution of vascular plants in Poland (ATPOL). Taxon 1978, 27, 481–484. [Google Scholar] [CrossRef]
- Net of ATPOL (Format SHP). Available online: https://botany.pl/atpol/SHP/SHP-Atpol.zip (accessed on 20 January 2020).
- Napierała-Filipiak, A.; Filipiak, M.; Łakomy, P. Changes in the Species Composition of Elms (Ulmus spp.) in Poland. Forests 2019, 10, 1008. [Google Scholar] [CrossRef] [Green Version]
- Nyland, R.D. Silviculture: Concepts and Applications, 2nd ed.; Waveland Prres, Inc.: Long Grove, IL, USA, 2007; p. 682. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; The Iowa State University Press: Ames, IA, USA, 1976; pp. 327–329. [Google Scholar]
- Interpretation Manual of European Union Habitats, Version EUR 28, 2013. Available online: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (accessed on 12 August 2021).
- Wojterski, T. Lasy z udziałem jodły w Polsce [Forests with fir in Poland.]. In Jodła Pospolita Abies alba Mill. Nasze Drzewa Leśne [European Silver Fir Abies alba Mill. Our Forest Trees]; Białobok, S., Ed.; PWN: Warszawa, Poland, 1983; Volume 4, pp. 431–482. [Google Scholar]
- Vrška, T.; Adam, D.; Hort, L.; Kolář, T.; Janík, D. European beech (Fagus sylvatica L.) and silver fir (Abies alba Mill.) Rotation in the Carpathians—A developmental cycle or a linear trend induced by man. For. Ecol. Manag. 2009, 258, 347–356. [Google Scholar] [CrossRef]
- Leibundgut, H. Europäische Urwälder der Bergstufe [European Primeval Forests of the Mountain Stage]; Paul Haupt Verlag: Bern, Switzerland; Stuttgart, Germany, 1982; p. 306. [Google Scholar]
- Mayer, H. Urwaldreste, Naturwaldreservate und Schützenswerte Naturwälder in Österreich [Remains of Primeval Forest, Natural Forest Reserves and Natural Forests Worthy of Protection in Austria]; Boden Kultur: Wien, Austria, 1987; p. 971. [Google Scholar]
- Modrý, M.; Hubený, D.; Rejšek, K. Differential response of naturally regenerated European shade tolerant tree species to soil type and light availability. For. Ecol. Manag. 2004, 188, 185–195. [Google Scholar] [CrossRef]
- Podlaski, R. A development cycle of the forest with fir (Abies alba Mill.) and beech (Fagus sylvatica L.) in its species composition in the Świętokrzyski National Park. J. For. Sci. 2004, 50, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Paluch, J.G. The influence of the spatial pattern of trees on forest floor vegetation and silver fir (Abies alba Mill.) regeneration in uneven-aged forests. For. Ecol. Manag. 2004, 205, 283–298. [Google Scholar] [CrossRef]
- Paluch, J. On the Consequences of Using Moving Window Segmentation to Analyze the Structural Stand Heterogeneity and Debatable Patchiness of Old-Growth Temperate Forests. Forests 2021, 12, 96. [Google Scholar] [CrossRef]
- Zoll, T. Podstawowe zagadnienia zagospodarowania lasów górskich w Sudetach [Basic issues in the development of mountain forests in the Sudetes]. Sylwan 1958, 102, 9–33. [Google Scholar]
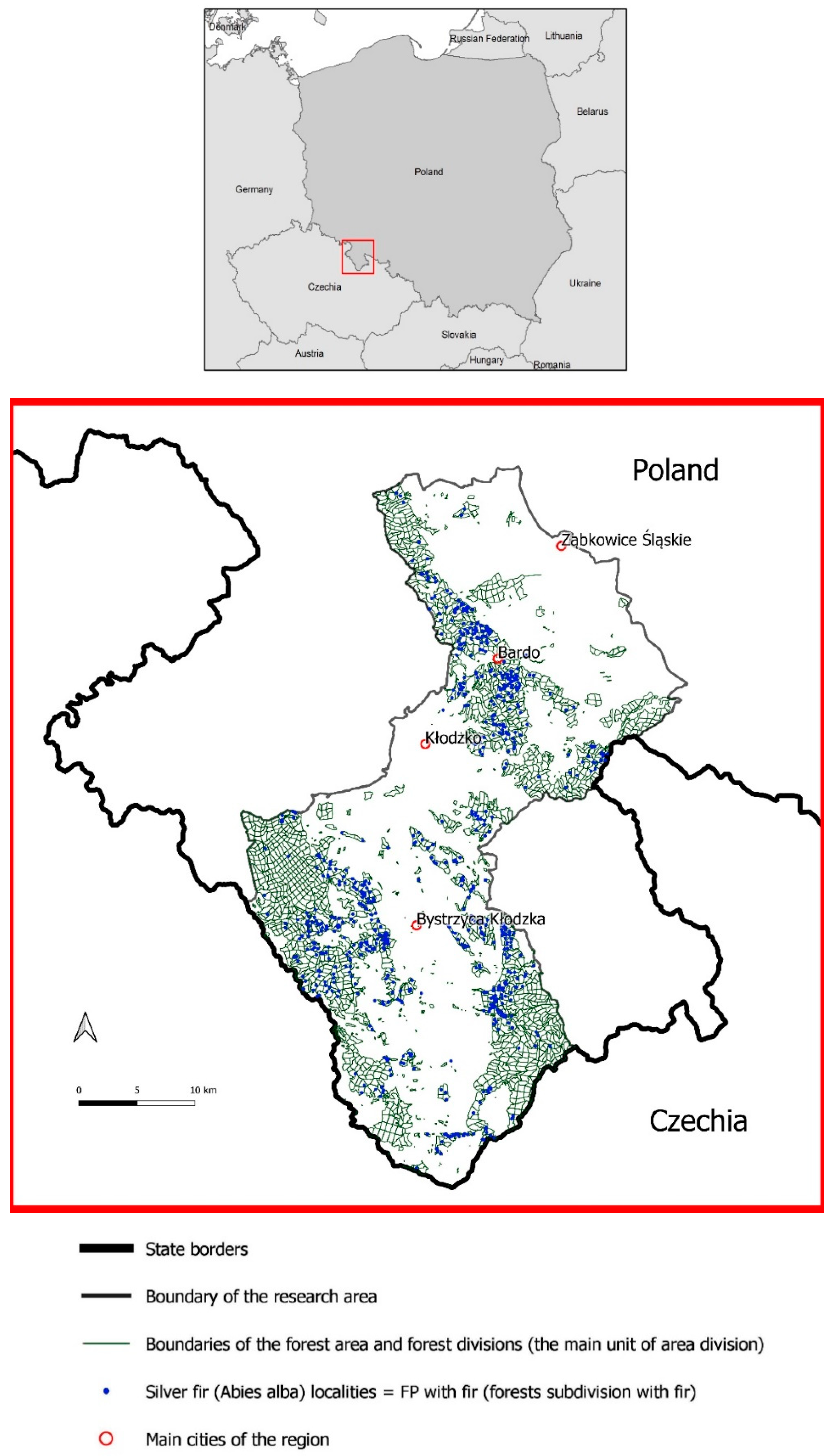
| Area | Forest Area | Forest Area | Average Annual Temperature | Vegetation Period | Average Annual Rainfall (mm) | Elevation |
|---|---|---|---|---|---|---|
| (km2) | (km2) | (%) | (°C) | (days) | m.a.s.l | |
| 1052 | 340.968 | 32 | 7.0 | 170–210 | 750–1000 | 350–1424 |
