Nonlinear Weather–Growth Relationships Suggest Disproportional Growth Changes of Norway Spruce in the Eastern Baltic Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Sampling, and Measurements
2.2. Data Analysis
3. Results
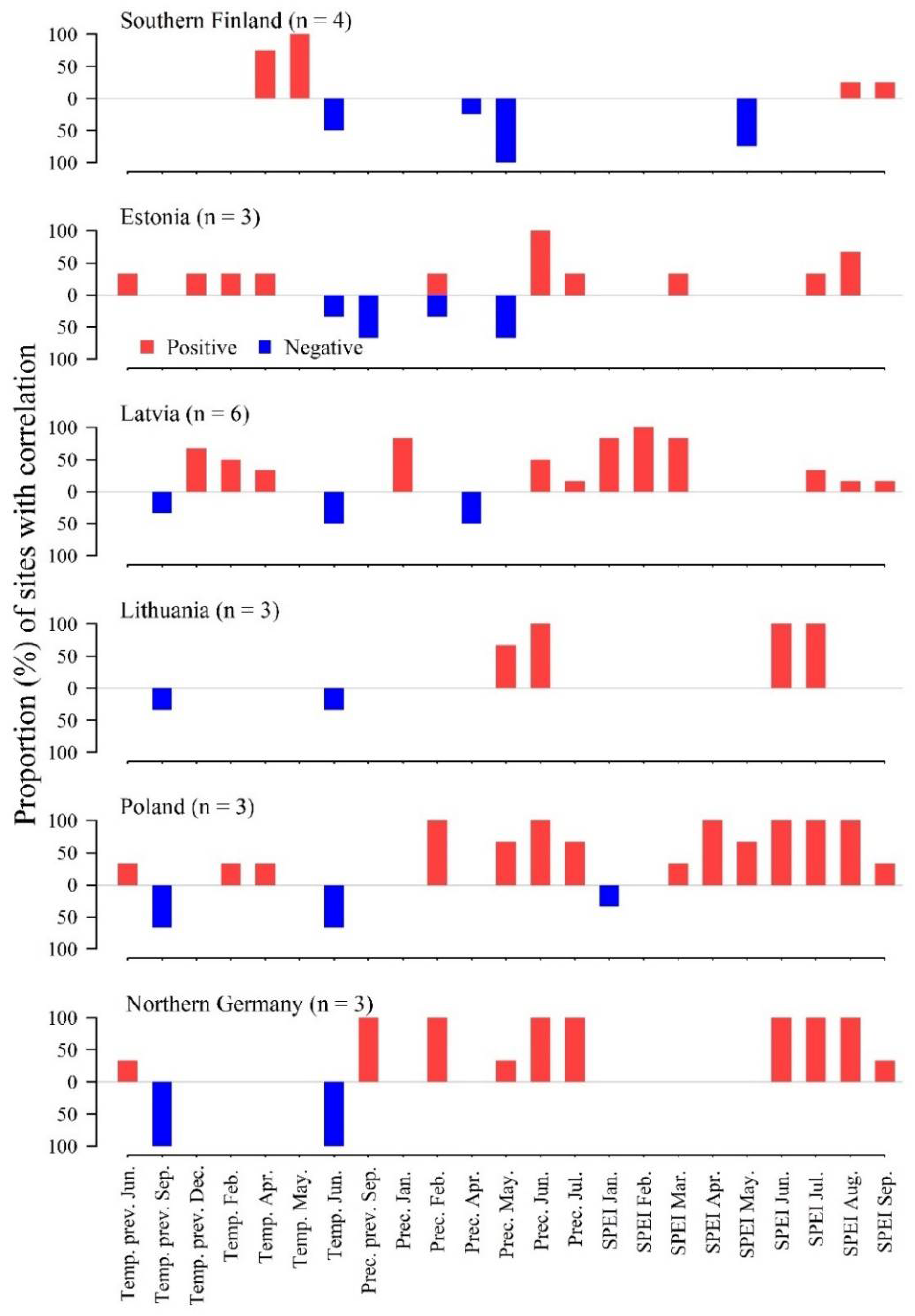
3.1. Local Weather–Growth Correlations
3.2. Nonstationarity of Local Correlations
3.3. Regional Weather–Growth Response Curves
4. Discussion
4.1. Plasticity and Stationarity of Weather–Growth Relationships
4.2. Regional Growth Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buras, A.; Menzel, A. Projecting Tree Species Composition Changes of European Forests for 2061–2090 Under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef] [PubMed]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.J.; Nabuurs, G.J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Nabuurs, G.-J.; Verkerk, P.J.; Schelhaas, M.-J.; Ramón González Olabarria, J.; Trasobares, A.; Cienciala, E. Climate-Smart Forestry: Mitigation impacts in three European regions. In From Science to Policy 6; European Forest Institute: Joensuu, Finland, 2018; p. 32. [Google Scholar]
- Yousefpour, R.; Temperli, C.; Bugmann, H.; Elkin, C.; Hanewinkel, M.; Meilby, H.; Jacobsen, J.B.; Thorsen, B.J. Updating beliefs and combining evidence in adaptive forest management under climate change: A case study of Norway spruce (Picea abies L. Karst) in the Black Forest, Germany. J. Environ. Manag. 2013, 122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.E.; Smiljanić, M.; Scharnweber, T.; Buras, A.; Cedro, A.; Cruz-García, R.; Drobyshev, I.; Janecka, K.; Jansons, Ā.; Kaczka, R.; et al. Tree growth influenced by warming winter climate and summer moisture availability in northern temperate forests. Glob. Chang. Biol. 2020, 26, 2505–2518. [Google Scholar] [CrossRef]
- Taeger, S.; Sparks, T.H.; Menzel, A. Effects of temperature and drought manipulations on seedlings of Scots pine provenances. Plant. Biol. 2015, 17, 361–372. [Google Scholar] [CrossRef]
- Ditmarova, L.; Kurjak, D.; Palmroth, S.; Kmet, J.; Strelcova, K. Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol. 2009, 30, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Bast, A.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Non-linear regional weather-growth relationships indicate limited adaptability of the eastern Baltic Scots pine. For. Ecol. Manag. 2021, 479, 118600. [Google Scholar] [CrossRef]
- Cavin, L.; Jump, A.S. Highest drought sensitivity and lowest resistance to growth suppression are found in the range core of the tree Fagus sylvatica L. not the equatorial range edge. Glob. Chang. Biol. 2017, 23, 362–379. [Google Scholar] [CrossRef]
- Restaino, C.M.; Peterson, D.L.; Littell, J. Increased water deficit decreases Douglas fir growth throughout western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 9557–9562. [Google Scholar] [CrossRef] [PubMed]
- Wilmking, M.; Maaten-Theunissen, M.; Maaten, E.; Scharnweber, T.; Buras, A.; Biermann, C.; Gurskaya, M.; Hallinger, M.; Lange, J.; Shetti, R.; et al. Global assessment of relationships between climate and tree growth. Glob. Chang. Biol. 2020, 26, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Castagneri, D.; Fonti, P.; von Arx, G.; Carrer, M. How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Ann. Bot. 2017, 119, mcw274. [Google Scholar] [CrossRef]
