How Elemental Stoichiometric Ratios in Microorganisms Respond to Thinning Management in Larix principis-rupprechtti Mayr. Plantations of the Warm Temperate Zone in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site Description and Thinning Experiment
2.2. Soil and Litter Sampling and Laboratory Analysis
2.3. Statistical Analyses
3. Results
3.1. Soil Properties
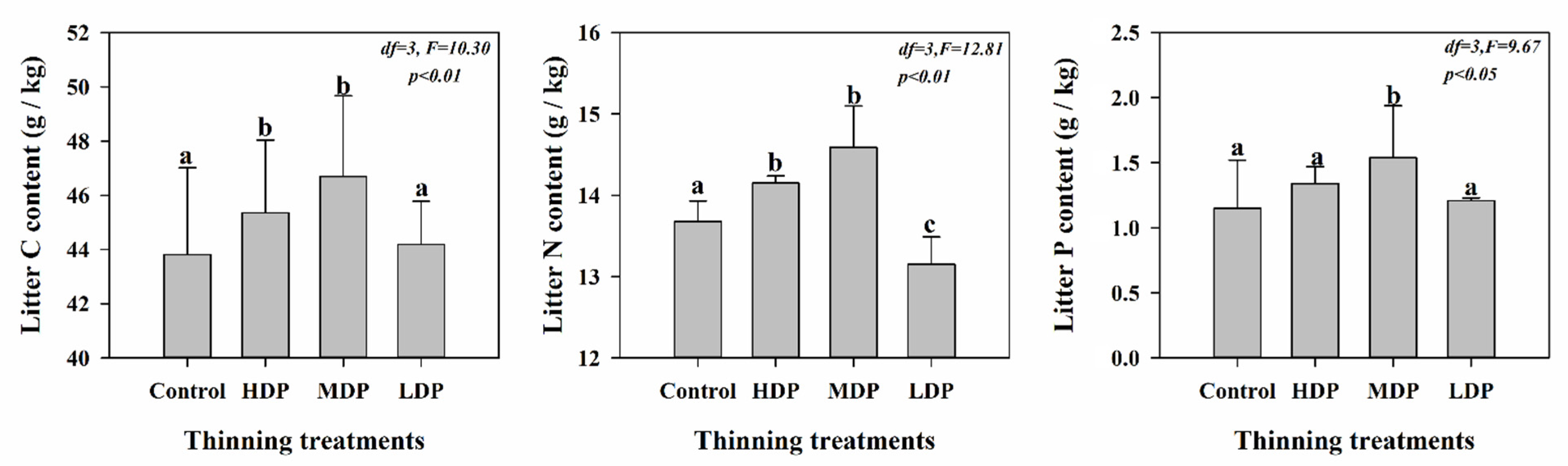
3.2. Understory Vegetation Characteristics and Litter Properties
3.3. The Stoichiometric Ratio of Soil Microbial Community
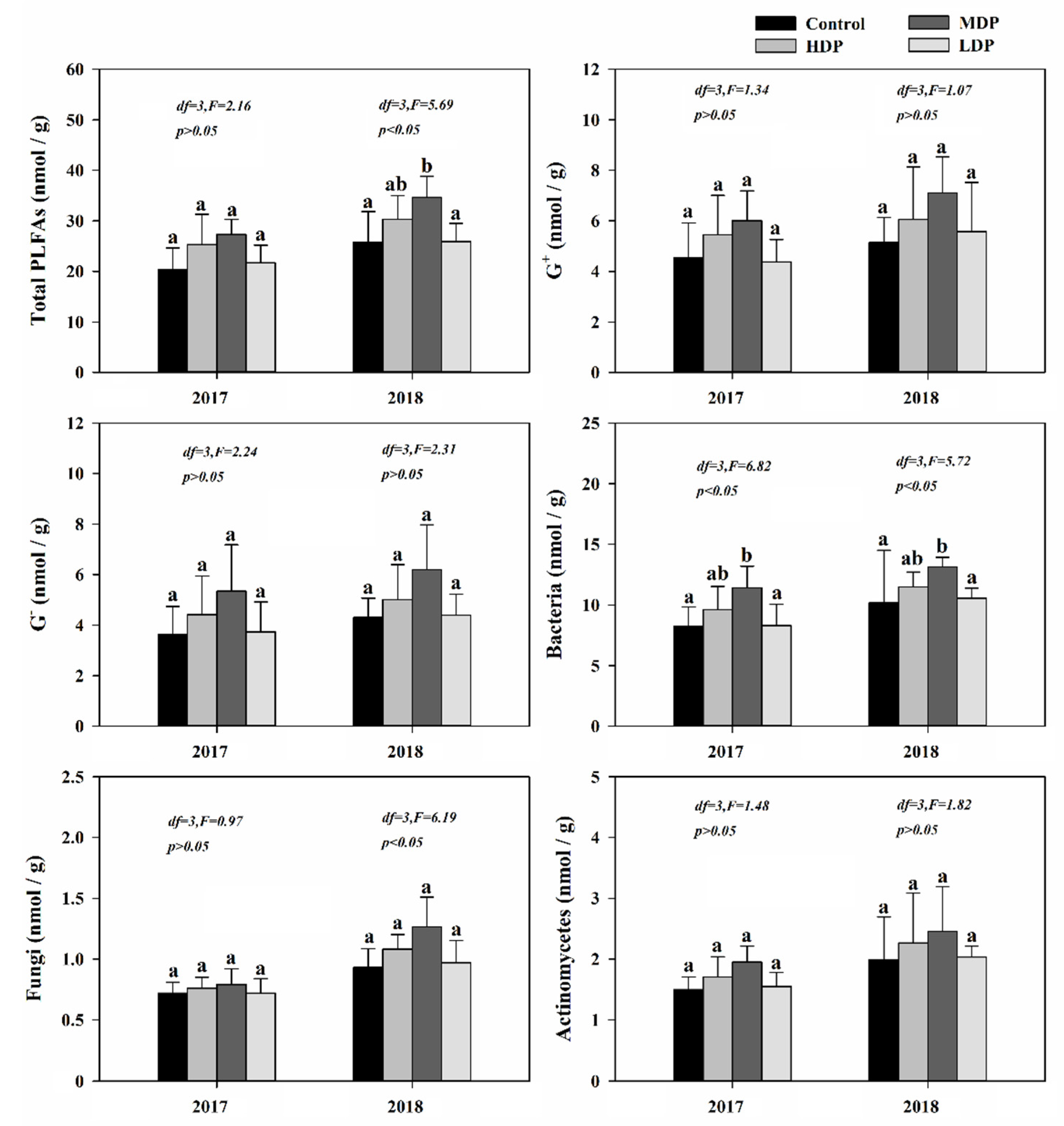
3.4. Soil Microorganism Community Composition
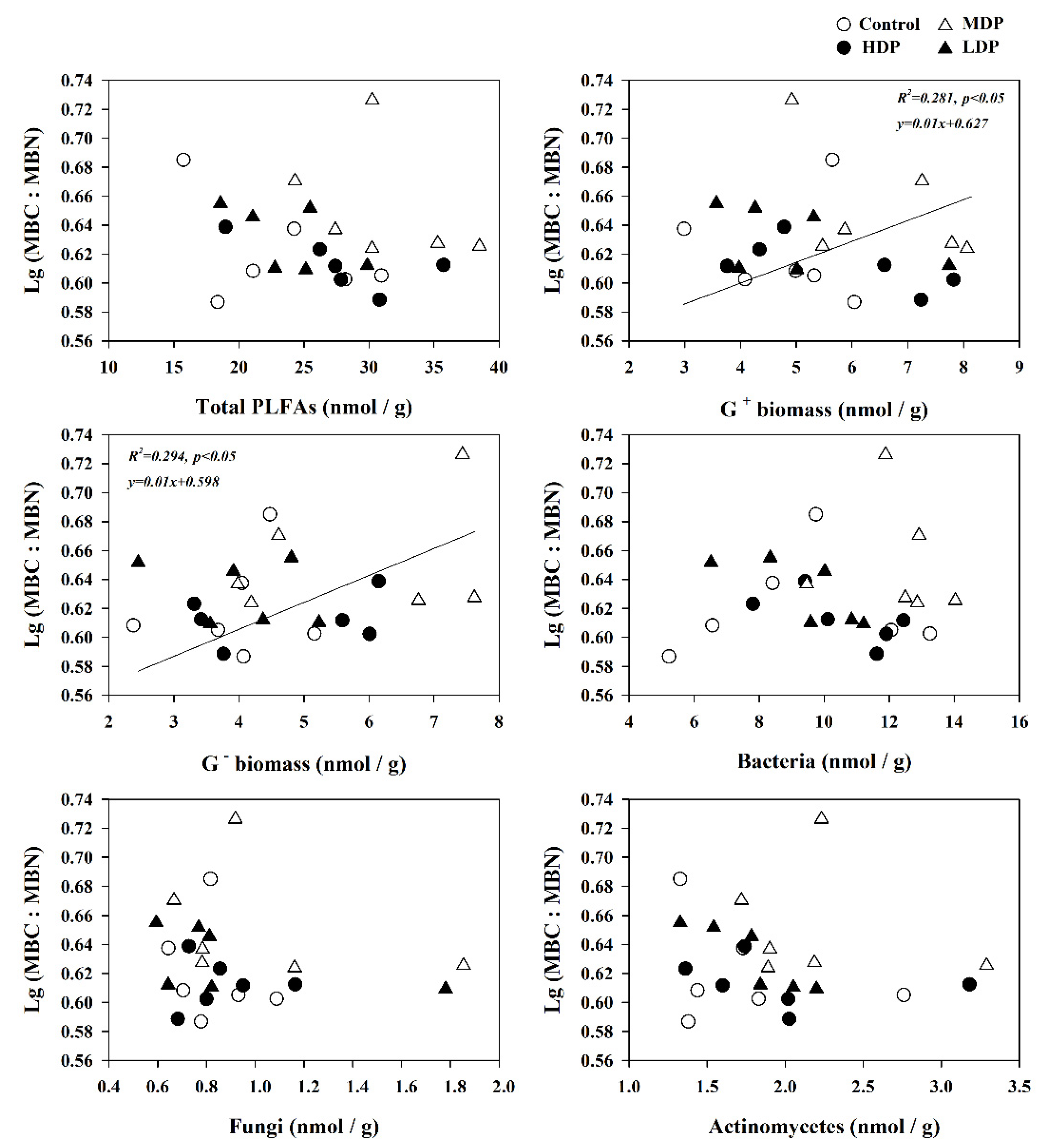
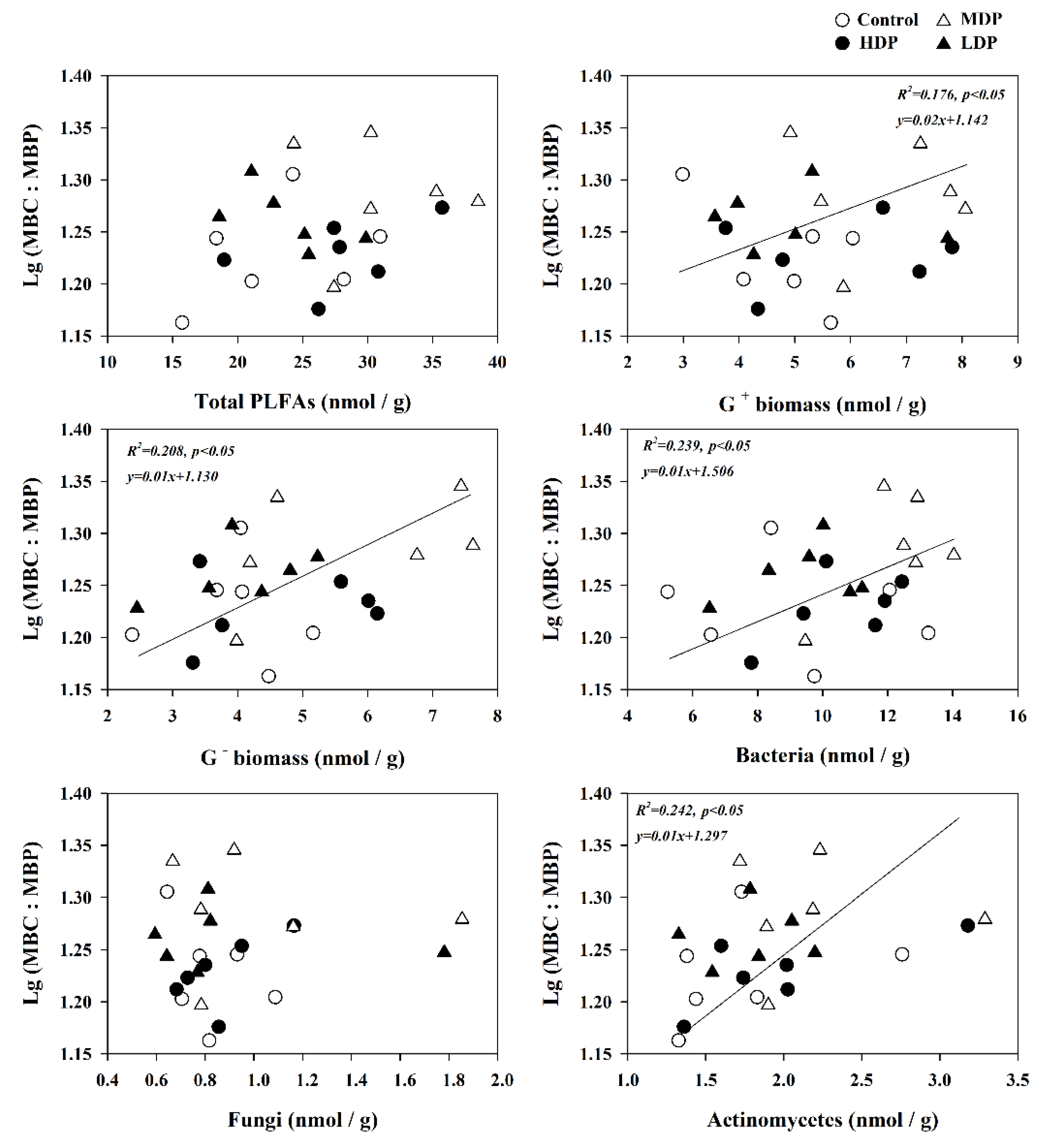
3.5. The Influence of Soil Properties and Microbial Communities on Microbial Stoichiometric Ratios
4. Discussion
4.1. Shifts in Ecological Factors Following Thinning
4.2. Shifts in Microbial Community Characteristics Following Thinning
4.3. Variation in Microbial Stoichiometry Linked to Microbe Groups and Soil Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Ma, J.; Ma, Z.; Li, L. Soil nutrient contents and stoichiometry as affected by land-use in an agro-pastoral region of northwest China. Catena 2017, 150, 146–153. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Buatois, B.; Hättenschwiler, S. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol. Lett. 2013, 16, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Sardans, J. Elementary factors. Nature 2009, 460, 803–804. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Bignell, D.E.; Brown, V.K.; Brussard, L.; Dangerfield, J.M.; Wall, D.H.; Wardle, D.A.; Coleman, D.C.; Giller, K.E.; Lavelle, P.; et al. Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: Patterns, mechanisms, and feedbacks. BioScience 2000, 50, 1049–1061. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NY, USA, 2002. [Google Scholar]
- Liu, J.; Li, S.; Ouyang, Z.; Tam, C.; Chen, X. Ecological and socioeconomic effects of China’s policies for ecosystem services. Proc. Natl. Acad. Sci. USA 2008, 105, 9477–9482. [Google Scholar] [CrossRef]
