Dwarf Mistletoe and Drought Contribute to Growth Decline, Dieback and Mortality of Junipers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Study Mistletoe and Host Species
2.3. Field Sampling and Measurements of Ring-Width Series
2.4. Processing Ring-Width Series
2.5. Climate Data and Drought Index
2.6. Statistical Analyses
3. Results
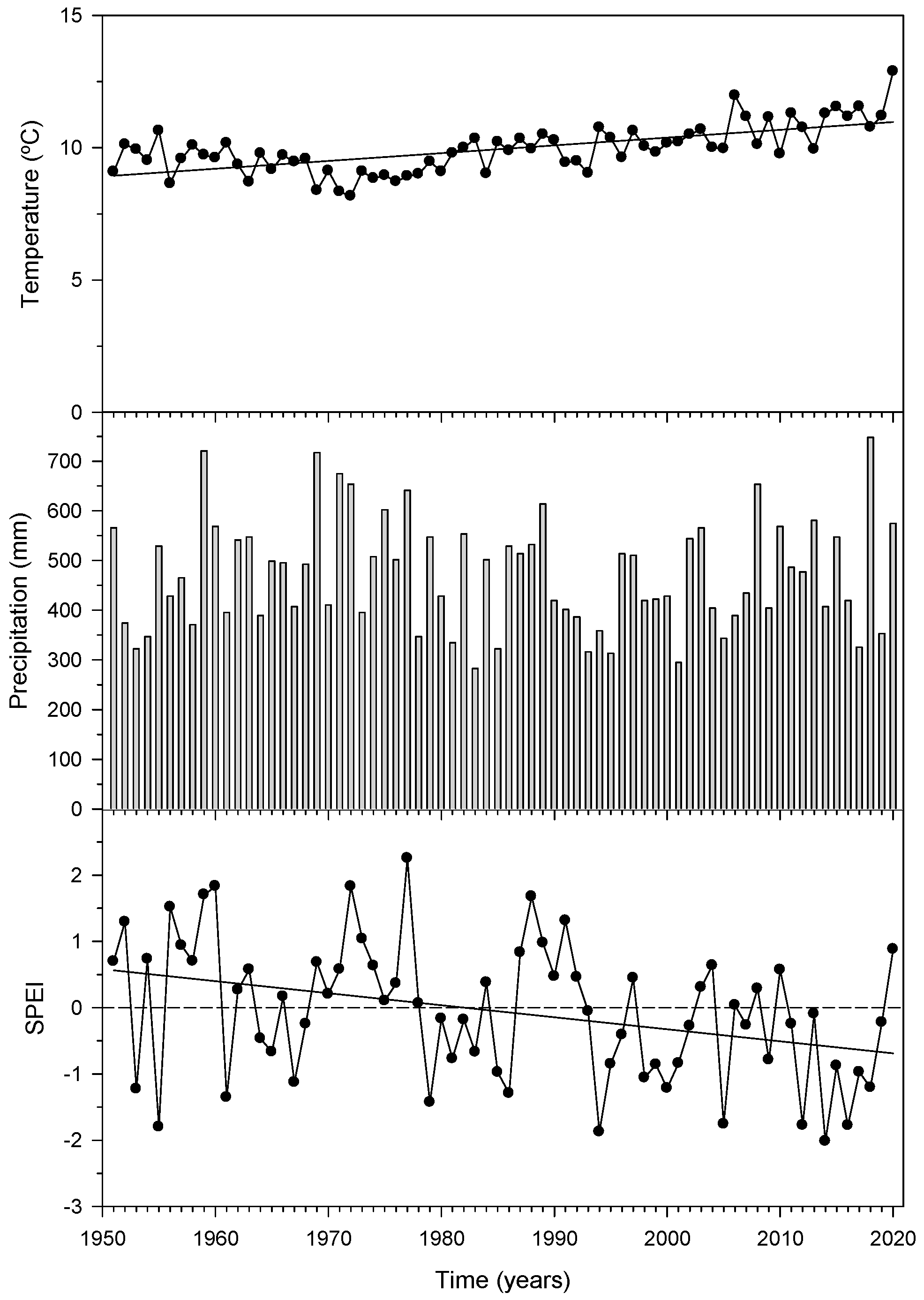
3.1. Temperature, Precipitation and Drought: Trends and Variability
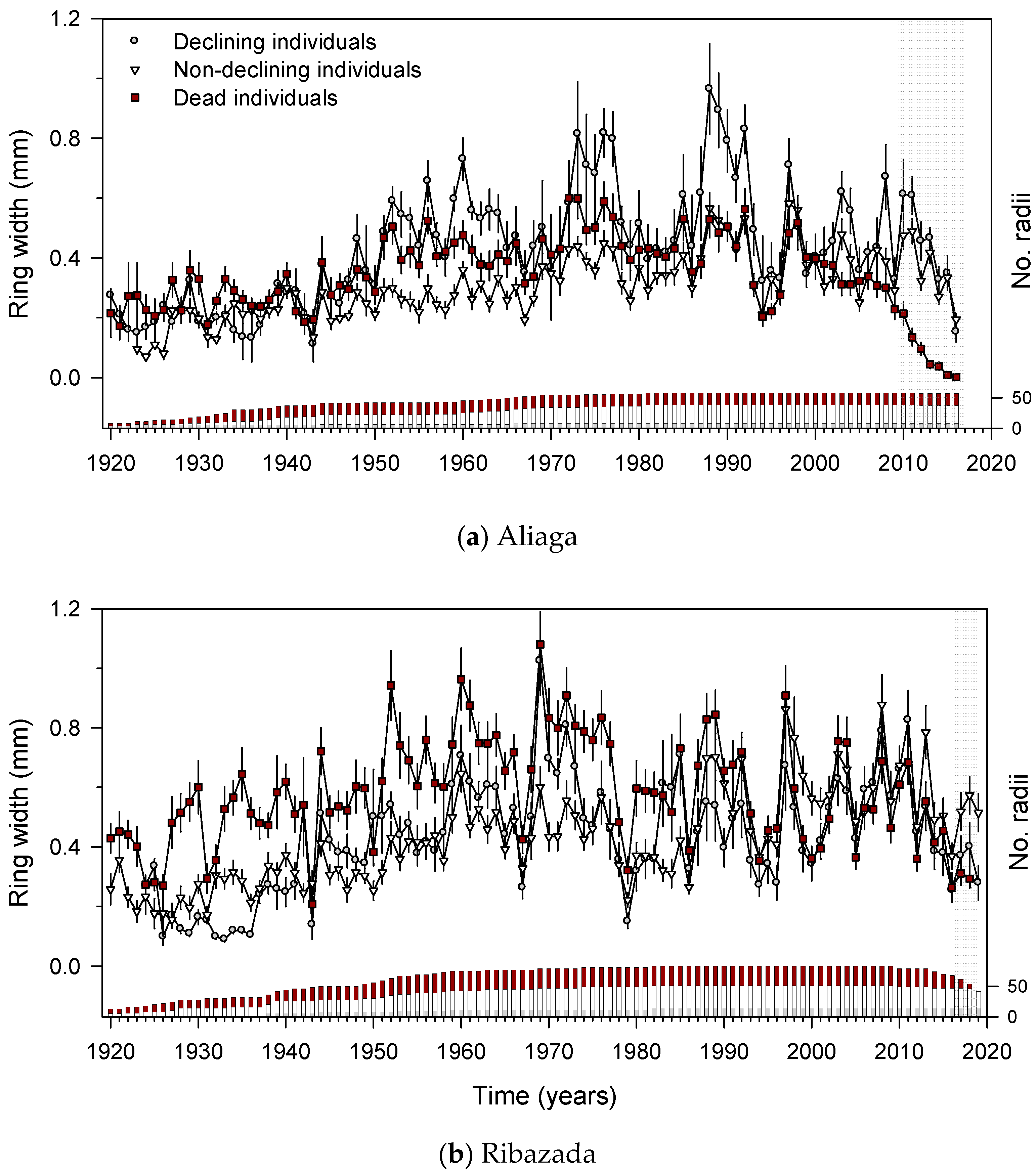
3.2. Junipers’ Features (Size, Health, Age, Growth Rate) and Dwarf Mistletoe Infestation
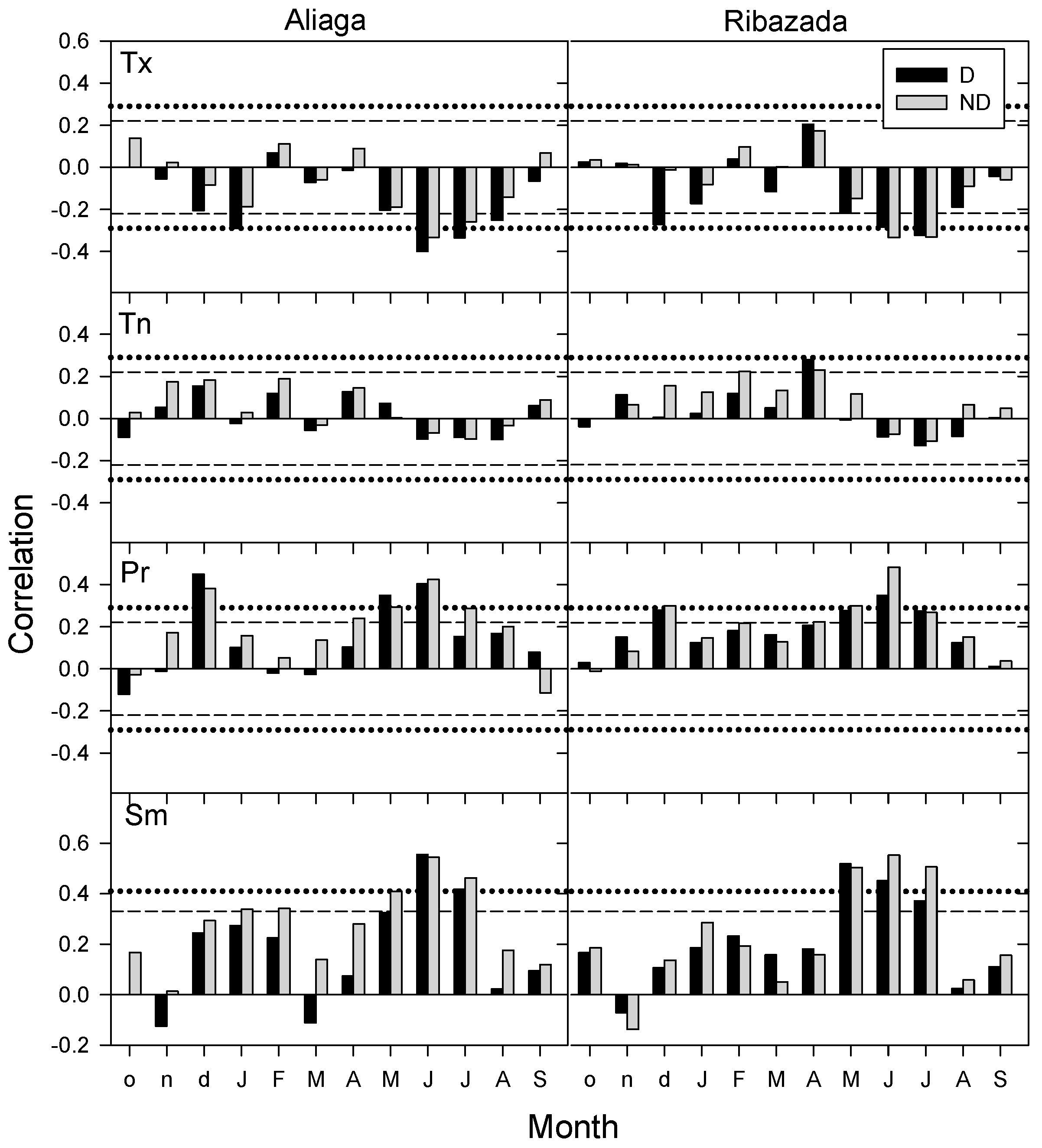
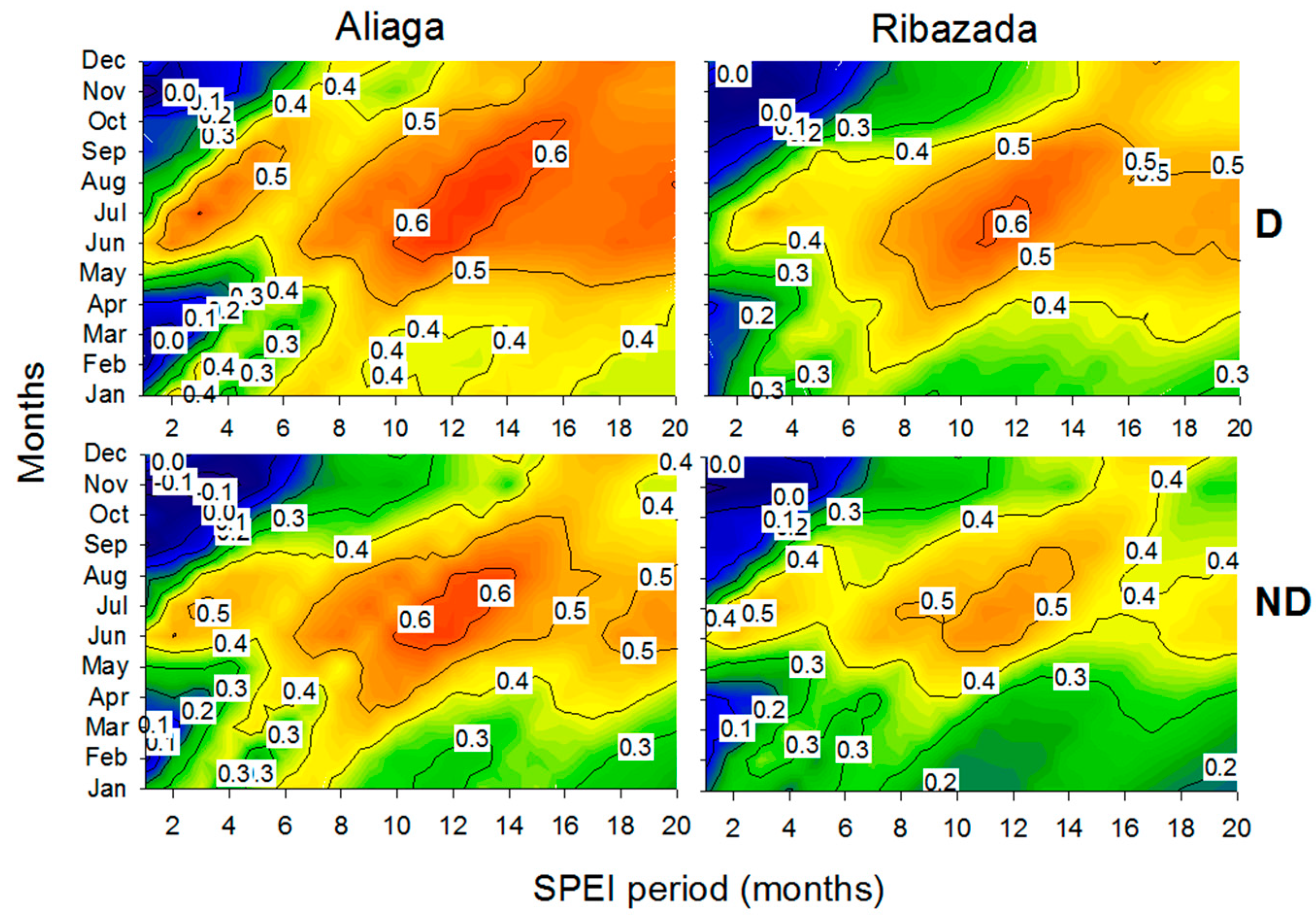
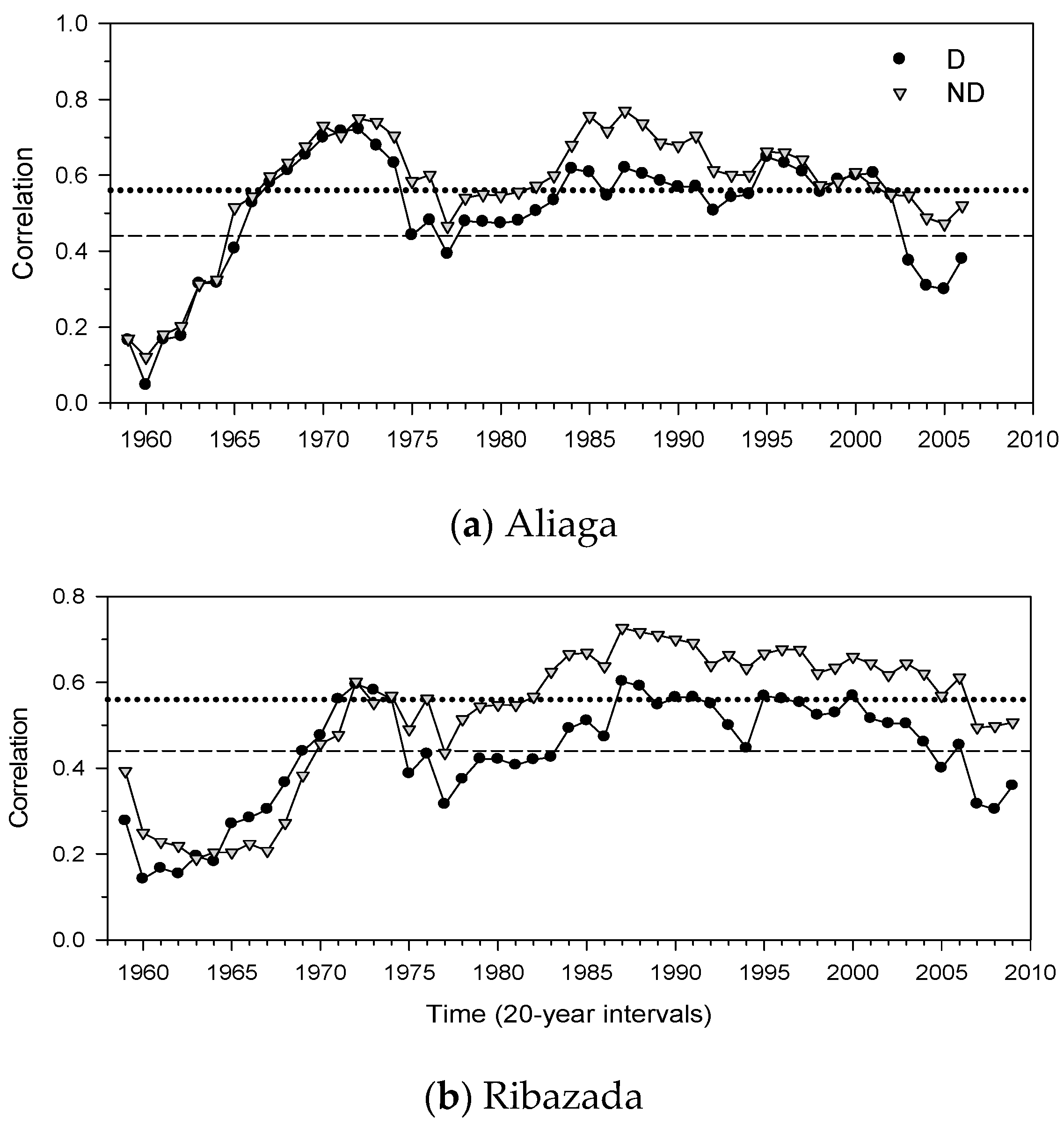
3.3. Growth Patterns and Climate-Growth Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Cook, B.I.; Anchukaitis, K.J.; Touchan, R.; Meko, D.M.; Cook, E.R. Spatiotemporal drought variability in the Mediterranean over the last 900 years. JGR Atmosph. 