Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia
Abstract
:1. Introduction
2. Methods
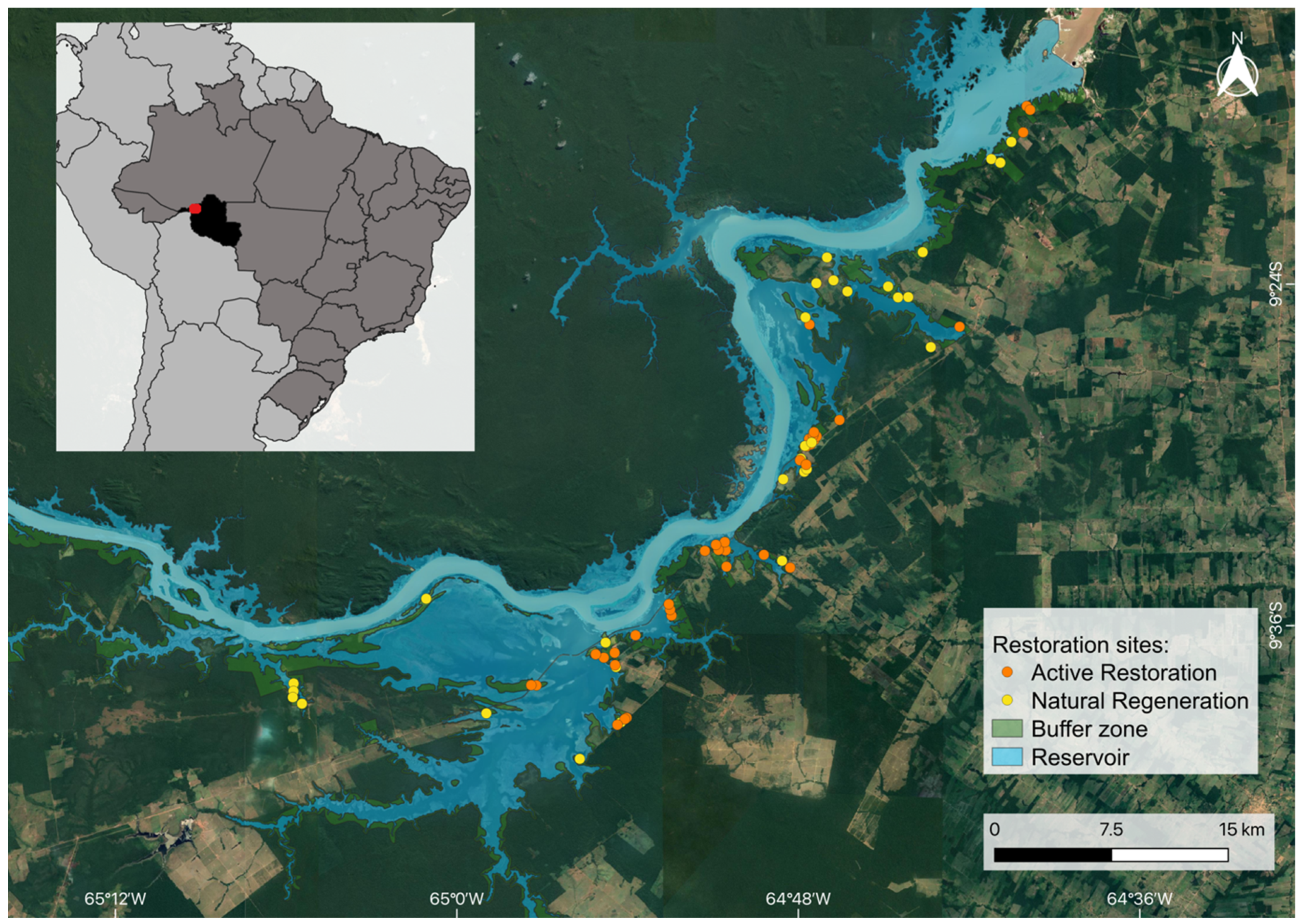
2.1. Study Area and Restoration Interventions
2.2. Sampling and Species Classification
2.3. Data Analysis
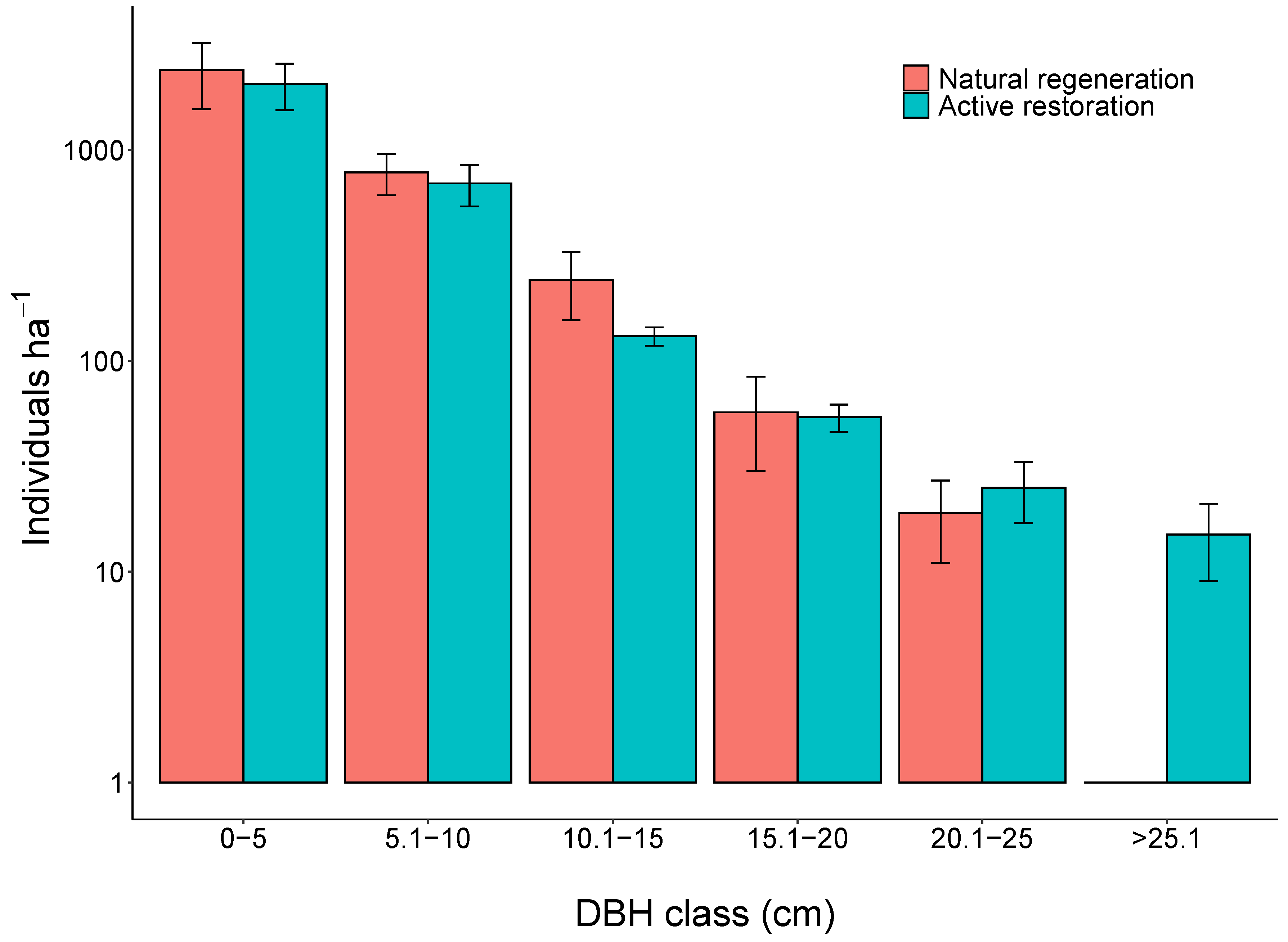
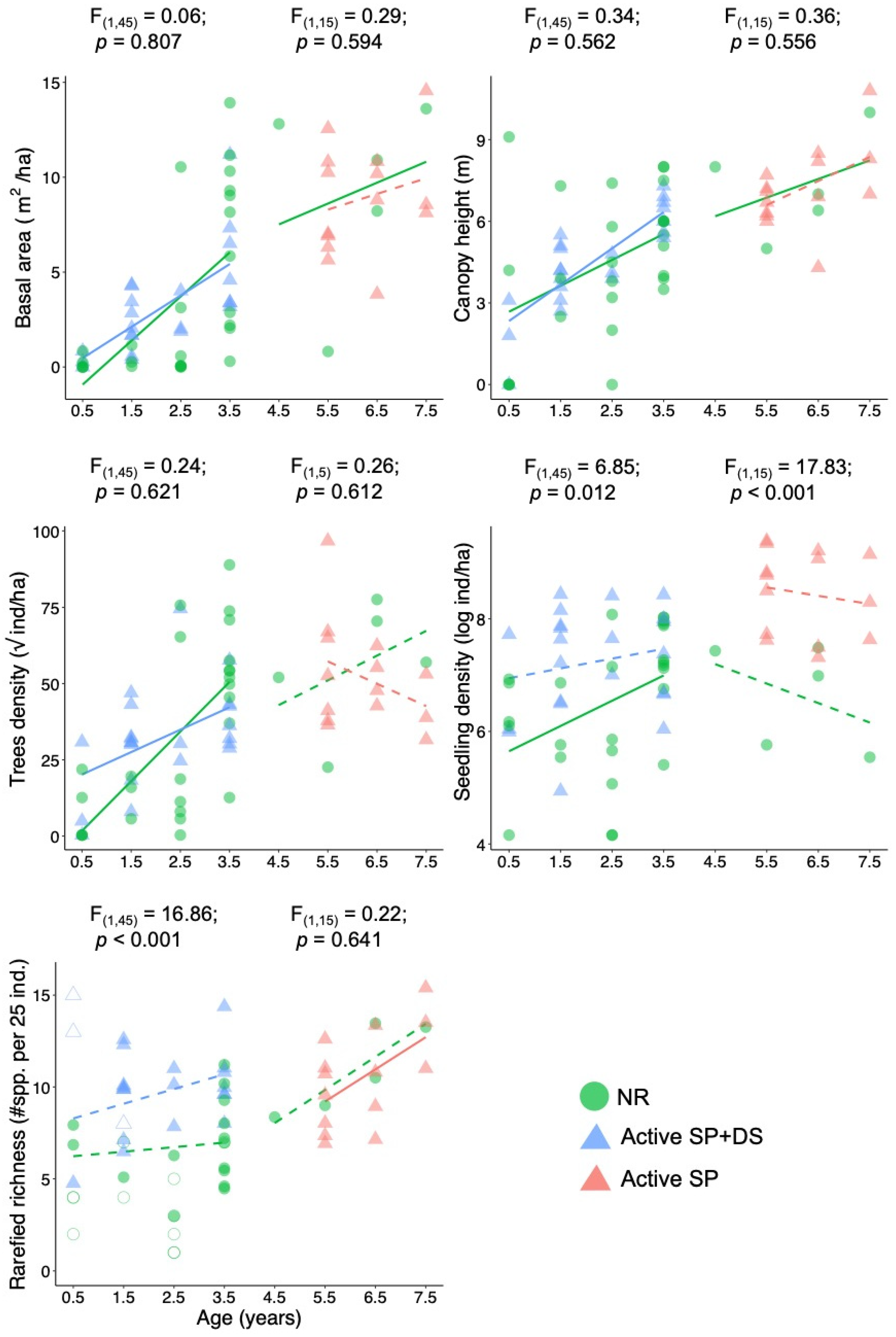
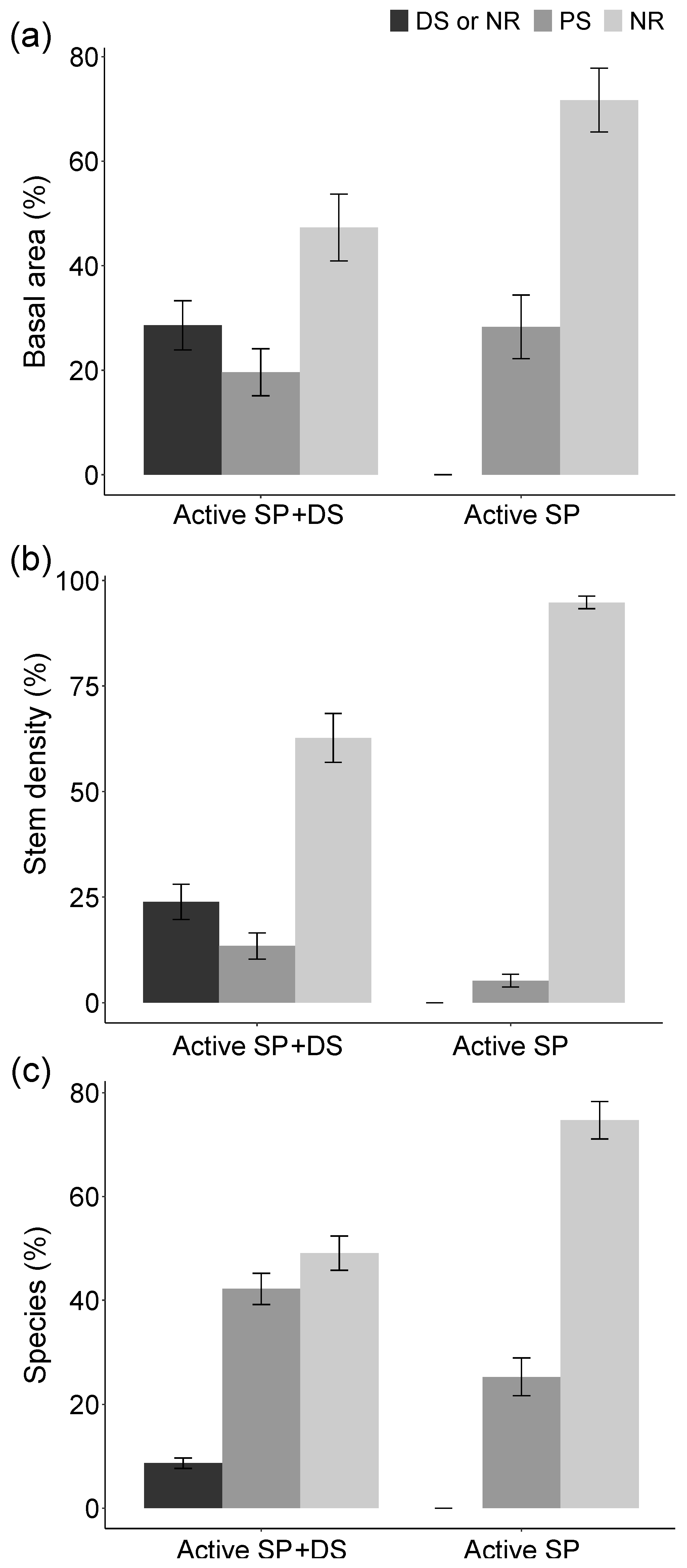
3. Results
4. Discussion
4.1. Active Restoration Is a Matter of Eliminating Regeneration Filters
4.2. Active Restoration and Naturally Regenerating Forests in Amazonia
4.3. Active Restoration or Natural Regeneration?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supporting Information
| Site–Age | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A 1-0.5 | A 2-0.5 | A 3-0.5 | A 1-1.5 | A 2-1.5 | A 3-1.5 | A 4-1.5 | A 5-1.5 | A 6-1.5 | A 7-1.5 | A 8-1.5 | A 9-1.5 | A 1-2.5 | A 2-2.5 | A 3-2.5 | A 1-3.5 | A 2-3.5 | A 3-3.5 | A 4-3.5 | A 5-3.5 | A 6-3.5 | A 7-3.5 | |
| Adenanthera pavonina | 2.4 | 1.5 | 2.6 | |||||||||||||||||||
| Anacardium occidentale | 2.8 | 1.8 | 1.4 | 4.0 | 3.0 | 0.9 | ||||||||||||||||
| Annona montana | 2.1 | 3.2 | ||||||||||||||||||||
| Apeiba tibourbou | 3.7 | 3.7 | ||||||||||||||||||||
| Bauhinia sp. | 1.7 | 1.5 | 0.1 | 1.6 | 1.8 | 1.9 | 0.8 | 0.6 | ||||||||||||||
| Bellucia grossularioides | 2.7 | |||||||||||||||||||||
| Bixa orellana | 15.1 | 15.1 | 11.8 | 4.0 | 2.5 | 1.0 | 1.9 | 2.0 | 0.5 | 5.0 | 11.2 | |||||||||||
| Byrsonima crassifolia | 1.0 | 5.0 | ||||||||||||||||||||
| Cecropia spp. | 1.1 | 1.1 | 2.8 | 0.5 | 0.5 | 1.3 | 1.0 | 0.1 | 1.9 | 1.4 | 2.2 | 1.9 | ||||||||||
| Ceiba sp. | 2.7 | 0.6 | 1.5 | |||||||||||||||||||
| Cochlospermum orinocense | 3.7 | 3.7 | 2.1 | |||||||||||||||||||
| Euterpe oleraceae | 1.2 | 1.2 | 1.2 | 12.1 | 2.1 | 1.8 | 0.5 | 7.0 | ||||||||||||||
| Handroanthus avellanedae | 0.9 | 0.4 | ||||||||||||||||||||
| Handroanthus chrysotrichus | 2.1 | 1.3 | 1.4 | 0.5 | 0.3 | |||||||||||||||||
| Himatanthus sucuuba | 0.7 | |||||||||||||||||||||
| Hymenaea courbaril | 2.0 | 6.0 | 2.8 | 4.8 | 3.0 | |||||||||||||||||
| Jenipa americana | 3.1 | 1.6 | 4.5 | 4.5 | ||||||||||||||||||
| Não identificada | 11.6 | 6.6 | 10.4 | |||||||||||||||||||
| Ochroma pyramidale | 4.1 | 4.1 | 3.9 | 0.8 | 2.7 | |||||||||||||||||
| Pachira sp. | 1.7 | |||||||||||||||||||||
| Schizolobium amazonicum | 1.0 | 1.0 | 4.1 | 3.0 | 1.2 | 1.7 | 1.0 | 1.9 | 0.2 | 2.9 | 5.7 | 2.9 | 6.5 | 4.9 | 2.6 | |||||||
| Sclerolobium paniculatum | 6.4 | 2.9 | 2.6 | 1.4 | 0.5 | 5.0 | 1.3 | |||||||||||||||
| Senna alata | 5.5 | 5.5 | 4.5 | 4.8 | 4.1 | 2.2 | 2.1 | 0.4 | 1.3 | 1.0 | 0.6 | 0.1 | 2.0 | 1.3 | 2.0 | 1.3 | 0.3 | |||||
| Solanum spp. | 48.1 | 48.1 | 19.9 | 1.0 | 1.2 | 1.6 | 4.5 | 4.5 | 2.3 | 3.0 | 11.1 | 0.3 | 0.3 | 1.9 | 1.2 | 1.7 | 1.0 | |||||
| Spondias mombin | 1.6 | 9.6 | 27.5 | 27.5 | 4.0 | 29.0 | ||||||||||||||||