- Verschuyl, J.; Riffell, S.; Miller, D.; Wigley, T.B. Biodiversity response to intensive biomass production from forest thinning in North American forests-a meta-analysis. For. Ecol. Manag. 2011, 261, 221–232. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Cañellas, I.; Del Río, M.; Roig, S.; Montero, G. Growth response to thinning in Q uercus Pyrenaica Willd. Coppice stands in Spanish Central Mountain. Ann. For. Sci. 2004, 61, 243–250. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, L.; He, Z.; Wang, R.; Wu, P.; Ma, X. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ. Sci. Pollut. Res. 2016, 23, 24135–24150. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Fu, X.; Xu, M.; Dai, X.; Wang, H. Thinning effect on understory community and photosynthetic characteristics in a subtropical Pinus Massoniana plantation. Can. J. For. Res. 2017, 47, 1104–1115. [Google Scholar] [CrossRef]
- He, F.; Barclay, H.J. Long-term response of understory plant species to thinning and fertilization in a Douglas-fir plantation on southern Vancouver Island, British Columbia. Can. J. For. Res. 2000, 30, 566–572. [Google Scholar] [CrossRef]
- Dang, P.; Gao, Y.; Liu, J.; Yu, S.; Zhao, Z. Effects of thinning intensity on understory vegetation and soil microbial communities of a mature Chinese pine plantation in the Loess Plateau. Sci. Total Environ. 2018, 630, 171–180. [Google Scholar] [CrossRef]
- Wolk, B.; Rocca, M.E. Thinning and chipping small-diameter ponderosa pine changes understory plant communities on the Colorado Front Range. For. Ecol. Manag. 2009, 257, 85–95. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, Y.; Zhou, H.; Zhao, G.; Wang, L. Effects of gaps inthe forest canopy on soil microbial communities and enzyme activity in a Chinese pine forest. Pedobiologia 2017, 61, 51–60. [Google Scholar] [CrossRef]
- Chen, X.; Han, Y.H.; Chen, X.; Wang, J.; Chen, B.; Wang, D.; Guan, Q. Soil labile organic carbon and carbon-cycle enzyme activities under different thinning intensities in Chinese fir plantations. Appl. Soil Ecol. 2016, 107, 162–169. [Google Scholar] [CrossRef]
- Yong, L.; Jinshui, W.; Shoulong, L.; Jianlin, S.; Daoyou, H.; Yirong, S.; Wenxue, W.; Syers, J.K. Is the C:N:P stoichiometry in soil and soil microbial biomass related to the landscape and land use in southern subtropical China? Global Biogeochem. Cy. 2012, 26, 4. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y.; Wang, N.; Zeng, L.; Wang, X. Labile organic carbon pools and enzyme activities of Pinus massoniana plantation soil as affected by understory vegetation removal and thinning. Sci. Rep. 2018, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Mouginot, C.; Kawamura, R.; Matulich, K.L.; Berlemont, R.; Allison, S.D.; Amend, A.S.; Martiny, A.C. Elemental stoichiometry of fungi and bacteria strains from grassland leaf litter. Soil Biol. Biochem. 2014, 76, 278–285. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C: N: P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chen, L.Y.; Peng, Y.F.; Ding, J.Z.; Li, F.; Yang, G.B.; Kou, D.; Liu, L.; Fang, K.; Zhang, B.B.; et al. Linking microbial C:N:P stoichiometry to microbial community and abiotic factors along a 3500-km grassland transect on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2016, 25, 1416–1427. [Google Scholar] [CrossRef]
- Wu, R.; Cheng, X.Q.; Han, H.R. The effect of forest thinning on soil microbial community structure and function. Forests. 2019, 10, 352. [Google Scholar] [CrossRef]
- Yuan, J.; Jose, S.; Hu, Z.; Pang, J.; Hou, L.; Zhang, S. Biometric and eddy covariance methods for examining the carbon balance of a Larix principis-rupprechtii forest in the Qinling Mountains, China. Forests 2018, 9, 67. [Google Scholar] [CrossRef]
- Li, H.L.; Crabbe, M.J.C.; Xu, F.L.; Wang, W.L.; Niu, R.L.; Gao, X.; Zhang, P.; Chen, H.K. Seasonal variations in carbon, nitrogen and phosphorus concentrations and C:N:P stoichiometry in the leaves of differently aged Larix principis-rupprechtii Mayr. Plantations. Forests 2017, 8, 373. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wu, J.S.; Lin, Q.M.; Huang, Q.Y.; Xiao, H.A. Soil Microbial Biomass—Methods and Applications; China Meteorological Press: Beijing, China, 2006. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Fichtner, A.; von Oheimb, G.; Härdtle, W.; Wilken, C.; Gutknecht, J.L.M. Effects of anthropogenic disturbances on soil microbial communities in oak forests persist for more than 100 years. Soil Biol. Biochem. 2014, 70, 79–87. [Google Scholar] [CrossRef]
- Kourtev, P.S.; Ehrenfeld, J.G.; Häggblom, M. Exotic plant species alter the microbial community structure and function in the soil. Ecology 2002, 83, 3152–3166. [Google Scholar] [CrossRef]
- Bossio, D.A.; Fleck, J.A.; Scow, K.M.; Fujii, R. Alteration of soil microbial communities and water quality in restored wetlands. Soil Biol. Biochem. 2006, 38, 1223–1233. [Google Scholar] [CrossRef]
