Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines
Abstract
1. Introduction
2. Materials and Methods
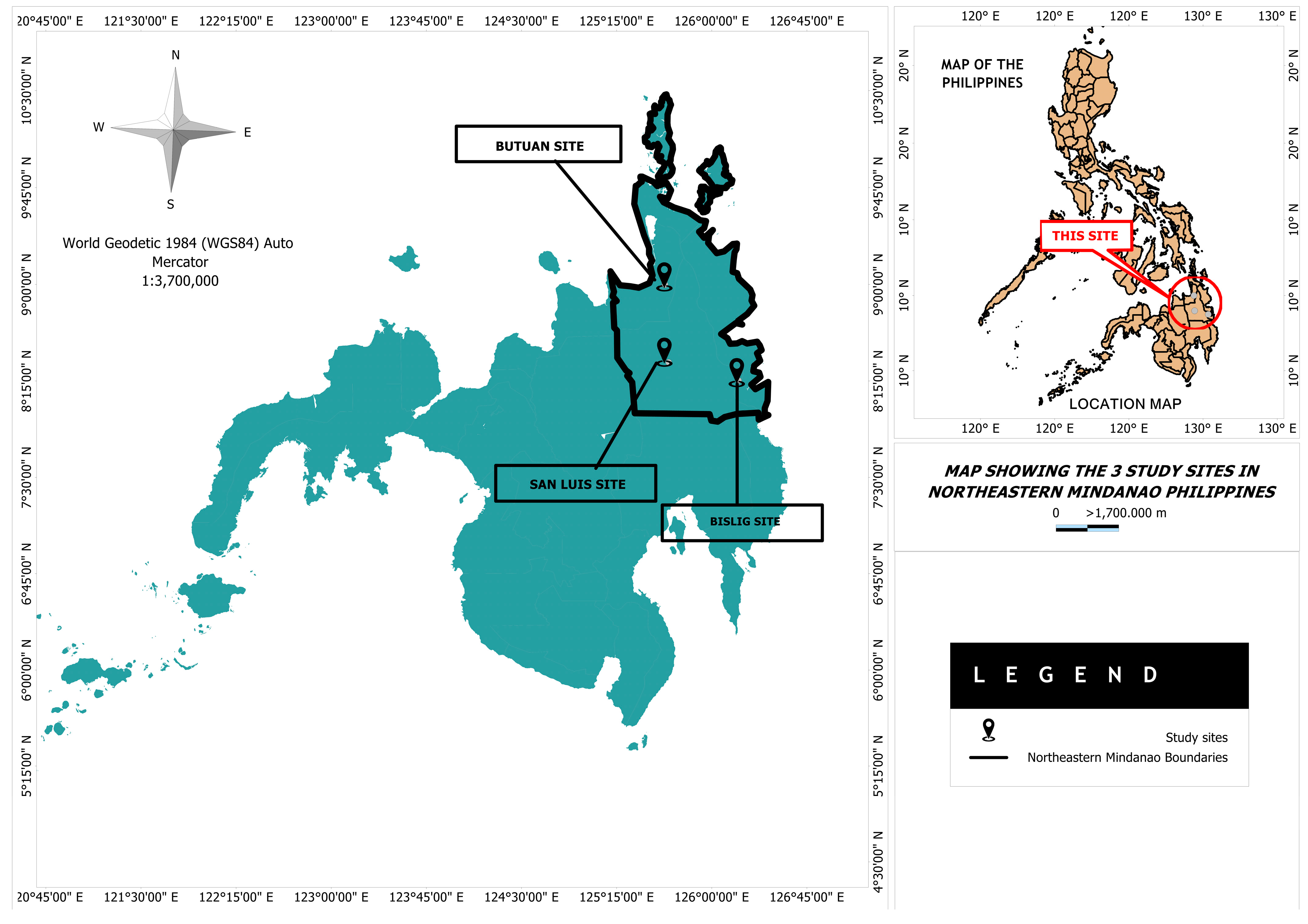
2.1. Study Sites
2.2. Species Information
2.3. Experimental Design, Establishment and Data Collection
2.4. DGR, HGR, and Survival Rate Determination
2.5. Climate Data Collection
2.6. Data Analysis
3. Results
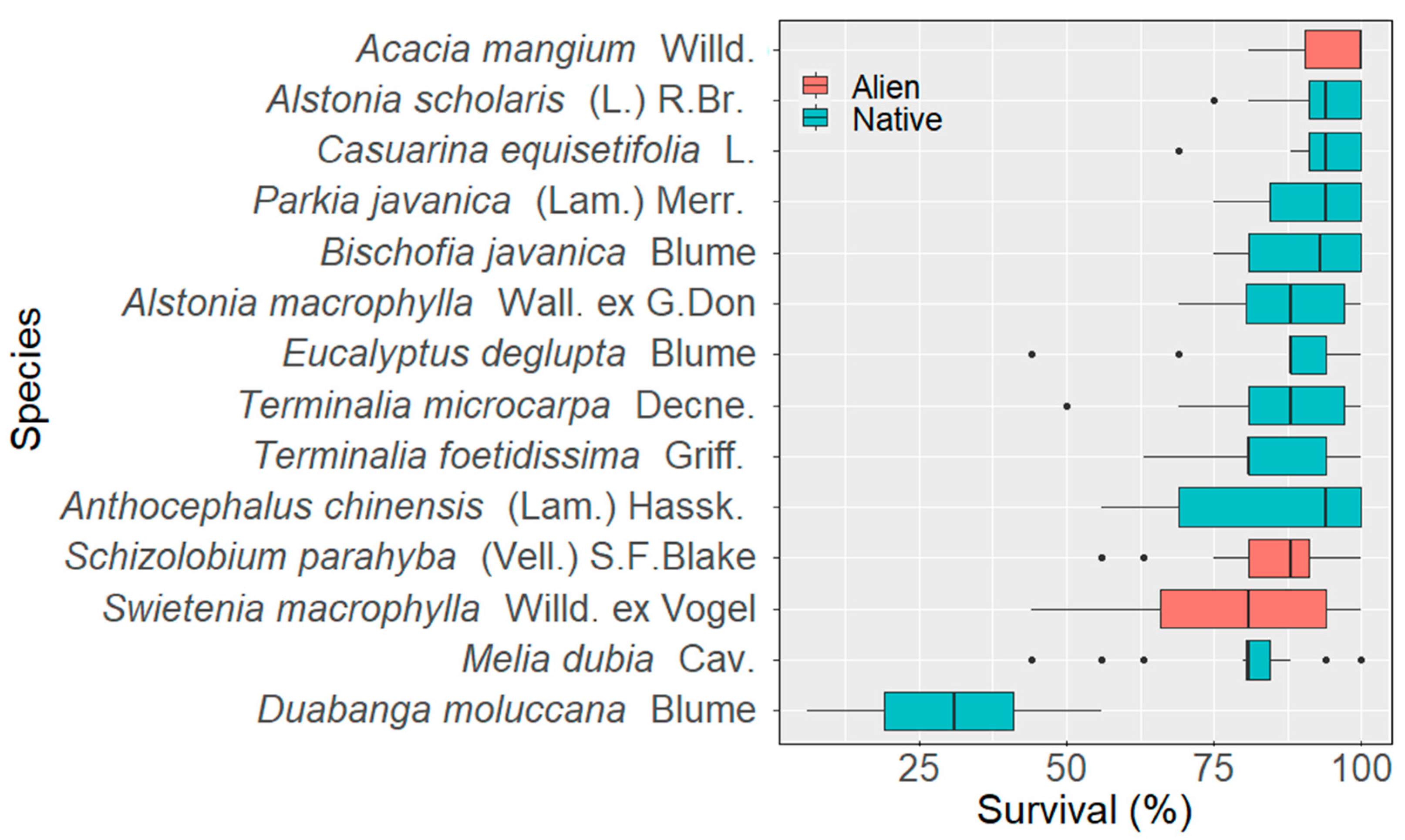
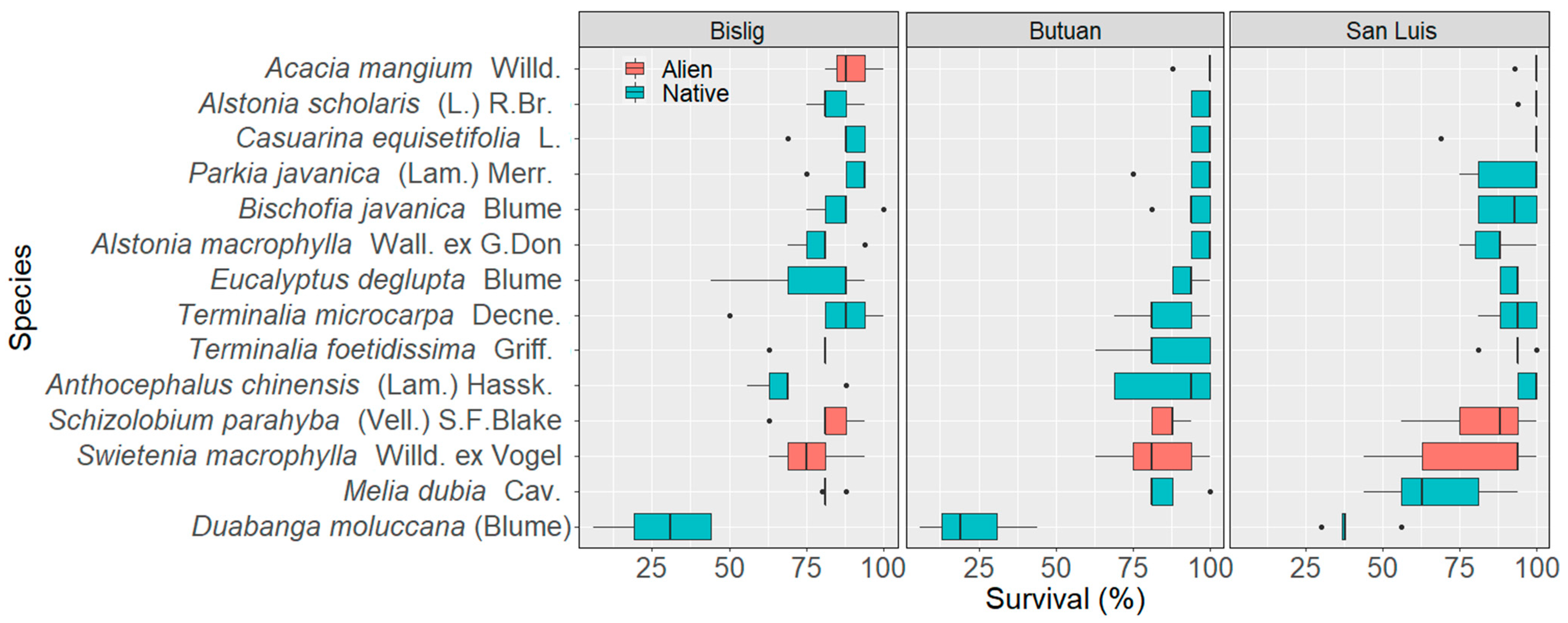
3.1. Survival Tendencies of Native and Alien Tree Species
3.1.1. Species Level
3.1.2. Site Level
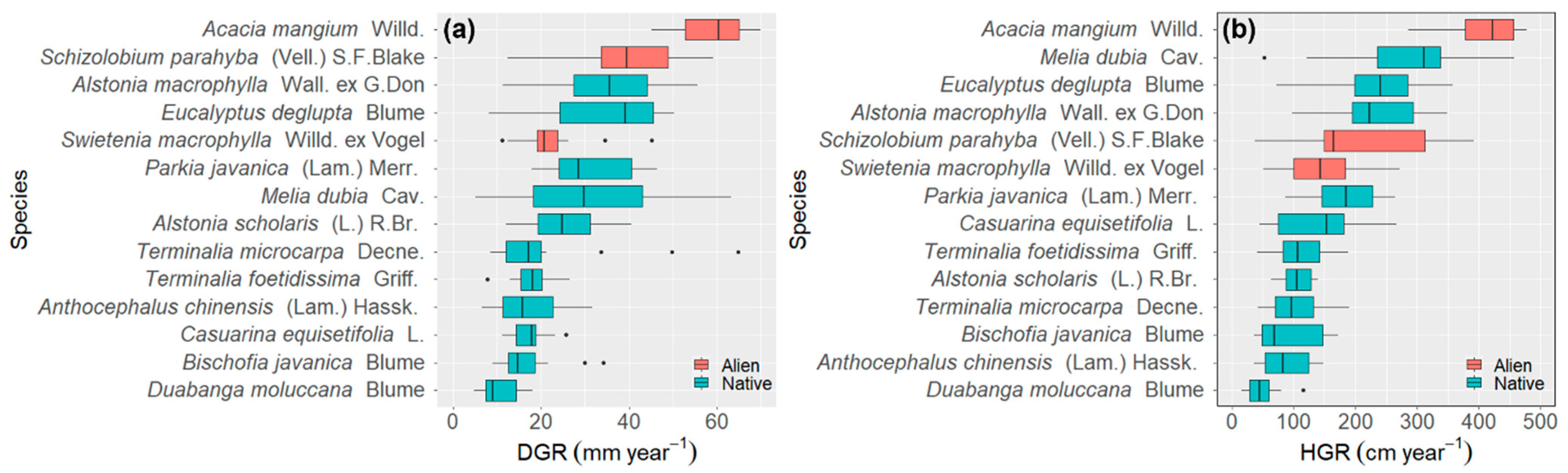
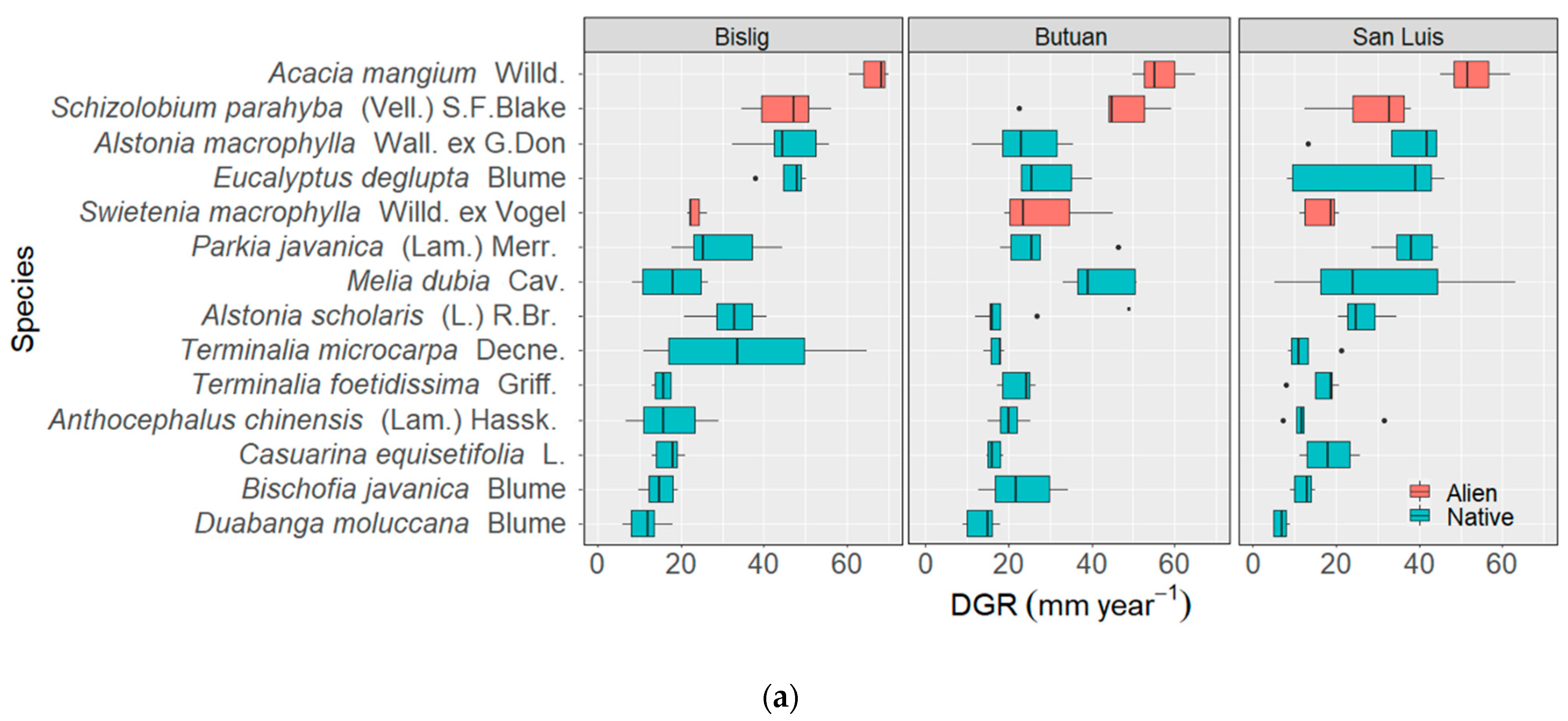
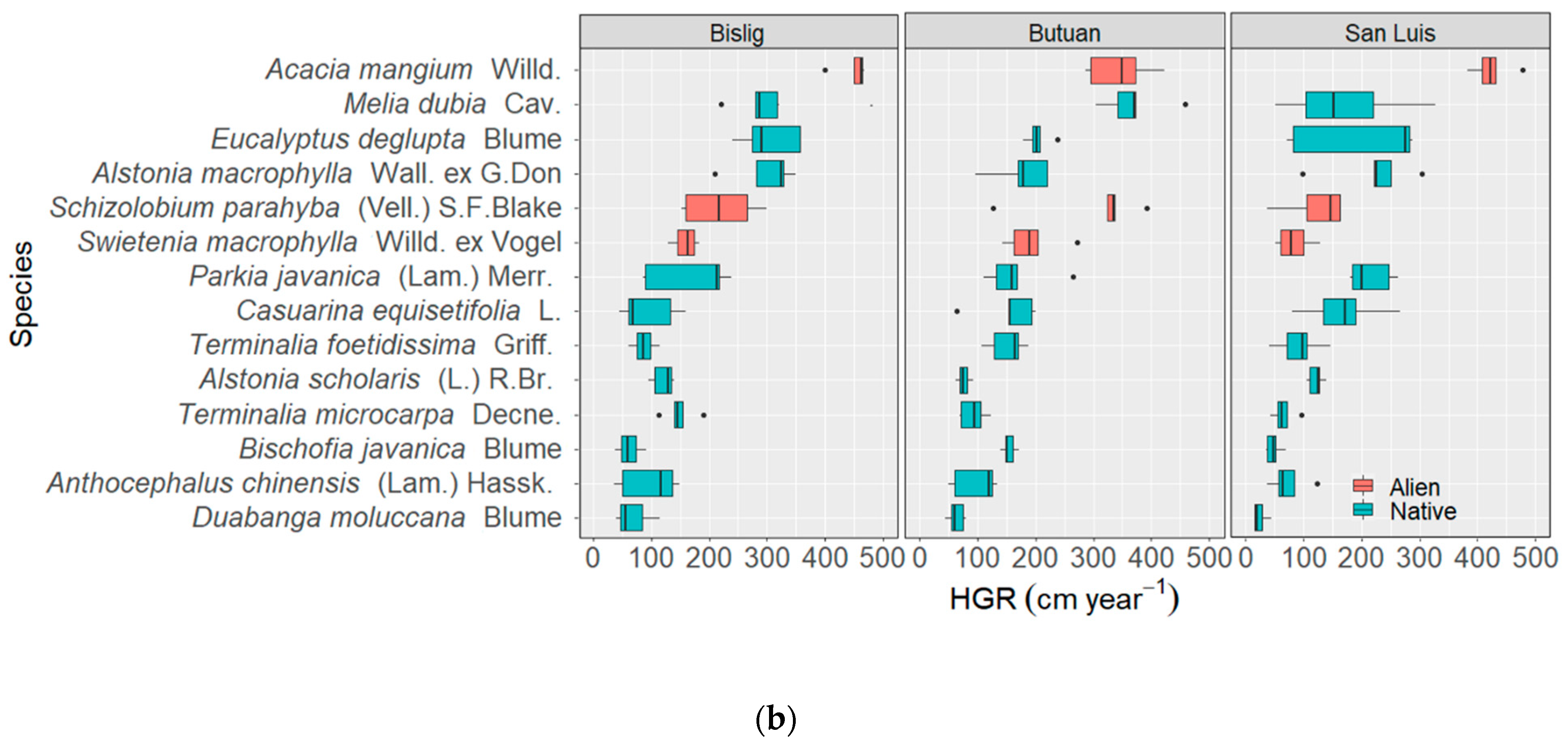
3.2. Diametric and Height Growth Rates of the Species
3.2.1. Species Level
3.2.2. Site Level
3.3. Site-Species Interactions
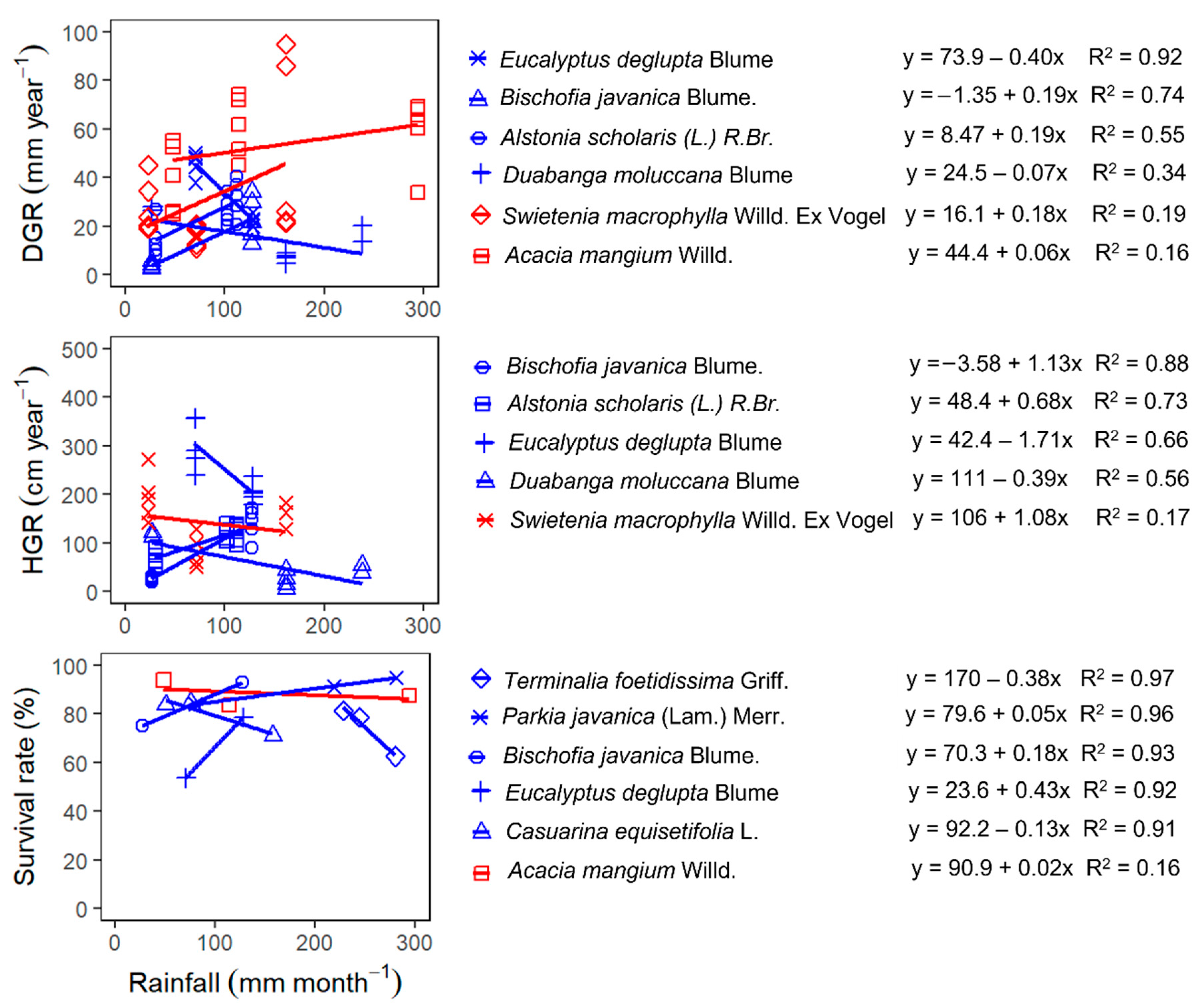
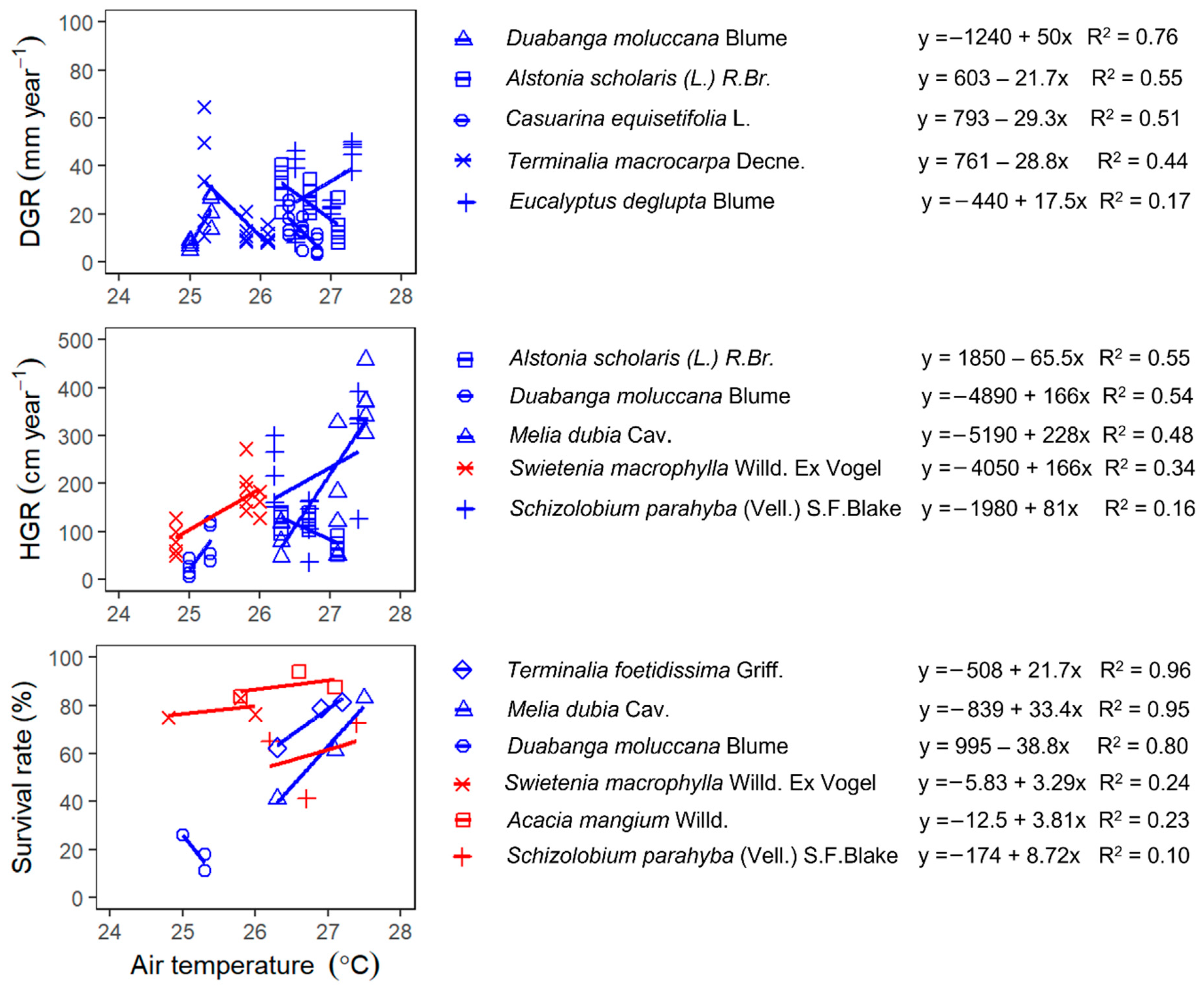
3.4. Climatic Factors Affecting the Growth and Survival of the Species
3.4.1. The Response of DGR, HGR, and Survival to Rainfall
3.4.2. The Response of DGR, HGR and Survival to Temperature
4. Discussion
4.1. Survival of Native and Alien Species
4.1.1. Species Level
4.1.2. Site Level
4.2. Height and Diameter Growth Rates of the Species
4.2.1. Species Level
4.2.2. Site Level
4.3. Climatic Factors Affecting the Growth and Survival of Native and Alien Species
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luna, M.P.G. Impact Assessment of the National Greening Program of the DENR: Scoping or Process Evaluation Phase-Institutional Component; 2016-29; Econstor: Quezon City, Philippines, 2016. [Google Scholar]
- Inail, M.A.; Hardiyanto, E.B.; Mendham, D.S. Growth responses of Eucalyptus pellita F. Muell plantations in south sumatra to macronutrient fertilisers following several rotations of Acacia mangium willd. Forests 2019, 10, 1054. [Google Scholar] [CrossRef]
- DENR-FMB. Forestry Statistics, 2011–2018; DENR: Quezon City, Philippines, 2018. [Google Scholar]
- Mukul, S.A.; Herbohn, J.; Firn, J. Rapid recovery of tropical forest diversity and structure after shifting cultivation in the Philippines uplands. Ecol. Evol. 2020, 10, 7189–7211. [Google Scholar] [CrossRef]
- Chokkalingam, A.P.; Carandang, A.P.; Pulhin, J.M.; Lasco, R.D.; Peras, R.; Toma, T. One Century of Forest Rehabilitation in the Philippines: Approaches, Outcomes and Lessons; Center for International Forestry Research (CIFOR), Department of Environment and Natural Resources, College of Forestry and Natural Resources, University of the Philippines Los Banos: Jakarta, Indonesia, 2006; ISBN 9792446435. [Google Scholar]