| Stryphnodendron sp. | 5.2 | 5.2 | 4.1 | 5.1 | 2.4 | 4.9 | 2.1 | 0.5 | 5.8 | 2.6 | ||||||||||||
| Syzygium jambolanum | 0.9 | 3.8 | 2.0 | 2.4 | 2.9 | 0.3 | ||||||||||||||||
| Tabebuia serratifolia | 2.6 | 0.3 | 0.5 | 0.1 | ||||||||||||||||||
| Tachigali tinctoria | 5.0 | 3.7 | 0.4 | 1.7 | 2.4 | |||||||||||||||||
| Tapirira obtusa | 3.0 | |||||||||||||||||||||
| Trema micrantha | 171.1 | 171.1 | 92.1 | 32.0 | 49.4 | 49.4 | 3.0 | 7.0 | 9.0 | 4.4 | 55.5 | 25.3 | 55.0 | 22.9 | 5.5 | |||||||
| Vismia antiscrophylla | 1.6 | 4.5 | 4.5 | 2.4 | ||||||||||||||||||
| Vockisia sp. | 0.7 | |||||||||||||||||||||
| Total | 259.4 | 259.4 | 140.1 | 55.9 | 25.0 | 16.3 | 18.9 | 46.4 | 90.9 | 90.9 | 6.8 | 37.6 | 12.4 | 25.7 | 75.0 | 21.0 | 5.0 | 80.2 | 40.1 | 80.5 | 41.7 | 10.2 |
| Active Restoration | Natural Regeneration | |
|---|---|---|
| Achariaceae | ||
| Lindackeria paludosa (Benth.) Gilg | x | |
| Anacardiaceae | ||
| Anacardium occidentale L. | x | |
| Mangifera indica L. | x | |
| Spondias mombin L. | x | |
| Tapirira guianensis Aubl. | x | x |
| Thyrsodium spruceanum Benth. | x | |
| Annonaceae | ||
| Annona amazonica R.E.Fr. | x | |
| Annona excellens R.E.Fr. | x | |
| Annona sp. 01 | x | |
| Annona sp. 02 | x | |
| Annonaceae 1 | x | |
| Bocageopsis multiflora (Mart.) R.E.Fr. | x | |
| Duguetia sp. | x | |
| Ephedranthus sp. | x | |
| Guatteria discolor R.E.Fr. | x | |
| Guatteria sp. | x | x |
| Rollinia exsucca (DC. Ex Dunal) A. D.C. | x | |
| Xylopia frutescens Aulb. | x | x |
| Xylopia sp. | x | |
| Apocynaceae | ||
| Apocinaceae 01 | x | |
| Aspidosperma macrocarpon Mart. | x | |
| Aspidosperma sp. | x | |
| Himatanthus articulatus (Vahl) Woodson | x | |
| Himatanthus sucuuba (Spruce ex Müll. Arg.) Woodson | x | |
| Lacmellea gracilis (Müll.Arg.) Markgr. | x | |
| Lacmellea sp. | x | |
| Tabernaemontana coriacea Link ex Roem. & Schult. | x | |
| Araliaceae | ||
| Schefflera morototoni (Aubl.) Maguire, Steyerm. & Frodin | x | |
| Arecaceae | ||
| Astrocaryum aculeatum G. Mey. | x | |
| Euterpe oleracea Mart. | x | |
| Euterpe precatoria Mart. | x | |
| Asteraceae | ||
| Vernonia sp. | x | |
| Bignoniaceae | ||
| Cybistax sp. | x | |
| Handroanthus impetiginosus (Mart. ex DC.) Mattos | x | |
| Handroanthus serratifolius (A.H.Gentry) S.Grose | x | x |
| Jacaranda copaia (Aubl.) D.Don | x | |
| Jacaranda sp. | x | |
| Tabebuia sp. | x | |
| Zeyheria tuberculosa (Vell.) Bureau | x | |
| Bixaceae | ||
| Bixa orellana L. | x | x |
| Cochlospermum orinocense (Kunth) Steud. | x | |
| Burseraceae | ||
| Protium sp. | x | |
| Protium amazonicum (Cuatrec.) Daly | x | |
| Protium unifoliolatum Engl. | x | |
| Tratinichia sp. | x | |
| Trattinnickia rhoifolia Willd. | x | |
| Cannabaceae | ||
| Trema micrantha (L.) Blume | x | x |
| Chrysobalanaceae | ||
| Couepia sp. | x | |
| Hirtella sp. | x | |
| Hirtella rodriguesii Prance | x | |