- Gavazov, K.; Ingrisch, J.; Hasibeder, R.; Mills, R.T.; Buttler, A.; Gleixner, G.; Pumpanen, J.; Bahn, M. Winter ecology of a subalpine grassland: Effects of snow removal on soil respiration, microbial structure and function. Sci. Total Environ. 2017, 590–591, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.T.; Zak, D.R.; White, D.C.; Peacock, A. Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci. Soc. Am. J. 2001, 65, 359–367. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Ren, T.; Tian, Z.; Wang, G.; He, X.; Tian, C. Short-term effect of tillage and crop rotation on microbial community structure and enzyme activities of a clay loam soil. Biol. Fertil. Soils 2014, 50, 1077–1085. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Ledebuhr, A.; Papen, H. Effects of forest management on soil N cycling in beech forests stocking on calcareous soils. Plant Soil 2006, 287, 279–300. [Google Scholar] [CrossRef]
- Baena, C.W.; Andrés-Abellán, M.; Lucas-Borja, M.E.; Martínez-García, E.; García-Morote, F.A.; Rubio, E.; Lópes-Serraano, F.R. Thinning and recovery effects on soil properties in two sites of a Mediterranean forest, in Cuenca Mountain (south-eastern of Spain). For. Ecol. Manag. 2013, 308, 223–230. [Google Scholar] [CrossRef]
- Tian, L.M.; Zhao, L.; Wu, X.D.; Fang, H.B.; Zhao, Y.H.; Hu, G.J.; Yue, G.Y.; Sheng, Y.; Wu, J.C.; Chen, J.; et al. Soil moisture and texture primarily control the soil nutrient stoichiometry across the Tibetan grassland. Sci. Total Environ. 2018, 622–623, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationship between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Settineri, G.; Mallamaci, C.; Mitrović, M.; Sidari, M.; Muscolo, A. Effects of different thinning intensities on soil carbon storage in Pinus laricio forest of Apenneine South Italy. Eur. J. For. Res. 2018, 137, 131–141. [Google Scholar] [CrossRef]
- Trentini, C.P.; Gampanello, P.I.; Villagra, M.; Ritter, L.; Ares, A.; Goldstein, G. Thinning of loblolly pine plantations in subtropical Argentina: Impact on microclimate and understory vegetation. For. Ecol. Manag. 2017, 384, 236–247. [Google Scholar] [CrossRef]