2016, 121, 2060–2074. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salguero, R.; Camarero, J.J.; Gutiérrez, E.; González Rouco, F.; Gazol, A.; Sangüesa-Barreda, G.; Andreu-Hayles, L.; Linares, J.C.; Seftigen, K. Assessing forest vulnerability to climate warming using a process-based model of tree growth: Bad prospects for rear-edges. Glob. Chang. Biol. 2017, 23, 2705–2719. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Gazol, A.; Camarero, J.J. The performance of Mediterranean subshrubs depends more on microsite than on regional climate conditions. J. Veg. Sci. 2012, 23, 1062–1070. [Google Scholar] [CrossRef]
- Quero, J.L.; Sterck, F.J.; Martínez-Vilalta, J.; Villar, R. Water-use strategies of six co-existing Mediterranean woody species during a summer drought. Oecologia 2011, 166, 45–57. [Google Scholar] [CrossRef]
- Altieri, S.; Mereu, S.; Cherubini, P.; Castaldi, S.; Sirignano, C.; Lubritto, C.; Battipaglia, G. Tree-ring carbon and oxygen isotopes indicate different water use strategies in three Mediterranean shrubs at Capo Caccia (Sardinia, Italy). Trees 2015, 29, 1593–1603. [Google Scholar] [CrossRef]
- Baquedano, F.J.; Castillo, F.J. Drought tolerance in the Mediterranean species Quercus coccifera, Quercus ilex, Pinus halepensis and Juniperus phoenicea. Photosynthetica 2007, 45, 229–238. [Google Scholar] [CrossRef]
- Gazol, A.; Sangüesa-Barreda, G.; Granda, E.; Camarero, J.J. Tracking the impact of drought on functionally different woody plants in a Mediterranean scrubland ecosystem. Plant Ecol. 2017, 218, 1009–1020. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Díaz-Delgado, R.; Casals, R. Dieback and mortality of junipers caused by drought: Dissimilar growth and wood isotope patterns preceding shrub death. Agric. For. Meteorol. 2020, 291, 108078. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J. Greater sensitivity to hotter droughts underlies juniper dieback and mortality in Mediterranean shrublands. Sci. Tot. Environ. 2020, 721, 137599. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Griebel, A.; Watson, D.; Pendall, E. Mistletoe, friend and foe: Synthesizing ecosystem implications of mistletoe infection. Environ. Res. Lett. 2017, 12, 115012. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Linares, J.C.; Camarero, J.J. Drought and mistletoe reduce growth and water-use efficiency of Scots pine. For. Ecol. Manag. 2013, 296, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Sala, A.; Carey, E.V.; Callaway, R.M. Dwarf mistletoe affects whole-tree water relations of Douglas fir western larch primarily through changes in leaf to sapwood ratios. Oecologia 2001, 126, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, R.; Bangerter, S.; Rigling, A.; Sterck, F.J. Pine and mistletoes: How to live with a leak in the water flow and storage system? J. Exp. Bot. 2012, 63, 2565–2578. [Google Scholar] [CrossRef] [Green Version]
- Dobbertin, M.; Rigling, A. Pine mistletoe (Viscum album ssp. austriacum) contributes to Scots pine (Pinus sylvestris) mortality in the Rhone valley of Switzerland. For. Pathol. 2006, 36, 309–322. [Google Scholar] [CrossRef]
- Adams, R.P. Junipers of the World: The Genus Juniperus; Trafford: Bloomington, IN, USA, 2014. [Google Scholar]
- Ramón, P.; De la Cruz, M.; Zavala, I.; Zavala, M.A. Factors influencing the dispersion of Arceuthobium oxycedri in Central Spain: Evaluation with a new null model for marked point patterns. For. Pathol. 2016, 46, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Hawksworth, F.G.; Wiens, D. Dwarf mistletoes: Biology, Pathology, and Systematics; Agriculture Handbook No. 450; US Department of Agriculture, Forest Service: Washington, DC, USA, 1996.