| Licania sp. | x | |
| Licania latifolia Benth. ex Hook. f. | x | |
| Licania longistyla (Hook.f.) Fritsch | x | |
| Licania pallida (Spruce ex Hook. f.) Spruce ex Sagot | x | |
| Licania tomentosa (Benth.) Fritsch | x | |
| Cordiaceae | ||
| Cordia sp. | x | |
| Cordia goeldiana Huber | x | |
| Cordia nodosa Lam. | x | |
| Cordia panicularis Rudge | x | |
| Cordia sellowiana Cham. | x | |
| Elaeocarpaceae | ||
| Sloanea sp. | x | |
| Erytrhoxylaceae | ||
| Erythroxylum macrophyllum Cav. | x | |
| Euphorbiaceae | ||
| Alchornea discolor Poepp. | x | |
| Croton sp. | x | |
| Croton matourensis Aubl. | x | x |
| Croton urucurana Baill. | x | |
| Hevea guianensis | x | |
| Mabea sp. | x | x |
| Mabea subsessilis Pax & K.Hoffm. | x | |
| Maprounea guianensis Aubl. | x | |
| Sapium glandulosum (L.) Morong | x | |
| Fabaceae | ||
| Abarema jupunba (Willd.) Britton & Killip | x | |
| Adenanthera pavonina L. | x | |
| Apuleia leiocarpa (Vogel) J.F. Macbr. | x | x |
| Bauhinia sp. 01 | x | |
| Bauhinia sp. 02 | x | |
| Bowdichia nitida Spruce ex Benth. | x | x |
| Clitoria fairchildiana R.A. Howard | x | |
| Dialium guianense (Aubl.) Sandwith | x | |
| Dinizia excelsa Ducke | x | |
| Dipteryx odorata (Aubl.) Willd. | x | x |
| Enterolobium schomburgkii (Benth.) Benth. | x | x |
| Enterolobium sp. | x | |
| Fabaceae 1 | x | |
| Fabaceae 2 | x | |
| Fabaceae 3 | x | |
| Hymenaea courbaril L. | x | |
| Hymenolobium sp. 01 | x | |
| Hymenolobium sp. 01 | x | |
| Inga brachystachys (Ducke) | x | |
| Inga cayennensis Sagot ex Benth. | x | |
| edulis Mart. | x | x |
| Inga graciliflora Benth. | x | |
| Inga heterophylla Willd. | x | |
| Inga leiocalycina Benth. | x | |
| Inga multinervis T.D.Penn. | x | |
| Inga obidensis Ducke | x | |
| Inga pezizifera Benth. | x | |
| Inga sp. 01 | x | |
| Inga sp. 01 | x | |
| Inga sp. 02 | x | |
| Inga sp. 02 | x | |
| Inga sp. 03 | x | |
| Inga sp. 04 | x | |
| Inga sp. 05 | x | |
| Inga sp. 06 | x | |
| Inga sp. 07 | x | |
| Inga thibaudiana DC. | x | |
| Inga vera Willd. | x | |
| Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz | x | |
| Ormosia grossa Rudd | x | |
| Parkia multijuga Benth. | x | |
| Parkia pendula (Willd.) Benth. ex Walp. | x | |
| Platymiscium sp. | x | |
| Pterocarpus sp. | x | |
| Pterocarpus amazonum (Mart. ex Benth.) Amshoff | x | |
| Pterodon emarginatus Vogel | x | |
| Samanea tubulosa (Benth.) Barneby & J.W. Grimes | x | |
| Schizolobium amazonicum Huber ex Ducke | x | |
| Senegalia sp. | x | |
| Senna alata (L.) Roxb. | x | |
| Senna multijuga (Rich.) H.S. Irwin & Barneby | x | x |
| Stryphnodendron sp. | x | |
| Stryphnodendron duckeanum Occhioni | x | |
| Stryphnodendron pulcherrimum (Willd.) Hochr. | x | |
| Swartizia arborescens (Aubl.) Pittier | x | |
| Swartzia sp. | x | |
| Swartzia kuhlmannii Hoehne | x | |
| Swartzia laurifolia Benth. | x | |