- Nath, C.D.; Schroth, G.; Burslem, D.F.R.P. Why do farmers plant more exotic than native trees? A case study from the Western Ghats, India. Agric. Ecosyst. Environ. 2016, 230, 315–328. [Google Scholar] [CrossRef]
- Richardson, D.M. Forestry trees as invasive aliens. Conserv. Biol. 1998, 12, 18–26. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Thébaud, C.; Simberloff, D. Are plants really larger in their introduced ranges? Am. Nat. 2001, 157, 231–236. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Kuzmin, I.V.; Silaeva, T.B. Anthropogenic drivers leading to regional extinction of threatened plants: Insights from regional Red Data Books of Russia. Biodivers. Conserv. 2020, 29, 2765–2777. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent Anthropogenic Plant Extinctions Differ in Biodiversity Hotspots and Coldspots. Curr. Biol. 2019, 29, 2912–2918.e2. [Google Scholar] [CrossRef]
- Kisa, M.; Sanon, A.; Thioulouse, J.; Assigbetse, K.; Sylla, S.; Spichiger, R.; Dieng, L.; Berthelin, J.; Prin, Y.; Galiana, A.; et al. Arbuscular mycorrhizal symbiosis can counterbalance the negative influence of the exotic tree species Eucalyptus camaldulensis on the structure and functioning of soil microbial communities in a sahelian soil. FEMS Microbiol. Ecol. 2007, 62, 32–44. [Google Scholar] [CrossRef][Green Version]
- Remigi, P.; Faye, A.; Kane, A.; Deruaz, M.; Thioulouse, J.; Cissoko, M.; Prin, Y.; Galiana, A.; Dreyfus, B.; Duponnois, R. The exotic legume tree species Acacia holosericea alters microbial soil functionalities and the structure of the arbuscular mycorrhizal community. Appl. Environ. Microbiol. 2008, 74, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Laface, V.L.A.; Musarella, C.M.; Ortiz, A.C.; Canas, R.Q.; Cannavò, S.; Spampinato, G. Three new alien taxa for europe and a chorological update on the alien vascular flora of calabria (Southern Italy). Plants 2020, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.; Erskine, P.D.; Parrota, J.A. Restoration of Degraded Tropical Forest Landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef]
- Calvo-Alvarado, J.C.; Arias, D.; Richter, D.D. Early growth performance of native and introduced fast growing tree species in wet to sub-humid climates of the Southern region of Costa Rica. For. Ecol. Manag. 2007, 242, 227–235. [Google Scholar] [CrossRef]
