Commercial Logging of Timber Species Enhances Amazon (Brazil) Nut Populations: Insights from Bolivian Managed Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Species Description
2.3. Community Involvement in Data Collection (Participatory Research)
2.4. Study Design
2.5. Collection of Demographic Data
2.6. Evaluation of Amazon Nut Production and Harvesting Intensity
2.7. Estimation of Timber Logging Intensity
2.8. Modeling Effects of Tree Conditions, Harvesting Intensity, and Management on Bertholletia Vital Rates
2.9. Matrix Construction
3. Results
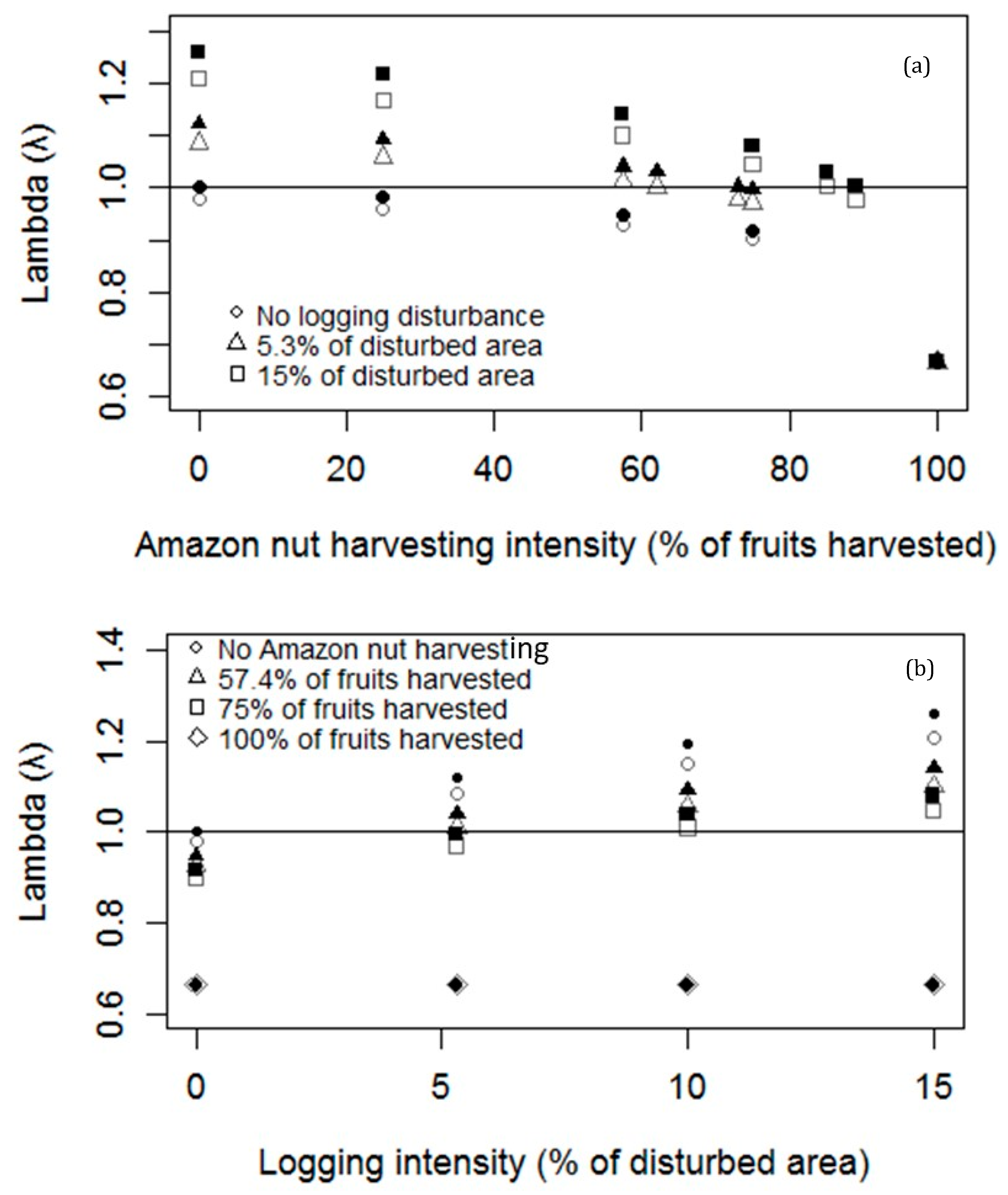
3.1. Effects of Amazon Nut Harvesting and Logging on Bertholletia Vital Rates
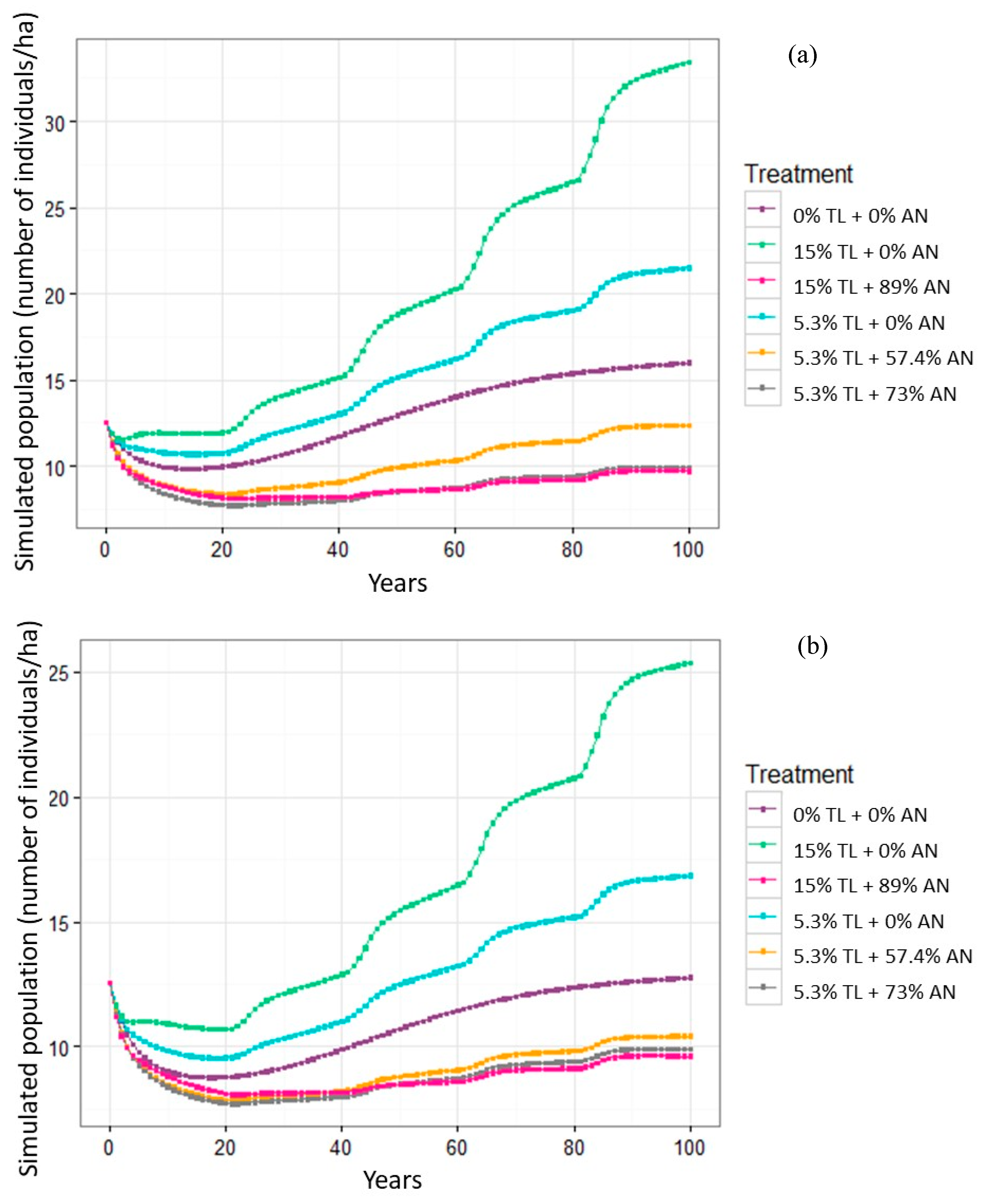
3.2. Bertholletia Populations under Amazon Nut Harvesting and Timber Logging Intensities
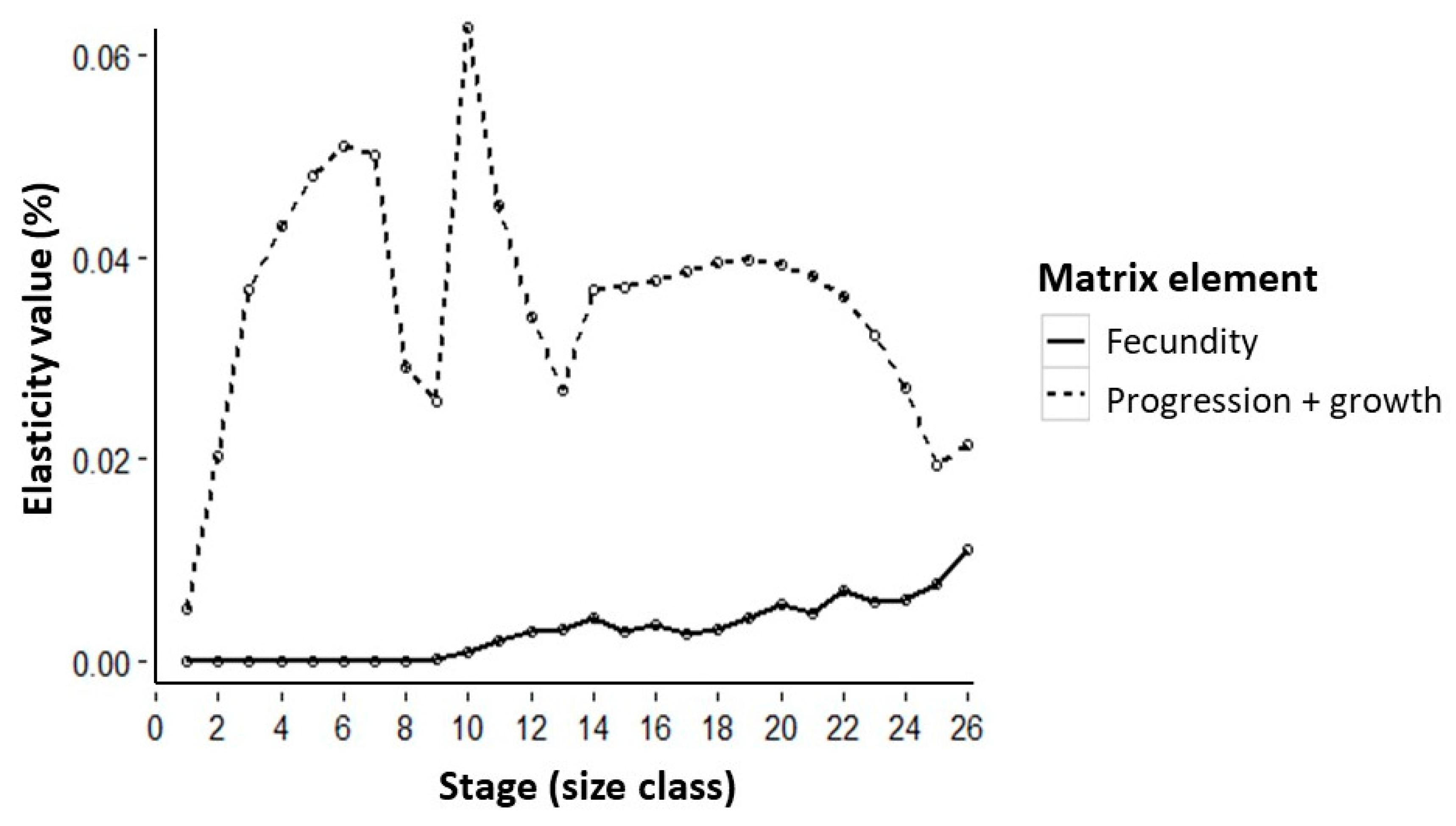
3.3. Liana Cutting Intensity Improves Bertholletia Population Growth Rate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- García-Fernández, C.; Ruiz-Pérez, M.; Wunder, S. Is multiple-use forest management widely implementable in the tropics? For. Ecol. Manag. 2008, 256, 1468–1476. [Google Scholar] [CrossRef]
- Sabogal, C.; Guariguata, M.; Broadhead, J.; Lescuyer, G. Multiple-Use Forest Management in the Humid Tropics: Opportunities and Challenges for Sustainable Forest Management; Food and Agriculture Organization on the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Cronkleton, P.; Guariguata, M.R.; Albornoz, M.A. Multiple use forestry planning: Timber and Brazil nut management in the community forests of Northern Bolivia. For. Ecol. Manag. 2012, 268, 49–56. [Google Scholar] [CrossRef]