| Swartzia lucida R.S. Cowan | x | x |
| Swartzia corrugata Benth. | x | |
| Tachigali sp. | x | |
| Tachigali chrysophylla (Poepp.) Zarucchi & Herend. | x | |
| Tachigali tinctoria (Benth.) Zarucchi & Herend. | x | |
| Vatairea fusca (Ducke) Ducke | x | |
| Vatairea sericea (Ducke) Ducke | x | |
| Zygia racemosa (Ducke) Barneby & J.W.Grimes | x | |
| Goupiaceae | ||
| Golpea sp. | x | |
| Goupia glabra Aubl. | x | x |
| Hypericaceae | ||
| Thyrsodium spruceanum Benth. | x | |
| Vismia cayennensis (Jacq.) Pers. | x | |
| Vismia gracilis Hieron. | x | x |
| Vismia guianensis (Aubl.) Choisy | x | x |
| Vismia sandwithii Ewan | x | x |
| Lacistemataceae | ||
| Lacistema grandifolium Schnizl. | x | |
| Lamiaceae | ||
| Vitex triflora Vahl | x | |
| Lauraceae | ||
| Aniba sp. | x | |
| Lauraceae 1 | x | |
| Mezilaurus itauba (Meisn.) Taub. ex Mez | x | |
| Ocotea minor Vicent. | x | |
| Ocotea nigrescens Vicent. | x | |
| Ocotea sp. | x | |
| Lechytidaceae | ||
| Couratari stellata A.C.Sm. | x | |
| Eschweilera coriacea (DC.) S.A.Mori | x | x |
| Eschweilera laevicarpa S.A.Mori | x | |
| Lecythis sp. | x | |
| Cariniana micrantha Ducke | x | |
| Couratari macrosperma A.C. Sm. | x | |
| Lythraceae | ||
| Physocalymma scaberrimum Pohl | x | x |
| Malpighiaceae | ||
| Byrsonima sp. | x | |
| Byrsonima sp. 01 | x | |
| Byrsonima sp. 02 | x | |
| Malvaceae | ||
| Apeiba tibourbou Aubl. | x | x |
| Ceiba samauma (Mart.) K. Schum. | x | |
| Eriotheca sp. | x | |
| Luehea sp. | x | |
| Ochroma pyramidale (Cav. ex Lam.) Urb. | x | |
| Pachira aquatica Aubl. | x | |
| Pachira sp. | x | |
| Sterculia sp. | x | |
| Theobroma grandiflorum (Willd. ex Spreng.) K. Schum. | x | |
| Theobroma speciosum Willd. ex Spreng. | x | x |
| Melastomataceae | ||
| Belluccia grossularioides (L.) Triana | x | x |
| Belluccia sp. | x | |
| Leandra cf dichotoma (Pav. ex D. Don) Cogn. | x | |
| Miconia argyrophylla DC. | x | |
| Miconia biglandulosa Gleason | x | |
| Miconia cuspidata Naudin | x | x |
| Miconia elaeodendron (DC.) Naudin | x | |
| Miconia ferruginea (Desr.) DC. | x | |
| Miconia phanerostila Pilg. | x | |
| Miconia poeppigii Triana | x | |
| Miconia pyrifolia Naudin | x | x |
| Miconia sp. 01 | x | |
| Miconia sp. 02 | x | |
| Miconia sp. 03 | x | |
| Miconia sp. 04 | x | |
| Mouriri sp. | x | |
| Tococa subciliata (DC.) Triana | x | |
| Meliaceae | ||
| Carapa guianensis Aubl. | x | |
| Cedrela fissilis Vell. | x | |
| Moraceae | ||
| Brosimum guianense (Aubl.) Huber | x | |
| Brosimum sp. | x | |
| Ficus sphenophylla Standl. | x | |
| Machira sp. | x | |
| Maquira calophylla (Poepp. & Endl.) C.C.Berg | x | |
| Moraceae 1 | x | |
| Perebea mollis (Poepp. & Endl.) Huber | x | |
| Sorocea sp. | x | |
| Muntingiaceae | ||
| Muntingia calabura L. | x | |
| Myristicaceae | ||
| Virola calophylla Warb. | x | |