- Zhang, Z.; Babst, F.; Bellassen, V.; Frank, D.; Launois, T.; Tan, K.; Ciais, P.; Poulter, B. Converging Climate Sensitivities of European Forests Between Observed Radial Tree Growth and Vegetation Models. Ecosystems 2018, 21, 410–425. [Google Scholar] [CrossRef]
- Heer, K.; Behringer, D.; Piermattei, A.; Bässler, C.; Brandl, R.; Fady, B.; Jehl, H.; Liepelt, S.; Lorch, S.; Piotti, A.; et al. Linking dendroecology and association genetics in natural populations: Stress responses archived in tree rings associate with SNP genotypes in silver fir (Abies alba Mill.). Mol. Ecol. 2018, 27, 1428–1438. [Google Scholar] [CrossRef]
- Housset, J.M.; Nadeau, S.; Isabel, N.; Depardieu, C.; Duchesne, I.; Lenz, P.; Girardin, M.P. Tree rings provide a new class of phenotypes for genetic associations that foster insights into adaptation of conifers to climate change. New Phytol. 2018, 218, 630–645. [Google Scholar] [CrossRef] [PubMed]
- McCullough, I.M.; Davis, F.W.; Williams, A.P. A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings. For. Ecol. Manag. 2017, 402, 223–233. [Google Scholar] [CrossRef]
- Shi, F.; Yang, B.; Linderholm, H.W.; Seftigen, K.; Yang, F.; Yin, Q.; Shao, X.; Guo, Z. Ensemble standardization constraints on the influence of the tree growth trends in dendroclimatology. Clim. Dyn. 2020, 54, 3387–3404. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Pattison, R.R.; Brownlee, A.H.; Cahoon, S.M.P.; Hollingsworth, T.N. Effect of tree-ring detrending method on apparent growth trends of black and white spruce in interior Alaska. Environ. Res. Lett. 2016, 11, 114007. [Google Scholar] [CrossRef]
- Tei, S.; Sugimoto, A.; Yonenobu, H.; Matsuura, Y.; Osawa, A.; Sato, H.; Fujinuma, J.; Maximov, T. Tree-ring analysis and modeling approaches yield contrary response of circumboreal forest productivity to climate change. Glob. Chang. Biol. 2017, 23, 5179–5188. [Google Scholar] [CrossRef]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of species responses to climate change. Sci. Adv. 2017, 3, e1603055. [Google Scholar] [CrossRef]
- Konter, O.; Büntgen, U.; Carrer, M.; Timonen, M.; Esper, J. Climate signal age effects in boreal tree-rings: Lessons to be learned for paleoclimatic reconstructions. Quat. Sci. Rev. 2016, 142, 164–172. [Google Scholar] [CrossRef]
- Hofgaard, A.; Ols, C.; Drobyshev, I.; Kirchhefer, A.J.; Sandberg, S.; Söderström, L. Non-stationary Response of Tree Growth to Climate Trends Along the Arctic Margin. Ecosystems 2019, 22, 434–451. [Google Scholar] [CrossRef]
- Billings, S.A.; Glaser, S.M.; Boone, A.S.; Stephen, F.M. Nonlinear tree growth dynamics predict resilience to disturbance. Ecosphere 2015, 6, art242. [Google Scholar] [CrossRef]
- Lloyd, A.H.; Duffy, P.A.; Mann, D.H. Nonlinear responses of white spruce growth to climate variability in interior Alaska. Can. J. For. Res. 2013, 43, 331–343. [Google Scholar] [CrossRef]
- Matisons, R.; Puriņa, L.; Adamovičs, A.; Robalte, L.; Jansons, Ā. European beech in its northeasternmost stands in Europe: Varying climate-growth relationships among generations and diameter classes. Dendrochronologia 2017, 45, 123–131. [Google Scholar] [CrossRef]