- Shanley, P.; da Serra Silva, M.; Melo, T.; Carmenta, R.; Nasi, R. From conflict of use to multiple use: Forest management innovations by small holders in Amazonian logging frontiers. For. Ecol. Manag. 2012, 268, 70–80. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Cronkleton, P.; Duchelle, A.E.; Zuidema, P. Revisiting the ‘cornerstone of Amazonian conservation’: A socioecological assessment of Brazil nut exploitation. Biodivers. Conserv. 2017, 26, 2007–2027. [Google Scholar] [CrossRef]
- Banco Ganadero. Bolivia: Exportación de Castañas; Boletín Electrónico Bisemanal Nº 840; IBCE CIFRAS: Santa Cruz, Bolivia, 2019. [Google Scholar]
- Soriano, M.; Mohren, F.; Ascarrunz, N.; Dressler, W.; Peña-Claros, M. Socio-ecological costs of Amazon nut and timber production at community household forests in the Bolivian Amazon. PLoS ONE 2017, 12, e0170594. [Google Scholar] [CrossRef]
- Myers, G.P.; Newton, A.C.; Melgarejo, O. The influence of canopy gap size on natural regeneration of Brazil nut (Bertholletia excelsa) in Bolivia. For. Ecol. Manag. 2000, 127, 119–128. [Google Scholar] [CrossRef]
- Schwartz, G.; Peña-Claros, M.; Lopes, J.C.A.; Mohren, G.M.J.; Kanashiro, M. Mid-term effects of reduced-impact logging on the regeneration of seven tree commercial species in the Eastern Amazon. For. Ecol. Manag. 2012, 274, 116–125. [Google Scholar] [CrossRef]