- Van Breugel, M.; Hall, J.S.; Craven, D.J.; Gregoire, T.G.; Park, A.; Dent, D.H.; Wishnie, M.H.; Mariscal, E.; Deago, J.; Ibarra, D.; et al. Early growth and survival of 49 tropical tree species across sites differing in soil fertility and rainfall in Panama. For. Ecol. Manag. 2011, 261, 1580–1589. [Google Scholar] [CrossRef]
- Aguilos, R.; Marquez, C.; Adornado, H.; Aguilos, M. Domesticating commercially important native tree species in the Philippines: Early growth performance level. Forests 2020, 11, 885. [Google Scholar] [CrossRef]
- Otsamo, R. Effect of nurse tree species on early growth of Anisoptera marginata Korth. (Dipterocarpaceae) on an Imperata cylindrica (L.) Beauv. grassland site in South Kalimantan, Indonesia. For. Ecol. Manag. 1998, 105, 303–311. [Google Scholar] [CrossRef]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Nath, C.D.; Pélissier, R.; Ramesh, B.R.; Garcia, C. Promoting native trees in shade coffee plantations of southern India: Comparison of growth rates with the exotic Grevillea robusta. Agrofor. Syst. 2011, 83, 107–119. [Google Scholar] [CrossRef]
- Aguilos, M.; Mitra, B.; Noormets, A.; Minick, K.; Prajapati, P.; Gavazzi, M.; Sun, G.; McNulty, S.; Li, X.; Domec, J.C.; et al. Long-term carbon flux and balance in managed and natural coastal forested wetlands of the Southeastern USA. Agric. For. Meteorol. 2020, 288–289, 108022. [Google Scholar] [CrossRef]
- Caravaca, F.; Alguacil, M.M.; Figueroa, D.; Barea, J.M.; Roldán, A. Re-establishment of Retama sphaerocarpa as a target species for reclamation of soil physical and biological properties in a semi-arid Mediterranean area. For. Ecol. Manag. 2003, 182, 49–58. [Google Scholar] [CrossRef]
- Climate Change in the Philippines; Department of Science and Technology-Philippine Atmospheric Geophysical and Astronomical Services Administration. Climatology and Agrometeorology Division (CAD): Diliman City, Philippines, 2011.
- Collado, W.B.; Obico, M.R. Result of the Soil Analysis on the Physical and Chemical Characteristics of Butuan Series; PhilRice AES: Agusan del Norte, Philippines, 2007. [Google Scholar]