| Virola cf. surinamensis (Rol. ex Rottb.) Warb. | x | |
| Virola multinervia Ducke | x | |
| Myrtaceae | ||
| Caliptrantes sp. | x | |
| Eugenia patrisii Vahl | x | |
| Eugenia sp. 01 | x | |
| Eugenia sp.02 | x | |
| Myrcia calycampa Amshoff | x | |
| Myrcia sp. 01 | x | |
| Myrcia sp. 01 | x | |
| Myrcia sp. 02 | x | |
| Myrcia sp. 02 | x | |
| Myrcia sp. 03 | x | |
| Myrcia sp. 04 | x | |
| Myrcia subsericea A. Gray | x | |
| Myrtaceae 1 | x | |
| Myrtaceae 1 | x | |
| Myrtaceae 2 | x | |
| Myrtaceae 3 | x | |
| Myrtaceae 4 | x | |
| Myrtaceae 5 | x | |
| Psidium guajava L. | x | x |
| Psidium sp. | x | |
| Syzygium cumini (L.) Skeels | x | x |
| Syzygium jambos (L.) Alston | x | |
| Non identified | x | |
| N. I. 01 | x | |
| N. I. 02 | x | |
| N. I. 01 | x | |
| N. I. 02 | x | |
| N. I. 03 | x | |
| N. I. 04 | x | |
| N. I. 05 | x | |
| N. I. 06 | x | |
| N. I. 07 | x | |
| N. I. 08 | x | |
| N. I. 09 | x | |
| N. I. 10 | x | |
| N. I. 11 | x | |
| N. I. 12 | x | |
| N. I. 13 | x | |
| N. I. 14 | x | |
| N. I. 15 | x | |
| N. I. 16 | x | |
| Nyctaginaceae | ||
| Neea theifera Oerst. | x | |
| Ochnaceae | ||
| Ouratea sp. | x | |
| Ouratea odora Engl. | x | |
| Olacaceae | ||
| Minquartia guianensis Aubl. | x | |
| Pentaphylacaceae | ||
| Ternstroemia dentata (Aubl.) Sw. | x | |
| Phyllanthaceae | ||
| Richeria grandis Vah | x | |
| Piperaceae | ||
| Piper aduncum L. | x | |
| Primulaceae | ||
| Cybianthus sp. | x | |
| Quiinaceae | ||
| Lacunaria macrosthachya (Tul.) A.C Sm. | x | |
| Rhizophoraceae | ||
| Sterigmapetalum obovatum Kuhlm. | x | |
| Rubiaceae | ||
| Alibertia sp. | x | |
| Capirona decorticans Spruce | x | |
| Cordiera concolor (Cham.) Kuntze | x | |
| Duroia sp. | x | |
| Duroia longiflora Ducke | x | |
| Ferdinandusa hirsuta Standl. | x | |
| Genipa americana L. | x | |
| Isertia hypoleuca Benth. | x | x |
| Psychotria sp. | x | |
| Richeria grandis Vah | x | |
| Rubiaceae 1 | x | |
| Rubiaceae 1 | x | |
| Warszewiczia coccinea (Vahl) Klotzsch | x | |
| Rutaceae | ||
| Citrus aurantifolia Swingle | x | |
| Citrus sp. | x | |
| Dictyoloma vandellianum A. Juss. | x | |
| Nycticalanthus sp. | x | |
| Zanthoxylum rhoifolium Lam. | x | |
| Salicaceae | ||
| Casearia duckeana Sleumer | x | |
| Casearia grandiflora Cambess. | x | |
| Casearia javitensis Kunth | x | x |
| Casearia negrensis Eichler | x | |
| Laetia procera (Poepp.) Eichler | x | |
| Ryania speciosa Vahl | x | |
| Sapindaceae | ||
| Cupania hispida Radlk. | x | |
| Cupania rubiginosa (Poir.) Radlk. | x | |
| Matayba sp. | x | |
| Matayba arborescens (Aubl.) Radlk. | x | |
| Talisia sp. | x | |
| Toulicia guianensis Aubl. | x | |
| Sapotaceae | ||
| Pouteria caimito (Ruiz & Pav.) Radlk. | x | |
| Pouteria sp. | x | |
| Simaroubaceae | ||
| Homalolepis sp. | x | |
| Quassia amara L. | x | |
| Simarouba amara Aubl. | x | x |