- Silva, J.N.M.; de Carvalho, J.D.; do Ca Lopes, J.; De Almeida, B.F.; Costa, D.H.M.; de Oliveira, L.D.; Vanclay, J.K.; Skovsgaard, J.P. Growth and yield of a tropical rain forest in the Brazilian Amazon 13 years after logging. For. Ecol. Manag. 1995, 71, 267–274. [Google Scholar] [CrossRef]
- Soriano, M.; Kainer, K.A.; Staudhammer, C.L.; Soriano, E. Implementing multiple forest management in Brazil nut-rich community forests: Effects of logging on natural regeneration and forest disturbance. For. Ecol. Manag. 2012, 268, 92–102. [Google Scholar] [CrossRef]
- Zuidema, P.A.; Boot, R.G.A. Demography of the Brazil nut tree (Bertholletia excelsa) in the Bolivian Amazon: Impact of seed extraction on recruitment and population dynamics. J. Trop. Ecol. 2002, 18, 1–31. [Google Scholar] [CrossRef]
- Duchelle, A.E.; Guariguata, M.R.; Less, G.; Albornoz, M.A.; Chavez, A.; Melo, T. Evaluating the opportunities and limitations to multiple use of Brazil nuts and timber in Western Amazonia. For. Ecol. Manag. 2012, 268, 39–48. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Licona, J.C.; Mostacedo, B.; Cronkleton, P. Damage to Brazil nut trees (Bertholletia excelsa) during selective timber harvesting in Northern Bolivia. For. Ecol. Manag. 2009, 258, 788–793. [Google Scholar] [CrossRef]
- Hall, P.; Bawa, K. Methods to Assess the Impact of Extraction of Non-Timber Tropical Forest Products on Plant Populations. Econ. Bot. 2010, 47, 234–247. [Google Scholar] [CrossRef]