- Deng, J.; Sun, P.; Zhao, F.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Soil C, N, P and its stratification ratio affected by artificial vegetation in subsoil, Loess Plateau China. PLoS ONE 2016, 11, e0151446. [Google Scholar] [CrossRef]
- Kim, S.; Li, G.; Han, S.; Kim, C.; Lee, S.; Son, Y. Microbial biomass and enzymatic responses to temperate oak and larch forest thinning: Influential factors for the site-specific changes. Sci. Total Environ. 2019, 651, 2068–2079. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.; Hui, D.; Zhang, Q.; Li, M.; Zhang, Q. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The links between ecosystem multi-functionality and above- and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Juodvalkis, A.; Kairiukstis, L.; Vasiliauskas, R. Effects of thinning on growth of six tree species in north-temperate forests of Lithuania. Eur. J. For. Res. 2005, 124, 187–192. [Google Scholar] [CrossRef]
- Geng, Y.; Dighton, J.; Gray, D. The effects of thinning and soil disturbance on enzyme activities under pitch pine soil in New Jersey Pinelands. Appl. Soil Ecol. 2012, 62, 1–7. [Google Scholar] [CrossRef]
- Xiao, W.; Fei, F.; Diao, J.; Chen, B.J.W.; Guan, Q. Thinning intensity affects microbial functional diversity and enzymatic activities associated with litter decomposition in a Chinese fir plantation. J. For. Res. 2018, 29, 1337–1350. [Google Scholar] [CrossRef]
- Maassen, S.; Wirth, S. Soil microbiological monitoring of a pine forest after partial thinning for stand regeneration with beech seedlings. Soil Sci. Plant Nutr. 2004, 50, 815–819. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Lado-Monserrat, L.; Lidón, A.; Bautista, I. Litterfall, litter decomposition and associated nutrient fluxes in Pinus halepensis: Influence of tree removal intensity in a Mediterranean forest. Eur. J. For. Res. 2015, 5, 833–844. [Google Scholar] [CrossRef]
- Thibodeau, L.; Raymond, P.; Camiré, C.; Munson, A.D. Impact of precommercial thinning in balsam fir stands on soil nitrogen dynamics, microbial biomass, decomposition, and foliar nutrition. Can. J. For. Res. 2000, 30, 229–238. [Google Scholar] [CrossRef]