- Watson, D.M. Mistletoe—a keystone resource in forests and woodlands worldwide. Ann. Rev. Ecol. Evol. Syst. 2001, 32, 219–249. [Google Scholar] [CrossRef] [Green Version]
- Ciesla, W.M.; Geils, B.W.; Adams, R.P. Hosts and geographic distribution of Arceuthobium oxycedri. (Revised 2004). RMRS-RN-11. Fort Collins, CO, U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 2002. Available online: http://www.fs.fed.us/rm/pubs/rmrs_rn11/ (accessed on 7 July 2021).
- Mathiasen, R.L. Dwarf mistletoes in forest canopies. Northwest Sci. 1996, 70, 61–71. [Google Scholar]
- Shaw, D.C.; Chen, J.; Freeman, E.A.; Braun, D.M. Spatial and population characteristics of dwarf mistletoe infected trees in an old growth Douglas-fir western hemlock forest. Can. J. For. Res. 2005, 35, 990–1001. [Google Scholar] [CrossRef] [Green Version]
- Quejeiro-Bolaños, M.; Cano-Santana, Z. Growth of Hartweg’s pine (Pinus hartwegii) parasitized by two dwarf mistletoe species (Arceuthobium spp.). Bot. Sci. 2016, 94, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Epp, B.; Tardif, J.C. Effects of lodgepole pine dwarf mistletoe, Arceuthobium americanum, on Jack pine, Pinus banksiana, growth in Manitoba. Can. Field Nat. 2004, 118, 595–601. [Google Scholar] [CrossRef] [Green Version]
- González-Elizondo, M.; Flores-Villegas, M.Y.; Álvarez-Zagoya, R.; González-Elizondo, M.S.; Mérquez-Linares, M.A.; Quiñonez-Barraza, S.; Howell, B.E.; Mathiasen, R.L. Effects of Mexican dwarf mistletoe (Arceuthobium vaginatum subsp. vaginatum) on the grouth of Pinus cooperi in Durango, Mexico—A case study. For. Pathol. 2018, 49, e12473. [Google Scholar]
- Meinzer, F.C.; Woodruff, D.R.; Shaw, D.C. Integrated responses of hydraulic architecture, water and carbon relations of western hemlock to dwarf mistletoe infection. Plant Cell Environ. 2004, 27, 937–946. [Google Scholar] [CrossRef]
- Logan, B.A.; Huhn, E.R.; Tissue, D.T. Photosynthetic characteristics of eastern dwarf mistletoe (Arceuthobium pusillum Peck) and its effects on the needles of host white spruce (Picea glauca Voss). Plant Biol. 2002, 4, 740–745. [Google Scholar] [CrossRef]
- Klutsch, J.; Erbilgin, N. Dwarf mistletoe infection in Jack pine alters growth-defense relationships. Tree Physiol. 2018, 38, 1538–1547. [Google Scholar] [CrossRef]
- Wahid, H.A.; Khan Barozai, M.Y.; Din, M. Dwarf mistletoe (Arceuthobium oxycedri) and damage caused by dwarf mistletoe to family Cupressaceae. Pure Appl. Biol. 2015, 4, 15–23. [Google Scholar] [CrossRef]
- Rey, L.; Sadik, A.; Renaudin, S. Trophic relations of the dwarf mistletoe Arceuthobium oxycedri with its host Juniperus oxycedri. J. Plants Physiol. 1991, 138, 411–416. [Google Scholar] [CrossRef]