- Gourlet-Fleury, S.; Mortier, F.; Fayolle, A.; Baya, F.; Ouédraogo, D.; Bénédet, F.; Picard, N. Tropical forest recovery from logging: A 24 year silvicultural experiment from Central Africa. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120302. [Google Scholar] [CrossRef]
- Rockwell, C.A.; Kainer, K.A.; Marcondes, N.; Baraloto, C. Ecological limitations of reduced-impact logging at the smallholder scale. For. Ecol. Manag. 2007, 238, 365–374. [Google Scholar] [CrossRef]
- Shenkin, A.; Bolker, B.; Peña-Claros, M.; Licona, J.C.; Putz, F.E. Fates of trees damaged by logging in Amazonian Bolivia. For. Ecol. Manag. 2015, 357, 50–59. [Google Scholar] [CrossRef]
- Van Rheenen (Jacaranda), H.M.P.J.B.; Boot, R.G.A.; Werger, M.J.A.; Ulloa Ulloa, M. Regeneration of timber trees in a logged tropical forest in North Bolivia. For. Ecol. Manag. 2004, 25, 39–48. [Google Scholar] [CrossRef]
- Schulze, M.; Grogan, J.; Landis, R.M.; Vidal, E. How rare is too rare to harvest? Management challenges posed by timber species occurring at low densities in the Brazilian Amazon. For. Ecol. Manag. 2008, 256, 1443–1457. [Google Scholar] [CrossRef]
- Gaoue, O.G.; Horvitz, C.C.; Ticktin, T. Non-timber forest products harvest in variable environments: Modeling the effects of harvesting as a stochastic sequence. Ecol. Appl. 2011, 21, 1604–1616. [Google Scholar] [CrossRef]
- Kainer, K.A.; Wadt, L.H.O.; Staudhammer, C.L. Explaining variation in Brazil nut fruit production. For. Ecol. Manag. 2007, 250, 244–255. [Google Scholar] [CrossRef]