- Kim, S.; Li, G.; Han, S.H.; Kim, H.-J.; Kim, C.; Lee, S.-T.; Son, Y. Thinning affects microbial biomass without changing enzyme activity in the soil of Pinus densiflora Sieb. et Zucc. forests after 7 years. Ann. For. Sci. 2018, 75, 13. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 1–11. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.Y.; Hu, B.; Bao, W.K.; Tian, G.L. Does thinning- induced gap size result in altered soil microbial community in pine plantation in eastern Tibetan Plateau? Ecol. Evol. 2017, 7, 2986–2993. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.C.; Peng, D.L.; Wang, H.B.; Wang, Z.Y.; Cheng, S. Minimum data set for evaluation of stand density effects on soil quality in Larix principis-rupprechtii plantations in North China. Ecol. Indic. 2019, 103, 236–247. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Li, J.W.; Liu, Y.L.; Hai, X.Y.; Shangguan, Z.P.; Lei, D. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Rev. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Huang, C. Spatial variability of soil total nitrogen and soil total phosphorus under different land uses in a small watershed on the Loess Plateau, China. Geoderma 2009, 150, 141–149. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant-microbial-soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Reich, P.B.; Khachane, A.N.; Campbell, C.D.; Thomas, N.; Freitag, T.E.; Abu Al-Soud, W.; Sørensen, S.; Bardgett, R.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Xu, X.F.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
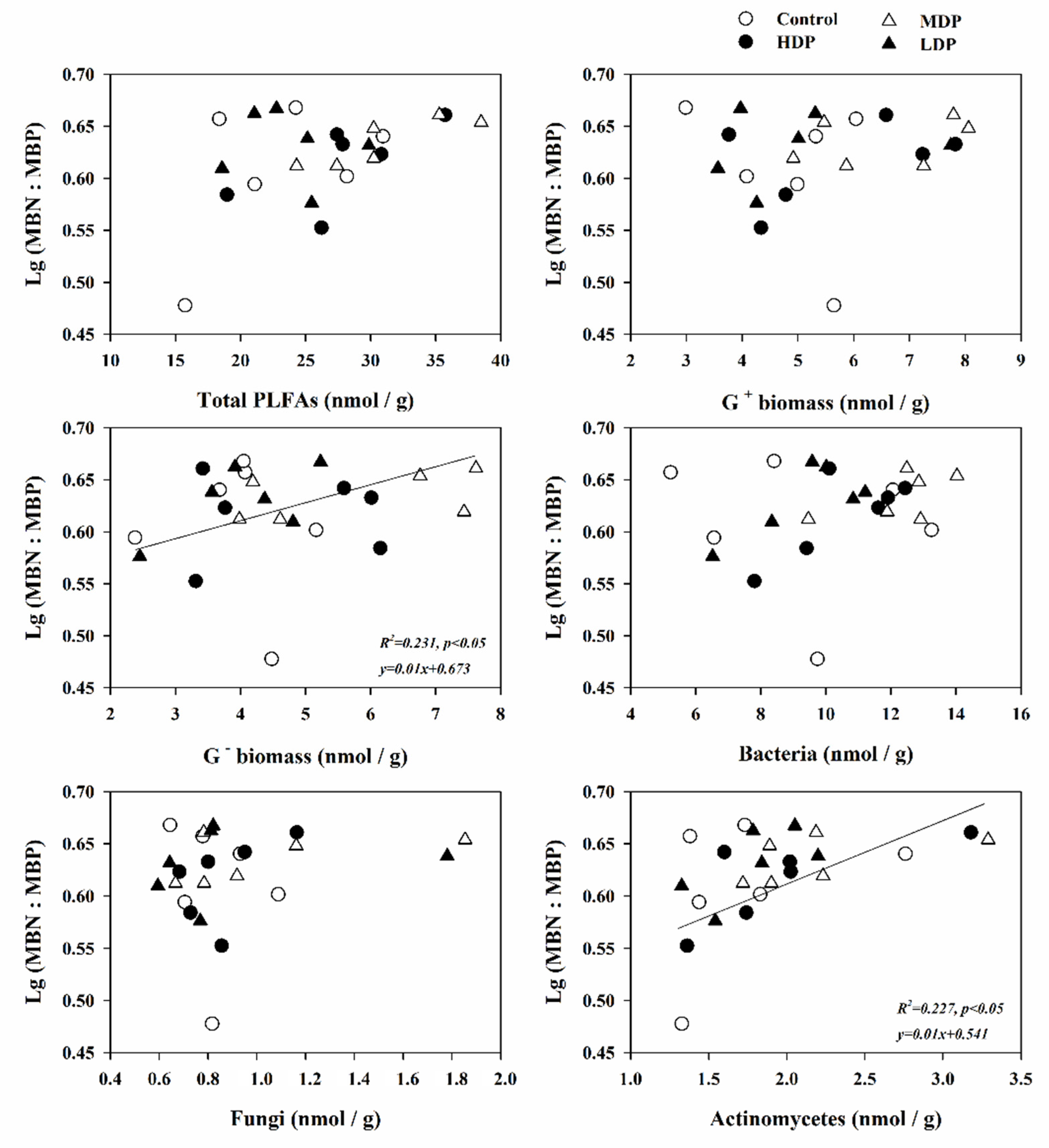
| Thinning Treatments | STC (g kg−1) | STN (g kg−1) | STP (g kg−1) | NO3−-N (mg kg−1) | NH4+-N (mg kg−1) | SAP (mg kg−1) | pH Value | SWC (%) |
|---|---|---|---|---|---|---|---|---|
| Control | 37.2 ± 1.0 a | 3.0 ± 0.4 a | 0.5 ± 0.1 a | 7.1 ± 2.2 a | 4.7 ± 0.6 a | 4.0 ± 0.1 a | 6.2 ± 0.1 a | 32.4 ± 1.3 a |
| HDP | 40.5 ± 1.3 b | 3.4 ± 0.6 b | 0.4 ± 0.1 a | 9.1 ± 2.9 b | 4.9 ± 0.6 a | 4.3 ± 0.2 a | 6.2 ± 0.1 a | 45.5 ± 2.7 b |
| MDP | 50.0 ± 1.4 c | 3.9 ± 0.6 c | 0.5 ± 0.1 a | 10.0 ± 2.5 b | 5.9 ± 0.5 b | 4.9 ± 0.2 a | 6.1 ± 0.1 a | 50.7 ± 2.5 b |
| LDP | 43.2 ± 1.3 b | 3.6 ± 0.4 b | 0.5 ± 0.0 a | 8.6 ± 1.7 ab | 5.1 ± 0.6 a | 4.3 ± 0.1 a | 6.2 ± 0.1 a | 44.0 ± 2.8 b |
| df | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| F | 10.19 | 9.86 | 2.43 | 7.34 | 6.41 | 1.36 | 0.95 | 8.91 |
| p | <0.01 | <0.01 | 0.37 | 0.02 | 0.03 | 0.43 | 0.56 | <0.01 |
| Thinning Treatment | Shrub Biodiversity | Herb Biodiversity | Litter Layer Biomass (t ha−1) | ||||
|---|---|---|---|---|---|---|---|