- Ohse, B.; Ohse, B.; Jansen, F.; Wilmking, M. Do limiting factors at Alaskan treelines shift with climatic regimes? Environ. Res. Lett. 2012, 7, 15505. [Google Scholar] [CrossRef]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; Kleunen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef]
- Booth, T.H. Estimating potential range and hence climatic adaptability in selected tree species. For. Ecol. Manag. 2016, 366, 175–183. [Google Scholar] [CrossRef]
- Seddon, A.W.R.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef]
- Nabais, C.; Hansen, J.K.; David-Schwartz, R.; Klisz, M.; López, R.; Rozenberg, P. The effect of climate on wood density: What provenance trials tell us? For. Ecol. Manag. 2018, 408, 148–156. [Google Scholar] [CrossRef]
- Chauvin, T.; Cochard, H.; Segura, V.; Rozenberg, P. Native-source climate determines the Douglas-fir potential of adaptation to drought. For. Ecol. Manag. 2019, 444, 9–20. [Google Scholar] [CrossRef]
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Klisz, M.; Buras, A.; Sass-Klaassen, U.; Puchałka, R.; Koprowski, M.; Ukalska, J. Limitations at the Limit? Diminishing of Genetic Effects in Norway Spruce Provenance Trials. Front. Plant. Sci. 2019, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Trouillier, M.; van der Maaten-Theunissen, M.; Scharnweber, T.; Würth, D.; Burger, A.; Schnittler, M.; Wilmking, M. Size matters—a comparison of three methods to assess age- and size-dependent climate sensitivity of trees. Trees Struct. Funct. 2019, 33, 183–192. [Google Scholar] [CrossRef]
- Wu, G.; Xu, G.; Chen, T.; Liu, X.; Zhang, Y.; An, W.; Wang, W.; Fang, Z.A.; Yu, S. Age-dependent tree-ring growth responses of Schrenk spruce (Picea schrenkiana) to climate—A case study in the Tianshan Mountain, China. Dendrochronologia 2013, 31, 318–326. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Makinen, H.; Prislan, P.; Rossi, S.; Del Castillo, E.M.; Campelo, F.; Vavrčík, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Cuny, H.E.; Fonti, P.; Rathgeber, C.B.K.; Arx, G.; Peters, R.L.; Frank, D.C. Couplings in cell differentiation kinetics mitigate air temperature influence on conifer wood anatomy. Plant. Cell Environ. 2019, 42, 1222–1232. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Buntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Loffler, J. Complex climate controls on 20th century oak growth in Central-West Germany. Tree Physiol. 2009, 29, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; O’Neill, G.A.; Aitken, S.N. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 2010, 20, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Parkatti, V.P.; Assmuth, A.; Rämö, J.; Tahvonen, O. Economics of boreal conifer species in continuous cover and rotation forestry. For. Policy Econ. 2019, 100, 55–67. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Bauhus, J. Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob. Chang. Biol. 2017, 23, 5108–5119. [Google Scholar] [CrossRef] [PubMed]
- Niinimäki, S.; Tahvonen, O.; Mäkelä, A.; Linkosalo, T. On the economics of Norway spruce stands and carbon storage. Can. J. For. Res. 2013, 43, 637–648. [Google Scholar] [CrossRef]