- Peña-Claros, M.; Fredericksen, T.S.; Alarcón, A.; Blate, G.M.; Choque, U.; Leaño, C.; Licona, J.C.; Mostacedo, B.; Pariona, W.; Villegas, Z.; et al. Beyond reduced-impact logging: Silvicultural treatments to increase growth rates of tropical trees. For. Ecol. Manag. 2008, 256, 1458–1467. [Google Scholar] [CrossRef]
- Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation, 2nd ed.; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2001. [Google Scholar]
- Herrero-Jáuregui, C.; García-Fernández, C.; Sist, P.L.J.; Casado, M.A. Recruitment dynamics of two low-density neotropical multiple-use tree species. Plant Ecol. 2011, 212, 1501–1512. [Google Scholar] [CrossRef]
- Klimas, C.A.; Cropper, W.P.; Kainer, K.A.; de Oliveira Wadt, L.H. Viability of combined timber and non-timber harvests for one species: A Carapa guianensis case study. Ecol. Model. 2012, 246, 147–156. [Google Scholar] [CrossRef]
- Salick, J.; Mejia, A.; Anderson, T. Non-timber forest products integrated with natural forest management, Rio San Juan, Nicaragua. Ecol. Appl. 1995, 5, 878–895. [Google Scholar] [CrossRef]
- Moll-Rocek, J.; Gilbert, M.E.; Broadbent, E.N. Brazil Nut (Bertholletia excelsa, Lecythidaceae) Regeneration in Logging Gaps in the Peruvian Amazon. Int. J. For. Res. 2014, 2014, 420764. [Google Scholar] [CrossRef]
- Cotta, J.N.; Kainer, K.A.; Wadt, L.H.O.; Staudhammer, C.L. Shifting cultivation effects on Brazil nut (Bertholletia excelsa) regeneration. For. Ecol. Manag. 2008, 256, 28–35. [Google Scholar] [CrossRef]