- Griscom, H.P.; Ashton, P.M.S.; Berlyn, G.P. Seedling survival and growth of native tree species in pastures: Implications for dry tropical forest rehabilitation in central Panama. For. Ecol. Manag. 2005, 218, 306–318. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Koutika, L.S.; Richardson, D.M. Acacia mangium willd: Benefits and threats associated with its increasing use around the world. For. Ecosyst. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Jusoh, I.; Suteh, J.K.; Adam, N.S. Growth and yield of Acacia mangium based on permanent sampling plots in a plantation. Trans. Sci. Technol. 2017, 4, 513–518. [Google Scholar]
- DENR. DENR Annual Report 2017; Department of Environment and Natural Resources: Quezon City, Philippines, 2017. [Google Scholar]
- Aide, M.; Cavelier, J. Barriers to lowland tropical forest restoration in the Sierra Nevada de Santa Marta, Colombia. Restor. Ecol. 1994, 2, 219–229. [Google Scholar] [CrossRef]
- Lestari, D.A.; Fiqa, A.P.; Fauziah, F.; Budiharta, S. Growth evaluation of native tree species planted on post coal mining reclamation site in East Kalimantan, Indonesia. Biodiversitas 2019, 20, 134–143. [Google Scholar] [CrossRef]
- Hardie, M.; Akhmad, N.; Mohammed, C.; Mendham, D.; Corkrey, R.; Gafur, A.; Siregar, S. Role of site in the mortality and production of Acacia mangium plantations in Indonesia. South. For. 2018, 80, 37–50. [Google Scholar] [CrossRef]
- Thangjam, U.; Sahoo, U.K.; Thong, P. Characterization of morphometric, reproductive and seedling traits of parkia timoriana in Northeast India. Silva Fenn. 2020, 54, 1–16. [Google Scholar] [CrossRef]
- Turnbull, J.; Crompton, H.; Pinyopusarerk, K. Recent developments in Acacia planting. In Proceedings of an International Workshop Held in Hanoi, Vietnam; Brown Prior Anderson Pty Ltd.: Brisbane, Australia, 1998. [Google Scholar]
- van Wilgen, B.W.; Richardson, D.M.; Maitre, D.C.L.E.; Marais, C.; Magadlela, D. The economic consequences of alien plant invasions: Examples of impacts and approaches to sustainable management in South Africa. Environ. Dev. Sustain. 2001, 3, 145–168. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Othman, F.E.; Kahar, R.S. Growth and competition between seedlings of an invasive plantation tree, Acacia mangium, and those of a native Borneo heath-forest species, Melastoma beccarianum. Ecol. Res. 2005, 20, 205–214. [Google Scholar] [CrossRef]
- De Wit, M.P.; Crookes, D.J.; Van Wilgen, B.W. Conflicts of interest in environmental management: Estimating. Biol. Invations 2001, 3, 167–178. [Google Scholar] [CrossRef]