- Netherer, S.; Panassiti, B.; Pennerstorfer, J.; Matthews, B. Acute Drought Is an Important Driver of Bark Beetle Infestation in Austrian Norway Spruce Stands. Front. For. Glob. Chang. 2019, 2, 39. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Appelberg, G.; Harding, S.; Bärring, L. Spatio-temporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Glob. Chang. Biol. 2009, 15, 486–499. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Jäger, D.; Lexer, M.J. Impact of bark beetle (Ips typographus L.) disturbance on timber production and carbon sequestration in different management strategies under climate change. For. Ecol. Manag. 2008, 256, 209–220. [Google Scholar] [CrossRef]
- Hlásny, T.; Barka, I.; Roessiger, J.; Kulla, L.; Trombik, J.; Sarvašová, Z.; Bucha, T.; Kovalčík, M.; Čihák, T. Conversion of Norway spruce forests in the face of climate change: A case study in Central Europe. Eur. J. For. Res. 2017, 136, 1013–1028. [Google Scholar] [CrossRef]
- Skrøppa, T. Picea abies—Technical Guidelines for Genetic Conservation and Use for Norway Spruce; Norwegian Forest Research Institute: Ås, Norway, 2003; p. 6. [Google Scholar]
- Sedmáková, D.; Sedmák, R.; Bosela, M.; Ježík, M.; Blaženec, M.; Hlásny, T.; Marušák, R. Growth-climate responses indicate shifts in the competitive ability of European beech and Norway spruce under recent climate warming in East-Central Europe. Dendrochronologia 2019, 54, 37–48. [Google Scholar] [CrossRef]
- Mäkinen, H.; Nöjd, P.; Kahle, H.P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Radial growth variation of Norway spruce (Picea abies (L.) Karst.) across latitudinal and altitudinal gradients in central and northern Europe. For. Ecol. Manag. 2002, 171, 243–259. [Google Scholar] [CrossRef]
- Weigel, R.; Muffler, L.; Klisz, M.; Kreyling, J.; Van Der Maaten-Theunissen, M.; Wilmking, M.; Van Der Maaten, E. Winter matters: Sensitivity to winter climate and cold events increases towards the cold distribution margin of European beech (Fagus sylvatica L.). J. Biogeogr. 2018, 45, 2779–2790. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Trajkovic, S. Temperature-Based Approaches for Estimating Reference Evapotranspiration. J. Irrig. Drain. Eng. 2005, 131, 316–323. [Google Scholar] [CrossRef]
- Hartmann, D.L.; Klein Tank, A.M.G.; Rusticucci, M.; Alexander, L.V.; Brönnimann, S.; Charabi, Y.A.R.; Dentener, F.J.; Dlugokencky, E.J.; Easterling, D.R.; Kaplan, A.; et al. Observations: Atmosphere and surface. In Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; Volume 9781107057999, pp. 159–254. ISBN 9781107415324. [Google Scholar]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Von Arx, G.; Arzac, A.; Fonti, P.; Frank, D.; Zweifel, R.; Rigling, A.; Galiano, L.; Gessler, A.; Olano, J.M. Responses of sapwood ray parenchyma and non-structural carbohydrates of Pinus sylvestris to drought and long-term irrigation. Funct. Ecol. 2017, 31, 1371–1382. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Dendroclimatic calibration in R: The bootRes package for response and correlation function analysis. Dendrochronologia 2013, 31, 68–74. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Sass-Klaassen, U.; Fonti, P.; Cherubini, P.; Gričar, J.; Robert, E.M.R.; Steppe, K.; Bräuning, A. A Tree-Centered Approach to Assess Impacts of Extreme Climatic Events on Forests. Front. Plant. Sci. 2016, 7, 1069. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.r-project.org/ (accessed on 5 December 2019).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression. Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 12 March 2021).