- D’Oliveira, M.V.N. Artificial regeneration in gaps and skidding trails after mechanised forest exploitation in Acre, Brazil. For. Ecol. Manag. 2000, 127, 67–76. [Google Scholar] [CrossRef]
- Rockwell, C.A.; Guariguata, M.R.; Menton, M.; Arroyo Quispe, E.; Quaedvlieg, J.; Warren-Thomas, E.; Fernandez Silva, H.; Jurado Rojas, E.E.; Kohagura Arrunátegui, J.A.H.; Meza Vega, L.A.; et al. Nut Production in Bertholletia excelsa across a Logged Forest Mosaic: Implications for Multiple Forest Use. PLoS ONE 2015, 10, e0135464. [Google Scholar] [CrossRef]
- Kainer, K.A.; Wadt, L.H.O.; Staudhammer, C.L. Testing a silvicultural recommendation: Brazil nut responses 10 years after liana cutting. J. Appl. Ecol. 2014, 51, 655–663. [Google Scholar] [CrossRef][Green Version]
- Marsik, M.; Stevens, F.R.; Southworth, J. Amazon deforestation: Rates and patterns of land cover change and fragmentation in Pando, northern Bolivia, 1986 to 2005. Prog. Phys. Geogr. 2011, 35, 353–374. [Google Scholar] [CrossRef]
- Hjortsø, C.N.; Jacobsen, J.B.; Bach, K.; Kamelarczyk, F.; Moraes, M.R. Economía forestal en Bolivia. Botánica Económica de Los Andes Centrales 2006, 2006, 533–557. [Google Scholar]
- Mostacedo, B.; Balcazar, J.; Montero, J.C. Tipos de bosque, diversidad y composición florística en la Amazonia sudoeste de Bolivia Forest types, diversity and floristic composition in the southwestern amazon of Bolivia. Ecol. Boliv. 2006, 41, 99–116. [Google Scholar]
- Zonificación Agroecológica y Establecimiento de Una Base de Datos y Red de Sistema de Información Geográfica en Bolivia; ZONISIG: La Paz, Bolivia, 1997; p. 271.