- Souza, A.O.; Chaves, M.d.P.S.R.; Barbosa, R.I.; Clement, C.R. Local ecological knowledge concerning the invasion of Amerindian lands in the northern Brazilian Amazon by Acacia mangium (Willd.). J. Ethnobiol. Ethnomed. 2018, 14, 1–14. [Google Scholar] [CrossRef]
- Gordon, D.R. Effects of invasive, non-indigenous plant species on ecosystem processes: Lessons from Florida. Ecol. Appl. 1998, 8, 975–989. [Google Scholar] [CrossRef]
- Rouget, M.; Richardson, D.M.; Nel, J.L.; Van Wilgen, B.W. Commercially important trees as invasive aliens-Towards spatially explicit risk assessment at a national scale. Biol. Invasions 2002, 4, 397–412. [Google Scholar] [CrossRef]
- Aguilos, M.; Sun, G.; Noormets, A.; Domec, J.C.; McNulty, S.; Gavazzi, M.; Minick, K.; Mitra, B.; Prajapati, P.; Yang, Y.; et al. Effects of land-use change and drought on decadal evapotranspiration and water balance of natural and managed forested wetlands along the southeastern US lower coastal plain. Agric. For. Meteorol. 2021, 303, 108381. [Google Scholar] [CrossRef]
- Aguilos, M.; Stahl, C.; Burban, B.; Hérault, B.; Courtois, E.; Coste, S.; Wagner, F.; Ziegler, C.; Takagi, K.; Bonal, D. Interannual and seasonal variations in ecosystem transpiration and water use efficiency in a tropical rainforest. Forests 2019, 10, 14. [Google Scholar] [CrossRef]
- Ile, O.J.; Aguilos, M.; Morkoc, S.; Minick, K.; Domec, J.-C.; King, J.S. Productivity of low-input short-rotation coppice American sycamore (Platanus occidentalis L.) grown at different planting densities as a bioenergy feedstock over two rotation cycles. Biomass Bioenergy 2021, 146. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Ueyama, M.; Fukuzawa, K.; Nomura, M.; Kishida, O.; Fukazawa, T.; Takahashi, H.; Kotsuka, C.; et al. Dynamics of ecosystem carbon balance recovering from a clear-cutting in a cool-temperate forest. Agric. For. Meteorol. 2014. [Google Scholar] [CrossRef]
- Agyeman, V.K.; Swaine, M.D.; Thompson, J. Responses of tropical forest tree seedlings to irradiance and the derivation of a light response index. J. Ecol. 1999, 87, 815–827. [Google Scholar] [CrossRef]
- Nussbaum, R.; Anderson, J.; Spencer, T. Factors limiting the growth of indigenous tree seedlings planted on degraded rainforest soils in Sabah, Malaysia. For. Ecol. Manag. 1995, 74, 149–159. [Google Scholar] [CrossRef]
- Schneider, T.; Ashton, M.S.; Montagnini, F.; Milan, P.P. Growth performance of sixty tree species in smallholder reforestation trials on Leyte, Philippines. New For. 2014, 45, 83–96. [Google Scholar] [CrossRef]