- Ríos-Insua, V. Contribución al estudio de la biología de Arceuthobium oxycedri (DC.) M. Bieb. Bol. San. Veg. Plagas 1987, 13, 53–62. [Google Scholar]
- Pellizzari, E.; Camarero, J.J.; Gazol, A.; Granda, E.; Shetti, R.; Wilmking, M.; Moiseev, P.; Pividori, M.; Carrer, M. Diverging shrub and tree growth from the Polar to the Mediterranean biomes across the European continent. Glob. Chang. Biol. 2017, 23, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.-A.; Larsson, P.O. CDendro and CooRecorder (v. 9.3.1) [Software]; Cybis Elektronik: Saltsjöbaden, Sweden, 2018. [Google Scholar]
- Holmes, R.L. Computer assisted quality control in tree ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Fritts, H.C. Tree-Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Cook, E.R.; Krusic, P. A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling; with Interactive Graphics; Tree-Ring Laboratory; Lamont Doherty Earth Observatory; Columbia University: New York, NY, USA, 2005. [Google Scholar]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; pp. 137–152. [Google Scholar]
- Wigley, T.M.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series; with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Cornes, R.; van der Schrier, G.; van den Besselaar, E.J.M.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation datasets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef] [Green Version]
- Berrisford, P.; Dee, D.P.; Poli, P.; Brugge, R.; Fielding, M.; Fuentes, M.; Kållberg, P.W.; Kobayashi, S.; Uppala, S.; Simmons, A.; et al. The ERA-Interim Archive, 2nd ed.; ERA Report Series No. 1; Shinfield Park: Reading, UK, 2011. [Google Scholar]
- Vicente-Serrano, S.M.; Tomas-Burguera, M.; Beguería, S.; Reig, F.; Latorre, B.; Peña-Gallardo, M.; Luna, M.Y.; Morata, A.; González-Hidalgo, J.C. A high resolution dataset of drought indices for Spain. Data 2017, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods; J. Wiley: New York, NY, USA, 1973. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-151. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 19 October 2020).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]

| Variables | Aliaga | Ribazada | ||||
|---|---|---|---|---|---|---|
| Declining Individuals | Non-Declining Individuals | Dead Individuals | Declining Individuals | Non-Declining Individuals | Dead Individuals | |
| No. measured individuals/radii | 15/28 | 15/29 | 11/22 | 20/40 | 20/36 | 3/6 |
| Basal diameter (cm) | 4.9 ± 1.6 | 4.4 ± 1.4 | 4.7 ± 1.0 | 8.1 ± 3.0 | 7.7 ± 2.0 | 9.4 ± 3.2 |
| Height (m) | 1.56 ±0.36 | 1.64 ± 0.28 | 1.81 ± 0.39 | 2.58 ± 0.48 | 2.81 ± 0.58 | 3.10 ± 0.46 |