| Simarouba versicolor A. St.-Hil. | x | |
| Pouteria sp. | x | |
| Simaroubaceae | ||
| Siparuna sp. | x | |
| Siparuna guianensis Aubl. | x | |
| Solanaceae | ||
| Solanum lycocarpum A. St.-Hil. | x | |
| Solanum spp. | x | |
| Solanum subinerme Jacq. | x | |
| Solanum viarum Dunal | x | |
| Urticaceae | ||
| Cecropia distachya Huber | x | x |
| Cecropia membranacea Trécul | x | |
| Cecropia purpurascens C.C. Berg | x | x |
| Cecropia sciadophylla Mart. | x | |
| Cecropia sp. | x | |
| Vochysiaceae | ||
| Qualea paraensis Ducke | x | |
| Rouisteranea sp. | x | |
| Ruizterania albiflora (Warm.) Marc.-Berti | x | |
| Ruizterania retusa (Spruce ex Warm.) Marc.-Berti | x | |
References
- Chazdon, R.L.; Uriarte, M. Natural regeneration in the context of large-scale forest and landscape restoration in the tropics. Biotropica 2016, 48, 709–715. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Guariguata, M.R. Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Holl, K.D.; Aide, T.M. When and where to actively restore ecosystems? Ecol. Manag. 2011, 261, 1558–1563. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Zambrano, A.M.A.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass resilience of Neotropical secondary forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef]
- Rezende, G.M.; Vieira, D.L.M. Forest restoration in southern Amazonia: Soil preparation triggers natural regeneration. Ecol. Manag. 2019, 433, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Jakovac, C.C.; Bongers, F.; Kuyper, T.; Mesquita, R.C.G.; Pena-Claros, M. Land use as a filter for species composition in Amazonian secondary forests. J. Veg. Sci. 2016, 27, 1104–1116. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, R.D.C.G.; Massoca, P.E.D.S.; Jakovac, C.C.; Bentos, T.V.; Williamson, G.B. Amazon Rain Forest Succession: Stochasticity or Land-Use Legacy? BioScience 2015, 65, 849–861. [Google Scholar] [CrossRef]
- Freitas, M.G.; Rodrigues, S.B.; Campos-Filho, E.M.; do Carmo, G.H.P.; da Veiga, J.M.; Junqueira, R.G.P.; Vieira, D.L.M. Evaluating the success of direct seeding for tropical forest restoration over ten years. Ecol. Manag. 2019, 438, 224–232. [Google Scholar] [CrossRef]
- VanWey, L.K.; Spera, S.; de Sa, R.; Mahr, D.; Mustard, J.F. Socioeconomic development and agricultural intensification in Mato Grosso. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120168. [Google Scholar] [CrossRef]
- Durigan, G.; Guerin, N.; da Costa, J.N.M.N. Ecological restoration of Xingu Basin headwaters: Motivations, engagement, challenges and perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120165. [Google Scholar] [CrossRef] [Green Version]
- Corbin, J.D.; Holl, K.D. Applied nucleation as a forest restoration strategy. For. Ecol. Manag. 2012, 265, 37–46. [Google Scholar] [CrossRef]