| Shannon Index | Richness Index | Evenness Index | Shannon Index | Richness Index | Evenness Index | ||
| Control | 0.37 ± 0.63 a | 1 ± 1 a | 0.12 ± 0.21 a | 4.18 ± 0.01 a | 18 ± 3 a | 1.13 ± 0.01 a | 55.71 ± 9.57 a |
| HDP | 0.46 ± 0.40 a | 2 ± 1 a | 0.23 ± 0.20 a | 4.69 ± 0.47 ab | 22 ± 5 a | 1.26 ± 0.13 ab | 60.44 ± 18.09 ab |
| MDP | 0.44 ± 0.77 a | 2 ± 2 a | 0.32 ± 0.55 a | 5.19 ± 0.76 b | 25 ± 6 a | 1.40 ± 0.20 b | 69.15 ± 11.61 b |
| LDP | 0.23 ± 0.40 a | 1 ± 1 a | 0.12 ± 0.20 a | 4.51 ± 0.43 ab | 23 ± 1 a | 1.21 ± 0.12 ab | 58.01 ± 14.27 a |
| df | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| F | 0.76 | 0.61 | 0.53 | 7.23 | 1.56 | 7.53 | 7.25 |
| p | 0.64 | 0.50 | 0.46 | 0.02 | 0.27 | 0.02 | 0.02 |
| Treatment Methods | Soil Microbial Biomass | Soil Microbial Biomass Stoichiometry | References | |||||
|---|---|---|---|---|---|---|---|---|
| Microbial Biomass C (mg kg−1) | Microbial Biomass N (mg kg−1) | Microbial Biomass P (mg kg−1) | MBC:MBN | MBC:MBP | MBN:MBP | |||
| 2017 | Control | 433 ± 2 a | 98 ± 2 a | 26 ± 6 a | 4.4 ± 0.4 ab | 16.9 ± 3.0 ab | 3.9 ± 0.8 a | In this paper |
| HDP | 512 ± 2 b | 124 ± 10 b | 32 ± 2 b | 4.1 ± 0.2 a | 16.0 ± 0.9 a | 3.9 ± 0.3 a | ||
| MDP | 737 ± 4 c | 154 ± 9 c | 37 ± 2 c | 4.8 ± 0.5 b | 19.8 ± 3.6 c | 4.1 ± 0.0 a | ||
| LDP | 547 ± 5 b | 122 ± 2 b | 40 ± 3 c | 4.5 ± 0.1 ab | 18.5 ± 1.7 b | 4.1 ± 0.4 a | ||
| df | 3 | 3 | 3 | 3 | 3 | 3 | ||
| F | 13.35 | 12.98 | 11.83 | 7.90 | 9.88 | 2.29 | ||
| p | <0.01 | <0.01 | <0.01 | <0.05 | <0.01 | >0.05 | ||
| 2018 | Control | 578 ± 5 a | 146 ± 2 a | 34 ± 2 a | 4.0 ± 0.1 a | 17.1 ± 0.9 a | 4.3 ± 0.3 a | |
| HDP | 617 ± 6 ab | 152 ± 2 a | 34 ± 1 a | 4.0 ± 0.1 a | 18.0 ± 0.8 ab | 4.4 ± 0.2 a | ||
| MDP | 726 ± 3 c | 172 ± 1 b | 38 ± 1 b | 4.2 ± 0.0 b | 19.1 ± 0.4 c | 4.5 ± 0.1 a | ||
| LDP | 653 ± 9 b | 160 ± 2 b | 36 ± 1 b | 4.1 ± 0.0 ab | 18.1 ± 0.8 b | 4.4 ± 0.2 a | ||
| df | 3 | 3 | 3 | 3 | 3 | 3 | ||
| F | 13.01 ** | 12.67 ** | 11.34 ** | 7.60 * | 9.34 ** | 2.02 | ||
| p | <0.01 | <0.01 | <0.01 | <0.05 | <0.01 | >0.05 | ||
| Global average | 680 | 105 | 161 | 7.6 | 42.4 | 5.6 | Xu et al., (2013) | |
| Temperate coniferous forest | 508 | 80 | 86 | 6.3 | 70.6 | 7.4 | Xu et al., (2013) | |
| Soil Microbial Biomass C, N, and P Ratios | Soil pH Value | SWC | Soil Temperature | STC | STN | STP | NO3−-N | NH4+-N | SAP |