| Age (years) | 83 ± 4 | 78 ± 6 | 82 ± 4 | 82 ± 5 | 79 ± 5 | 77 ± 5 |
| Crown cover (%) | 45.0 ± 21.9 b | 96.0 ± 6.0 c | 0.0 ± 0.0 a | 34. 2 ± 12.0 b | 93. 8 ± 5.0 c | 0.0 ± 0.0 a |
| Mistletoe infestation (%) | 26.0 ± 7.8 b | 1.0 ± 0.0 a | 31.4 ± 8.09 b | 38.0 ± 10.1 b | 0.9 ± 0.1 a | 40.0±0.00 b |
| Ring width (mm) 1 | 0.39 ± 0.04 | 0.34 ± 0.03 | 0.39 ± 0.06 | 0.56 ± 0.04 | 0.50 ± 0.03 | 0.52 ±0.05 |
| First-order autocorrelation 1 | 0.67 ± 0.06 | 0.57 ± 0.06 | 0.61 ± 0.05 | 0.55 ± 0.03 | 0.54 ± 0.02 | 0.55 ± 0.06 |
| Mean sensitivity 1 | 0.35 ± 0.02 | 0.37 ± 0.02 | 0.34 ± 0.02 | 0.42 ± 0.01 | 0.39 ± 0.02 | 0.39 ± 0.03 |
| Correlation among series (rbar) 1 | 0.23 ± 0.02 a | 0.34 ± 0.03 b | 0.19 ± 0.01 a | 0.30 ± 0.02 b | 0.25 ± 0.01 a | 0.29 ± 0.01 b |
| Time span | 1908–2016 | 1923–2016 | 1894–2016 | 1886–2019 | 1907–2019 | 1931–2016 |
| Best-replicated time span 2 | 1943–2016 | 1944–2016 | 1946–2016 | 1943–2019 | 1939–2019 | 1942–2016 |
| Variable | Slope | SE | t value | p | ||||
|---|---|---|---|---|---|---|---|---|
| Aliaga | Ribazada | Aliaga | Ribazada | Aliaga | Ribazada | Aliaga | Ribazada | |
| Intercept | −33.28 | −8.62 | 0.71 | 0.51 | −46.85 | −16.94 | <0.01 | <0.01 |
| Year | 0.02 | 0.01 | 0.01 | 0.01 | 48.16 | 17.39 | <0.01 | <0.01 |
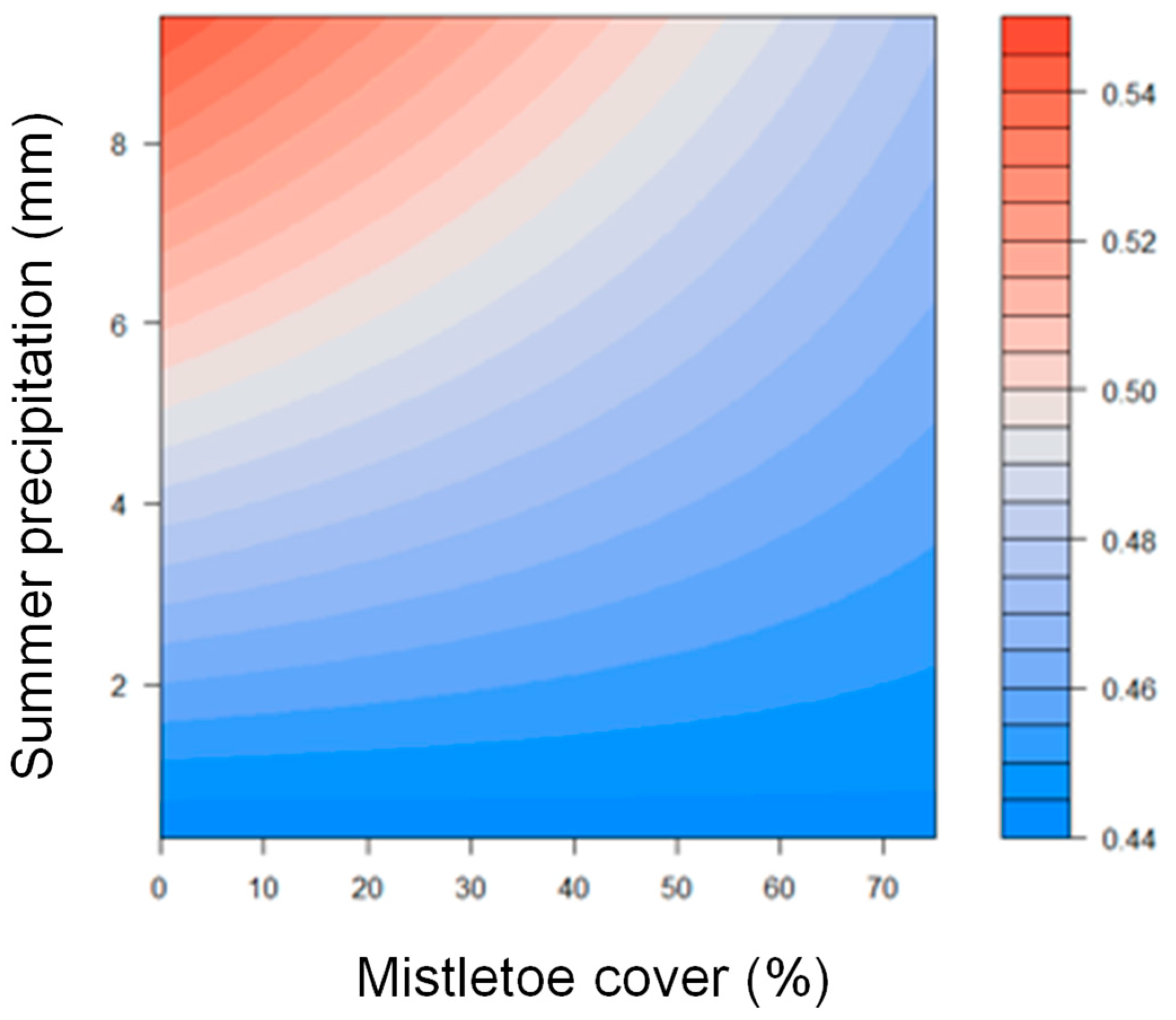
| Mistletoe infestation | −0.01 | 0.04 | 0.03 | 0.02 | −0.35 | 2.29 | 0.73 | 0.03 |
| Summer prec. | 0.01 | 0.01 | 0.01 | 0.01 | 2.22 | 12.85 | 0.03 | <0.01 |
| Year x infestation | 0.01 | −0.01 | 0.01 | 0.01 | 0.52 | −2.29 | 0.61 | 0.02 |
| Infestation x summer prec. | −0.01 | −0.01 | 0.01 | 0.01 | −0.93 | −3.54 | 0.35 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamudo, E.; Camarero, J.J.; Sangüesa-Barreda, G.; Anadón, J.D. Dwarf Mistletoe and Drought Contribute to Growth Decline, Dieback and Mortality of Junipers. Forests 2021, 12, 1199. https://doi.org/10.3390/f12091199
Tamudo E, Camarero JJ, Sangüesa-Barreda G, Anadón JD. Dwarf Mistletoe and Drought Contribute to Growth Decline, Dieback and Mortality of Junipers. Forests. 2021; 12(9):1199. https://doi.org/10.3390/f12091199
Chicago/Turabian StyleTamudo, Elisa, J. Julio Camarero, Gabriel Sangüesa-Barreda, and José Daniel Anadón. 2021. "Dwarf Mistletoe and Drought Contribute to Growth Decline, Dieback and Mortality of Junipers" Forests 12, no. 9: 1199. https://doi.org/10.3390/f12091199
APA StyleTamudo, E., Camarero, J. J., Sangüesa-Barreda, G., & Anadón, J. D. (2021). Dwarf Mistletoe and Drought Contribute to Growth Decline, Dieback and Mortality of Junipers. Forests, 12(9), 1199. https://doi.org/10.3390/f12091199