- Rodrigues, S.B.; Freitas, M.G.; Campos-Filho, E.M.; do Carmo, G.H.P.; da Veiga, J.M.; Junqueira, R.P.; Vieira, D.L.M. Direct seeded and colonizing species guarantee successful early restoration of South Amazon forests. For. Ecol. Manag. 2019, 451, 117559. [Google Scholar] [CrossRef]
- Sansevero, J.B.B.; Prieto, P.V.; de Moraes, L.F.D.; Rodrigues, P.J.F.P. Natural regeneration in plantations of native trees in lowland Brazilian Atlantic forest: Community structure, diversity, and dispersal syndromes. Restor. Ecol. 2011, 19, 379–389. [Google Scholar] [CrossRef]
- César, R.G.; Moreno, V.S.; Coletta, G.D.; Chazdon, R.L.; Ferraz, S.F.B.; de Almeida, D.R.A.; Brancalion, P.H.S. Early ecological outcomes of natural regeneration and tree plantations for restoring agricultural landscapes. Ecol. Appl. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Weidlich, E.W.A.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H.S. Controlling invasive plant species in ecological restoration: A global review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Camargo, J.L.C.; Ferraz, I.D.K.; Imakawa, A.M. Rehabilitation of degraded areas of central Amazonia using direct sowing of forest tree seeds. Restor. Ecol. 2002, 10, 636–644. [Google Scholar] [CrossRef]
- Cochrane, T.T.; Cochrane, T.A. Amazon Forest and Savanna Lands: A Guide to the Climates, Vegetation, Landscapes and Soils of Central Tropical South America; CreateSpace: Scotts Valley, CA, USA, 2010. [Google Scholar]
- Rocha, G.P.E.; Vieira, D.L.M.; Simon, M.F. Fast natural regeneration in abandoned pastures in southern Amazonia. Ecol. Manag. 2016, 370, 93–101. [Google Scholar] [CrossRef]
- Kishy, I.; Reis, F.; Solidera, D.; Solidera, D.; Borges, A.; Rezende, G.M.; Vieira, D.L.M. How a company is restoring its 3,000 hectares of forest liability and supporting local communities. In Forest Landscape Restoration and Social Opportunities in the Tropical World; Pinto, S.R.R., Santos, F.C., Prescott, C., Eds.; CEPAN: Recife, Brazil, 2020; pp. 249–258. [Google Scholar]
- Gehring, C.; Park, S.; Denich, M. Close relationship between diameters at 30cm height and at breast height (DBH). Acta Amaz. 2008, 38, 71–76. [Google Scholar] [CrossRef]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M. Which landscape size best predicts the influence of forest cover on restoration success? A global meta-analysis on the scale of effect. J. Appl. Ecol. 2016, 53, 440–448. [Google Scholar] [CrossRef]
- Shoo, L.P.; Freebody, K.; Kanowski, J.; Catterall, C.P. Slow recovery of tropical old-field rainforest regrowth and the value and limitations of active restoration. Conserv. Biol. 2015, 30, 121–132. [Google Scholar] [CrossRef]
- Suganuma, M.S.; Durigan, G. Indicators of restoration success in riparian tropical forests using multiple reference ecosystems. Restor. Ecol. 2015, 23, 238–251. [Google Scholar] [CrossRef]