| Numerical Designation and Name of the Plant Community on the Map of Potential Natural Vegetation | Description of the Plant Community [20,21,23,24,52] | N [FL] | S [ha] | N/S [FL/ha] |
|---|---|---|---|---|
| 02—Salici-Populetum | Lowland willow-poplar floodplain forest; regularly flooded—(dynamic complex of Salici-Populetum, Salicetum triandro-viminalis etc.) developed on sandy alluvial soils over larger rivers within the range of annual inundations | 0 | 97.59 | 0.00 |
| 03—Ficario-Ulmetum typicum | Lowland ash-elm floodplain forest; occasionally flooded developed mainly in the valleys of large rivers. on the highest situated floodplain terraces and very fertile typical fine-grained alluvial soils | 3 | 163.26 | 0.02 |
| 04—Ficario-Ulmetum chrysospl. | Lowland eutrophic forb-rich elm-oak forests on the ground-water soils out of floodplains associated with wet depressions, valleys of small watercourses, lakes, ravines and black turf soils | 5 | 229.89 | 0.02 |
| 06—Alnetum incanae | Submontane/montane grey alder floodplain forest developed on highly fertile mountain alluvial soils with a thickness of several dozen centimeters on rocky ground, made of pebbles deposited by water | 0 | 187.49 | 0.00 |
| 07—Carici remotae-Fraxinetum | Submontane forb-rich ash forests along streams and little rivers developing on the lowest alluvial terraces of small streams, with wet soil due to the constant seeping of water near the spreading springs. | 7 | 636.40 | 0.01 |
| 10—Galio-Carpinetum, Sil./Gr.-Pol., poor 2 | Middle European lowland oak-hornbeam forest; Silesia/Great-Poland-vicariant, mesotrophic (“poor”) communities developed on moderately fertile habitats in the lowlands and highlands; soils formed on boulders light clays and sands of glacial accumulation, as well as on river sands of accumulation terraces; brown leached soils, brown podzolic and grey-brown podzolic soils | 3 | 84.34 | 0.04 |
| 11—Galio-Carpinetum, Sil./Gr.-Pol., rich 1 | Middle European lowland oak-hornbeam forest; Silesia/Great-Poland-vicariant, eutrophic (“rich”) communities developed on fertile fresh and moderately moist habitats in lowlands and highlands; soils formed on boulders clays and clay sands; typical, fluvic and gleyic brown soils, black turf soil | 52 | 1001.42 | 0.05 |
| 12—Galio Carpinetum submont poor 2 | Middle European submontane oak-hornbeam forest; Silesia/Great-Poland-vicariant, mesotrophic (“poor”) communities developed on moderately fertile habitats in the foothills; soils formed on boulders light clays and sands of glacial accumulation, as well as on river sands of accumulation terraces; brown leached soils, brown podzolic and grey-brown podzolic soils | 52 | 3370.97 | 0.02 |
| 13—Galio Carpinetum submont rich 1 | Middle European submontane oak-hornbeam forest; Silesia/Great-Poland-vicariant, eutrophic (“rich”) communities developed on fertile fresh and moderately moist habitats in the foothills; soils formed on boulders clays and clay sands; typical, fluvic and gleyic brown soils, black turf soil | 124 | 3330.88 | 0.04 |
| 28—Aceri Tilietum | Submontane maple-lime forest on the rocky slopes developed on acidic brown soils in the upper and middle parts of the slopes, typical and diluvial brown soils in the lower parts, and black soils and brown marshes at the bottom of valleys. | 0 | 8.31 | 0.00 |
| 30—Dentario enneaphyllidis-Fagetum submontane 3 | Submontane forb-rich sudetian beech forest developed on medium-deep and deep brown soils. | 54 | 750.1 | 0.07 |