- Tolentino, E.L. Restoration of Philippine Native Forest by Smallholder Tree Farms; Snelder, D.J., Lasco, R.D., Eds.; Springer: New York City, NY, USA, 2008; pp. 319–346. [Google Scholar]
- Redondo-Brenes, A.; Montagnini, F. Growth, productivity, aboveground biomass, and carbon sequestration of pure and mixed native tree plantations in the Caribbean lowlands of Costa Rica. For. Ecol. Manag. 2006, 232, 168–178. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Watanabe, Y.; Goto, S.; Takahashi, Y.; Mukai, H.; Sasa, K. Soil warming in a cool-temperate mixed forest with peat soil enhanced heterotrophic and basal respiration rates but Q10 remained unchanged. Biogeosciences Discuss. 2011, 8, 6415–6445. [Google Scholar] [CrossRef]
- Aguilos, M.; Takagi, K.; Liang, N.; Watanabe, Y.; Teramoto, M.; Goto, S.; Takahashi, Y.; Mukai, H.; Sasa, K. Sustained large stimulation of soil heterotrophic respiration rate and its temperature sensitivity by soil warming in a cool-temperate forested peatland. Tellus, Ser. B Chem. Phys. Meteorol. 2013. [Google Scholar] [CrossRef]
- Park, A.; van Breugel, M.; Ashton, M.S.; Wishnie, M.; Mariscal, E.; Deago, J.; Ibarra, D.; Cedeño, N.; Hall, J.S. Local and regional environmental variation influences the growth of tropical trees in selection trials in the Republic of Panama. For. Ecol. Manag. 2010, 260, 12–21. [Google Scholar] [CrossRef]
- Wishnie, M.H.; Dent, D.H.; Mariscal, E.; Deago, J.; Cedeño, N.; Ibarra, D.; Condit, R.; Ashton, P.M.S. Initial performance and reforestation potential of 24 tropical tree species planted across a precipitation gradient in the Republic of Panama. For. Ecol. Manag. 2007, 243, 39–49. [Google Scholar] [CrossRef]
- Booth, T.H.; Broadhurst, L.M.; Pinkard, E.; Prober, S.M.; Dillon, S.K.; Bush, D.; Pinyopusarerk, K.; Doran, J.C.; Ivkovich, M.; Young, A.G. Native forests and climate change: Lessons from eucalypts. For. Ecol. Manag. 2015, 347, 18–29. [Google Scholar] [CrossRef]
- Hughes, L.; Cawsey, E.M.; Westoby, M. Climatic Range Sizes of Eucalyptus Species in Relation to Future Climate Change. Glob. Ecol. Biogeogr. Lett. 1996, 5, 23–29. [Google Scholar] [CrossRef]