- Williamson, G.B.; Bentos, T.V.; Longworth, J.B.; Mesquita, R.C.G. Convergence and divergence in alternative successional pathways in Central Amazonia. Plant Ecol. Divers. 2012, 7, 341–348. [Google Scholar] [CrossRef]
- Norden, N.; Mesquita, R.C.G.; Bentos, T.V.; Chazdon, R.L.; Williamson, G.B. Contrasting community compensatory trends in alternative successional pathways in central Amazonia. Oikos 2011, 120, 143–151. [Google Scholar] [CrossRef]
- Uhl, C.; Buschbacher, R.; Serrao, E.A.S. Abandoned Pastures in Eastern Amazonia. I. Patterns of Plant Succession. J. Ecol. 1988, 76, 663–681. [Google Scholar] [CrossRef]
- Silveira, M.; Trevelin, L.; Port-Carvalho, M.; Godoi, S.; Mandetta, E.N.; Cruz-Neto, A.P. Frugivory by phyllostomid bats (Mammalia: Chiroptera) in a restored area in Southeast Brazil. Acta Oecologica 2011, 37, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, V.H.; Dalagnol, R.; Cassol, H.L.; Rosan, T.M.; de Almeida, C.T.; Junior, C.H.S.; Campanharo, W.A.; House, J.I.; Sitch, S.; Hales, T.C.; et al. Large carbon sink potential of secondary forests in the Brazilian Amazon to mitigate climate change. Nat. Commun. 2021, 12, 1785. [Google Scholar] [CrossRef]
- Gardon, F.R.; dos Santos, R.F.; Rodrigues, R.R. Brazil’s forest restoration, biomass and carbon stocks: A critical review of the knowledge gaps. For. Ecol. Manag. 2020, 462, 117972. [Google Scholar] [CrossRef]
- Giudice Badari, C.; Bernardini, L.E.; de Almeida, D.R.A.; Brancalion, P.H.S.; César, R.G.; Gutierrez, V.; Chazdon, R.L.; Gomes, H.B.; Viani, R.A.G. Ecological outcomes of agroforests and restoration 15 years after planting. Restor. Ecol. 2020, 28, 1135–1144. [Google Scholar] [CrossRef]
- Rajão, R.; Soares-Filho, B.; Nunes, F.; Börner, J.; Machado, L.; Assis, D.; Oliveira, A.; Pinto, L.; Ribeiro, V.; Rausch, L.; et al. The rotten apples of Brazil’s agribusiness. Science 2020, 369, 246–248. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, D.L.M.; Rodrigues, S.B.; Jakovac, C.C.; da Rocha, G.P.E.; Reis, F.; Borges, A. Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia. Forests 2021, 12, 1022. https://doi.org/10.3390/f12081022
Vieira DLM, Rodrigues SB, Jakovac CC, da Rocha GPE, Reis F, Borges A. Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia. Forests. 2021; 12(8):1022. https://doi.org/10.3390/f12081022
Chicago/Turabian StyleVieira, Daniel Luis Mascia, Silvia Barbosa Rodrigues, Catarina Conte Jakovac, Gustavo Paiva Evangelista da Rocha, Fagno Reis, and Augusto Borges. 2021. "Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia" Forests 12, no. 8: 1022. https://doi.org/10.3390/f12081022
APA StyleVieira, D. L. M., Rodrigues, S. B., Jakovac, C. C., da Rocha, G. P. E., Reis, F., & Borges, A. (2021). Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia. Forests, 12(8), 1022. https://doi.org/10.3390/f12081022