| 31—Dentario enneaphyllidis-Fagetum montane 3 | Montane forb-rich sudetian beech forest developed on neutral or near-neutral medium-deep and deep brown mountain soils, with mild humus (mull) | 106 | 3561.71 | 0.04 |
| 36—Cephalanthero-Fagenion 3 | Calciphilous and subthermophilous beech forests with many orchid species in undergrowth developed on calcareous, often superficial, soils, usually of steep slopes, of the medio-European and Atlantic domains of Western Europe and of central and northern Central Europe | 32 | 269.93 | 0.11 |
| 38—Luzulo luzuloidis-Fagetum 4 | montane/submontane acidophilous beech forest with graminoids and/or dwarf-shrubs in undergrowth developed on brown, acidic and podzolic soils, sometimes brown podzolic soils, with a base of crystalline, metamorphic rocks | 330 | 18,441.21 | 0.02 |
| 39—Acerenion pseudoplatani | Montane sycamore forest with tall forbs in undergrowth developed on fertile soils which undergo strong slope denudation | 5 | 165.07 | 0.03 |
| 45—Calamagrostio-Quercetum | Middle-European lowland acidophilous oak forest developed on grey-brown podzolic soils, brown podzolic soils, brown leached soils and stagnant-gleyic soils | 0 | 193.73 | 0.00 |
| 46—Luzulo luzuloidis-Quercetum | Submontane Middle-European acidophilous oak forests occur on nutrient-poor different acidic soils. | 64 | 2428.72 | 0.02 |
| 57—Abieti-Piceetum montanum | Lower-montane spruce- (rarely fir-spruce- forests, developed on leached brown soils, grey-brown podzolic soils and typical podzolic soils. | 13 | 541.89 | 0.02 |
| 58_Calamagrostio villosae-Piceetum | Sudetian higher-montane spruce forest occurs mainly on acidic podzols, but also on gleyic soils or on rankers over talus slopes. | 1 | 1389.09 | 0.00 |
| N Up to 10% | N 11%–49% | N 50% or More | N Total | S | |
|---|---|---|---|---|---|
| N up to 10% | 1.618 | 2.738 | 0.165 | 6.894 | |
| N 11%–49% | 0.674 | 0.767 | 13.442 | ||
| N 50% or more | 1.842 | 16.886 | |||
| N total | 8.482 | ||||
| S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipiak, M.; Gubański, J.; Jaworek-Jakubska, J.; Napierała-Filipiak, A. The Strong Position of Silver Fir (Abies alba Mill.) in Fertile Variants of Beech and Oak-Hornbeam Forests in the Light of Studies Conducted in the Sudetes. Forests 2021, 12, 1203. https://doi.org/10.3390/f12091203
Filipiak M, Gubański J, Jaworek-Jakubska J, Napierała-Filipiak A. The Strong Position of Silver Fir (Abies alba Mill.) in Fertile Variants of Beech and Oak-Hornbeam Forests in the Light of Studies Conducted in the Sudetes. Forests. 2021; 12(9):1203. https://doi.org/10.3390/f12091203
Chicago/Turabian StyleFilipiak, Maciej, Janusz Gubański, Justyna Jaworek-Jakubska, and Anna Napierała-Filipiak. 2021. "The Strong Position of Silver Fir (Abies alba Mill.) in Fertile Variants of Beech and Oak-Hornbeam Forests in the Light of Studies Conducted in the Sudetes" Forests 12, no. 9: 1203. https://doi.org/10.3390/f12091203
APA StyleFilipiak, M., Gubański, J., Jaworek-Jakubska, J., & Napierała-Filipiak, A. (2021). The Strong Position of Silver Fir (Abies alba Mill.) in Fertile Variants of Beech and Oak-Hornbeam Forests in the Light of Studies Conducted in the Sudetes. Forests, 12(9), 1203. https://doi.org/10.3390/f12091203