- Bojanic, A.J. Balance is Beautiful: Assessing Sustainable Development in the Rain Forests of the Bolivian Amazon; PROMAB Scientific Series: Riberalta, Bolivia, 2001. [Google Scholar]
- Pacheco, P.; Ormachea, E.; Cronkleton, P.; Albornoz, M.A.; Paye, L. Trayectorias y Tendencias de La Economía Forestal Extractiva en el Norte Amazónico de Bolivia; CIFOR-CEDLA: La Paz, Bolivia, 2009. [Google Scholar]
- Kainer, K.A.; Duryea, M.L.; De Macêdo, N.C.; Williams, K. Brazil nut seedling establishment and autecology in extractive reserves of Acre, Brazil. Ecol. Appl. 1998, 8, 397–410. [Google Scholar] [CrossRef]
- Zuidema, P. Ecology and Management of the Brazil Nut Tree (Bertholletia excelsa); PROMAB (Scientific Series 6): Riberalta, Bolivia, 2003. [Google Scholar]
- Paiva, P.M.; Guedes, M.C.; Funi, C. Brazil nut conservation through shifting cultivation. For. Ecol. Manag. 2011, 261, 508–514. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, A.C.; Gribel, R.; Wegge, P.; Haugaasen, T.M.J. Fruit Removal and Natural Seed Dispersal of the Brazil Nut Trre (Bertholletia excelsa) in Central Amazonia, Brazil. Biotropica 2012, 44, 205–210. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Zuidema, P.A. Lifetime growth patterns and ages of Bolivian rain forest trees obtained by tree ring analysis. J. Ecol. 2006, 94, 481–493. [Google Scholar] [CrossRef]
- Levis, C.; Costa, F.R.C.; Bongers, F.; Peña-Claros, M.; Clement, C.R.; Junqueira, A.B.; Neves, E.G.; Tamanaha, E.K.; Figueiredo, F.O.G.; Salomão, R.P.; et al. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 2017, 355, 925–931. [Google Scholar] [CrossRef]
- Shepard, G.H., Jr.; Ramirez, H. “Made in Brazil”: Human Dispersal of the Brazil Nut (Bertholletia excelsa, Lecythidaceae) in Ancient Amazonia. Econ. Bot. 2011, 65, 44–65. [Google Scholar] [CrossRef]
- Dawkins, H.C.; Field, D.R.B. A Long-term Surveillance System for British Woodland Vegetation; University of Oxford: Oxford, UK, 1978. [Google Scholar]
- Metcalf, C.J.E.; Clark, J.S.; Clark, D.A. Tree growth inference and prediction when the point of measurement changes: Modelling around buttresses in tropical forests. J. Trop. Ecol. 2009, 25, 1–12. [Google Scholar] [CrossRef]
- Cushman, K.C.; Muller-Landau, H.C.; Condit, R.S.; Hubbell, S.P. Improving estimates of biomass change in buttressed trees using tree taper models. Methods Ecol. Evol. 2014, 5, 573–582. [Google Scholar] [CrossRef]
- Contreras, F.; Cordero, W.; Fredericksen, T.S. Evaluación del Aprovechamiento Forestal; BOLFOR: Santa Cruz, Bolivia, 2001. [Google Scholar]
- The R Development Core Team. R: A Language for Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Staudhammer, C.L.; Wadt, L.H.O.; Kainer, K.A. Tradeoffs in basal area growth and reproduction shift over the lifetime of a long-lived tropical species. Oecologia 2013, 173, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Villegas, Z.; Peña-Claros, M.; Mostacedo, B.; Alarcón, A.; Licona, J.C.; Leaño, C.; Pariona, W.; Choque, U. Silvicultural treatments enhance growth rates of future crop trees in a tropical dry forest. For. Ecol. Manag. 2009, 258, 971–977. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Firth, D. Package “MASS”: Modern Applied Statistics with S.(R Package Version 7.3–47); Springer: Berlin, Germany, 2016; Available online: http://www.stats.ox.ac.uk/pub/MASS4/ (accessed on 24 February 2021).
- Harrell, F.E. Package “rms”: Regression Modelling Strategies (Version 5.1–1); Springer: Berlin, Germany, 2016; Available online: http://biostat.mc.vanderbilt.edu/rms (accessed on 24 February 2021).