|---|---|---|---|---|---|---|---|---|---|
| Soil microbial C | 0.26 | −0.16 | 0.46 * | 0.61 ** | 0.16 | 0.24 | 0.14 | 0.57 ** | 0.35 |
| Soil microbial N | 0.26 | −0.19 | −0.23 | 0.58 ** | 0.30 | 0.16 | 0.18 | 0.45* | 0.39 |
| Soil microbial P | 0.25 | −0.04 | −0.28 | 0.27 | 0.11 | 0.08 | 0.04 | 0.20 | 0.42 * |
| MBC: MBN | −0.17 | 0.20 | 0.51 * | −0.27 | 0.18 | −0.28 | 0.20 | −0.21 | −0.56 * |
| MBC: MBP | −0.24 | 0.09 | 0.24 | 0.36 * | 0.44 * | 0.49 * | 0.07 | −0.14 | −0.12 |
| MBN: MBP | −0.11 | 0.25 | 0.49 * | −0.13 | −0.09 | −0.18 | −0.25 | −0.15 | −0.19 |
| Predicted Variables | R2 | Sig. | Contribution of the Individual Predictor (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Actinomycete | Gram-Positive Bacteria | Gram-Negative Bacteria | STP | Soil Temperature | SAP | |||
| Soil microbial C:N | 0.274 | <0.05 | 14.5 | 39.3 | 20.4 | 25.8 | |||
| Soil microbial C:P | 0.530 | <0.01 | 34.1 | 16.9 | 13.7 | 11.8 | 23.5 | ||
| Soil microbial N:P | 0.201 | <0.05 | 65.1 | 17.6 | 17.3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Xing, S.; Cheng, X.; Liu, L.; Peng, X.; Shang, T.; Han, H. How Elemental Stoichiometric Ratios in Microorganisms Respond to Thinning Management in Larix principis-rupprechtti Mayr. Plantations of the Warm Temperate Zone in China. Forests 2021, 12, 684. https://doi.org/10.3390/f12060684
Cai M, Xing S, Cheng X, Liu L, Peng X, Shang T, Han H. How Elemental Stoichiometric Ratios in Microorganisms Respond to Thinning Management in Larix principis-rupprechtti Mayr. Plantations of the Warm Temperate Zone in China. Forests. 2021; 12(6):684. https://doi.org/10.3390/f12060684
Chicago/Turabian StyleCai, Mengke, Shiping Xing, Xiaoqing Cheng, Li Liu, Xinhao Peng, Tianxiong Shang, and Hairong Han. 2021. "How Elemental Stoichiometric Ratios in Microorganisms Respond to Thinning Management in Larix principis-rupprechtti Mayr. Plantations of the Warm Temperate Zone in China" Forests 12, no. 6: 684. https://doi.org/10.3390/f12060684
APA StyleCai, M., Xing, S., Cheng, X., Liu, L., Peng, X., Shang, T., & Han, H. (2021). How Elemental Stoichiometric Ratios in Microorganisms Respond to Thinning Management in Larix principis-rupprechtti Mayr. Plantations of the Warm Temperate Zone in China. Forests, 12(6), 684. https://doi.org/10.3390/f12060684