- Palma, R.A.; Carandang, W.M. Carbon Sequestration and Climate Change Impact on the Yield of Bagras (Eucalyptus deglupta Blume) in Bagras-Corn Boundary Planting Agroforestry System in Misamis Oriental and Bukidnon, Philippines. J. Environ. Sci. Manag. 2014, 17, 29–37. [Google Scholar]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- McKenzie, D.; Hessl, A.E.; Peterson, D.L. Recent growth of conifer species of western North America: Assessing spatial patterns of radial growth trends. Can. J. For. Res. 2001, 31, 526–538. [Google Scholar] [CrossRef]
| Local Name | Order | Family Name | Scientific Name | Place of Seed Collection | Geographical Coordinates | Seed Collection Date | ||
|---|---|---|---|---|---|---|---|---|
| Latitude | Longitude | |||||||
| Native species | ||||||||
| 1 | Kaatoan bangkal | Gentianales | Rubiaceae | Anthocephalus chinensis (Lam.) Hassk. | Surigao del Sur | 8°13′46.06″ | 26°16′38.05″ | April 2018 |
| Butuan City | 8°56′43.47″ | 125°35′55.24″ | December 2018 | |||||
| 2 | Batino | Gentianales | Apocynaceae | Alstonia macrophylla Wall. ex G.Don | Palawan | 9°41′56.60″ | 118°29′30.91″ | February 2018 |
| 3 | Dita | Gentianales | Apocynaceae | Alstonia scholaris (L.) R.Br. | Surigao del Sur | 8°15′32.28″ | 126°16′34.44″ | April 2018 |
| Misamis Oriental | 8°35′56.58″ | 124°49′8.61″ | December 2018 | |||||
| 4 | Tuai | Malpighiales | Phyllanthaceae | Bischofia javanica Blume | Misamis Oriental | 8°37′57.02″ | 124°56′44.64″ | January 2019 |
| 5 | Agoho | Fagales | Casuarinaceae | Casuarina equisetifolia L. | Agusan Norte; | 9°26′17.26″ | 125°33′10.70″ | May 2018 |
| Surigao del Sur | 8°11′18.12″ | 126°21′45.87″ | May 2018 | |||||
| 6 | Loktob | Myrtales | Lythraceae | Duabanga moluccana Blume | Surigao del Sur | 8°17′56.00″ | 126°17′21.00″ | July 2018 |
| Butuan City | 8°56′36.39″ | 125°35′51.03″ | July 2018 | |||||
| 7 | Bagras | Myrtales | Myrtaceae | Eucalyptus deglupta Blume | Bukidnon | 8°7′33.64″ | 125°5′58.35″ | May 2018 |
| Agusan del Norte | 9°26′20.44″ | 125°33′9.53″ | July 2018 | |||||
| 8 | Bagalunga | Sapindales | Meliaceae | Melia dubia Cav. | Agusan del Sur | 8°37′27.00″ | 125°54′33.00″ | August 2018 |
| Cabadbaran City | 9°5′55.00″ | 125°38′8.00″ | August 2018 | |||||
| Butuan City | 9°0′38.00″ | 125°39′12.00″ | September 2018 | |||||
| Zamboanga del Sur | 6°58′31.08″ | 122°4′8.52″ | September 2018 | |||||
| 9 | Kupang | Fabales | Fabaceae | Parkia javanica (Lam.) Merr. | Bukidnon | 8°9′57.75″ | 125°7′5.73″ | June 2018 |
| Butuan City | 8°57′7.00″ | 125°29′29.00″ | April 2018 | |||||