- Staudhammer, C.L.; Wadt, L.H.O.; Kainer, K.A.; da Cunha, T.A. Comparative models disentangle drivers of fruit production variability of an economically and ecologically important long-lived Amazonian tree. Sci. Rep. 2021, 11, 2563. [Google Scholar] [CrossRef] [PubMed]
- Moral-Pajares, E.; Martínez-Alcalá, C.; Gallego-Valero, L.; Caviedes-Conde, A.A. Transparency Index of the Supplying Countries’ Institutions and Tree Cover Loss: Determining Factors of EU Timber Imports? Forests 2020, 11, 1009. [Google Scholar] [CrossRef]
| Response | Size Class | R-squared | Significant Predictors | Estimate | p-Value |
|---|---|---|---|---|---|
| Survival | <1 cm in DBH | 0.11 | Intercept | 0.617 | 0.145 |
| Initial height (m) | 1.921 | 0.018 * | |||
| >1 cm in DBH | 0.40 | Intercept | 13.687 | 0.021 * | |
| Initial DBH (cm) | −0.056 | 0.074 | |||
| Growth | <1 cm in DBH | 0.08 | Intercept | 0.054 | 0.046 * |
| Percentage of area disturbed due to logging | 0.011 | 0.003 ** | |||
| Years since last logging | −0.008 | 0.064 | |||
| 1–50 cm in DBH | 0.2 | Intercept | 0.282 | <0.001 *** | |
| Initial DBH (cm) | 0.022 | <0.001 *** | |||
| >50 cm in DBH | 0.15 | Intercept | 0.466 | 0.247 | |
| Initial DBH (cm) | −0.007 | 0.002 ** | |||
| Full light | 0.601 | 0.013 * | |||
| Vertical light | 0.313 | 0.279 | |||
| Perfect crown | 0.614 | 0.039 * | |||
| Good crown | 0.302 | 0.297 | |||
| Fairly good crown | 0.293 | 0.353 | |||
| Probability of being reproductive | >30 cm in DBH | 0.30 | Intercept | −9.43 | <0.001 *** |
| Initial DBH (cm) | 0.222 | <0.001 ** | |||
| Fruit production | >30 cm in DBH | 0.55 | Intercept | 1.367 | 0.004 ** |
| Initial DBH (cm) | 0.012 | <0.001 *** | |||
| Amazon nut harvesting intensity (% of harvested fruits) | 0.033 | <0.001 *** | |||
| Liana cutting | 0.497 | 0.037 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, M.; Zuidema, P.A.; Barber, C.; Mohren, F.; Ascarrunz, N.; Licona, J.C.; Peña-Claros, M. Commercial Logging of Timber Species Enhances Amazon (Brazil) Nut Populations: Insights from Bolivian Managed Forests. Forests 2021, 12, 1059. https://doi.org/10.3390/f12081059
Soriano M, Zuidema PA, Barber C, Mohren F, Ascarrunz N, Licona JC, Peña-Claros M. Commercial Logging of Timber Species Enhances Amazon (Brazil) Nut Populations: Insights from Bolivian Managed Forests. Forests. 2021; 12(8):1059. https://doi.org/10.3390/f12081059
Chicago/Turabian StyleSoriano, Marlene, Pieter A. Zuidema, Cristina Barber, Frits Mohren, Nataly Ascarrunz, Juan Carlos Licona, and Marielos Peña-Claros. 2021. "Commercial Logging of Timber Species Enhances Amazon (Brazil) Nut Populations: Insights from Bolivian Managed Forests" Forests 12, no. 8: 1059. https://doi.org/10.3390/f12081059
APA StyleSoriano, M., Zuidema, P. A., Barber, C., Mohren, F., Ascarrunz, N., Licona, J. C., & Peña-Claros, M. (2021). Commercial Logging of Timber Species Enhances Amazon (Brazil) Nut Populations: Insights from Bolivian Managed Forests. Forests, 12(8), 1059. https://doi.org/10.3390/f12081059