| Laguna | 14°9′20.88″ | 121°14′9.13″ | April 2018 | |||||
| 10 | Talisay gubat | Myrtales | Combretaceae | Terminalia foetidissima Griff. | Misamis Oriental | 8°32′43.00″ | 124°19′11.00″ | February 2018 |
| 11 | Kalumpit | Myrtales | Combretaceae | Terminalia microcarpa Decne. | Butuan City | 8°57′16.70″ | 125°35′49.70″ | July 2018 |
| Cagayan de Oro City | 8°25′29.50″ | 124°41′42.64″ | August 2018 | |||||
| Alien species | ||||||||
| 12 | Mangium | Fabales | Fabaceae | Acacia mangium Willd. | Agusan del Norte | 9°26′14.75″ | 125°33′12.38″ | April 2018 |
| Cagayan de Oro | 8°23′56.95″ | 124°42′45.74″ | April 2018 | |||||
| 13 | Brazillian Fire Tree | Fabales | Fabaceae | Schizolobium parahyba (Vell.) S.F.Blake | Bukidnon | 8°9′24.64″ | 125°7′59.73″ | April 2018 |
| 14 | Mahogany | Sapindales | Meliaceae | Swietenia macrophylla Willd. ex Vogel | Butuan City | 8°56′36.82″ | 125°35′49.91″ | December 2018 |
| Bislig City | 8°14′40.03″ | 126°16′38.55″ | December 2019 | |||||
| Parameter | Source of Variation | DF | F values | Pr (>F) |
|---|---|---|---|---|
| DGR | ||||
| Block | 4 | 3.97 | 0.04 * | |
| Site | 2 | 8.67 | <0.0001 *** | |
| Species | 13 | 14.90 | <0.0001 *** | |
| Site × Species | 26 | 3.50 | <0.0001 *** | |
| HGR | ||||
| Block | 4 | 3.98 | 0.04 * | |
| Site | 2 | 5.83 | <0.001 ** | |
| Species | 13 | 21.47 | <0.0001 *** | |
| Site × Species | 26 | 4.05 | <0.0001 *** | |
| Survival Rate | ||||
| Block | 4 | 4.57 | 0.03 * | |
| Site | 2 | 3.48 | <0.0001 *** | |
| Species | 13 | 4.47 | <0.0001 *** | |
| Site × Species | 26 | 3.87 | <0.0001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez, C.; Aguilos, R.; Bacsal, R.; Adornado, H.; Aguilos, M. Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines. Forests 2021, 12, 909. https://doi.org/10.3390/f12070909
Marquez C, Aguilos R, Bacsal R, Adornado H, Aguilos M. Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines. Forests. 2021; 12(7):909. https://doi.org/10.3390/f12070909
Chicago/Turabian StyleMarquez, Conrado, Rustum Aguilos, Renato Bacsal, Henry Adornado, and Maricar Aguilos. 2021. "Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines" Forests 12, no. 7: 909. https://doi.org/10.3390/f12070909
APA StyleMarquez, C., Aguilos, R., Bacsal, R., Adornado, H., & Aguilos, M. (2021). Early Growth of 11 Native and Three Alien Tree Species in Northeastern Mindanao, Philippines. Forests, 12(7), 909. https://doi.org/10.3390/f12070909