- Mina, M.; Martin-Benito, D.; Bugmann, H.; Cailleret, M. Forward modeling of tree-ring width improves simulation of forest growth responses to drought. Agric. For. Meteorol. 2016, 221, 13–33. [Google Scholar] [CrossRef]
- Potokina, E.K.; Kiseleva, A.A.; Nikolaeva, M.A.; Ivanov, S.A.; Ulianich, P.S.; Potokin, A.F. Analysis of the polymorphism of organelle DNA to elucidate the phylogeography of Norway spruce in the East European Plain. Russ. J. Genet. Appl. Res. 2015, 5, 430–439. [Google Scholar] [CrossRef]
- Loehle, C. Height growth rate tradeoffs determine northern and southern range limits for trees. J. Biogeogr. 1998, 25, 735–742. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol. 2006, 170, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, R.; Jiang, S.; Bagila, M.; Ainur, U.; Yu, S. On the ‘Divergence Problem’ in the Alatau Mountains, Central Asia: A Study of the Responses of Schrenk Spruce Tree-Ring Width to Climate under the Recent Warming and Wetting Trend. Atmosphere 2019, 10, 473. [Google Scholar] [CrossRef]
- Allen, K.J.; Villalba, R.; Lavergne, A.; Palmer, J.G.; Cook, E.C.; Fenwick, P.; Drew, D.M.; Turney, C.S.M.; Baker, P.J. A comparison of some simple methods used to detect unstable temperature responses in tree-ring chronologies. Dendrochronologia 2018, 48, 52–73. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Wilson, R.; Liepert, B.; Cherubini, P. On the “Divergence Problem” in Northern Forests: A review of the tree-ring evidence and possible causes. Glob. Planet. Chang. 2008, 60, 289–305. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Lee, J.-E.; Rossini, M.; Joiner, J.; Munger, J.W.; Kornfeld, A.; Richardson, A.D. Solar-induced chlorophyll fluorescence that correlates with canopy photosynthesis on diurnal and seasonal scales in a temperate deciduous forest. Geophys. Res. Lett. 2015, 42, 2977–2987. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual tracheid production of Norway spruce and Scots pine across a latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Motta, R.; Urbinati, C. Contrasting tree-ring growth to climate responses of Abies alba toward the southern limit of its distribution area. Oikos 2010, 119, 1515–1525. [Google Scholar] [CrossRef]
- Pallardy, S.G. Physiology of Woody Plants, 3rd ed.; Elsevier: London, UK, 2008. [Google Scholar]
- Strand, M.; Löfvenius, M.O.; Bergsten, U.; Lundmark, T.; Rosvall, O. Height growth of planted conifer seedlings in relation to solar radiation and position in Scots pine shelterwood. In Forest Ecology and Management; Elsevier: Amsterdam, The Netherlands, 2006; Volume 224, pp. 258–265. [Google Scholar]
- Tei, S.; Sugimoto, A. Time lag and negative responses of forest greenness and tree growth to warming over circumboreal forests. Glob. Chang. Biol. 2018, 24, 4225–4237. [Google Scholar] [CrossRef] [PubMed]
- Hacket-Pain, A.; Ascoli, D.; Berretti, R.; Mencuccini, M.; Motta, R.; Nola, P.; Piussi, P.; Ruffinatto, F.; Vacchiano, G. Temperature and masting control Norway spruce growth, but with high individual tree variability. For. Ecol. Manag. 2019, 438, 142–150. [Google Scholar] [CrossRef]
- Heide, O.M. Growth and Dormancy in Norway Spruce Ecotypes. II. After-effects of Photoperiod and Temperature on Growth and Development in Subsequent Years. Physiol. Plant. 1974, 31, 131–139. [Google Scholar] [CrossRef]
- Pearce, R. Plant Freezing and Damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Hansen, J.; Beck, E. Seasonal changes in the utilization and turnover of assimilation products in 8-year-old Scots pine (Pinus sylvestris L.) trees. Trees 1994, 8, 172–182. [Google Scholar] [CrossRef]
- Beck, E.H.; Heim, R.; Hansen, J. Plant resistance to cold stress: Mechanisms and environmental signals triggering frost hardening and dehardening. J. Biosci. 2004, 29, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ogren, E. Relationship between temperature, respiratory loss of sugar and premature dehardening in dormant Scots pine seedlings. Tree Physiol. 1997, 17, 47–51. [Google Scholar] [CrossRef]
- Burt, T.P.; Pinay, G.; Matheson, F.E.; Haycock, N.E.; Butturini, A.; Clement, J.C.; Danielescu, S.; Dowrick, D.J.; Hefting, M.M.; Hillbricht-Ilkowska, A.; et al. Water table fluctuations in the riparian zone: Comparative results from a pan-European experiment. J. Hydrol. 2002, 265, 129–148. [Google Scholar] [CrossRef]
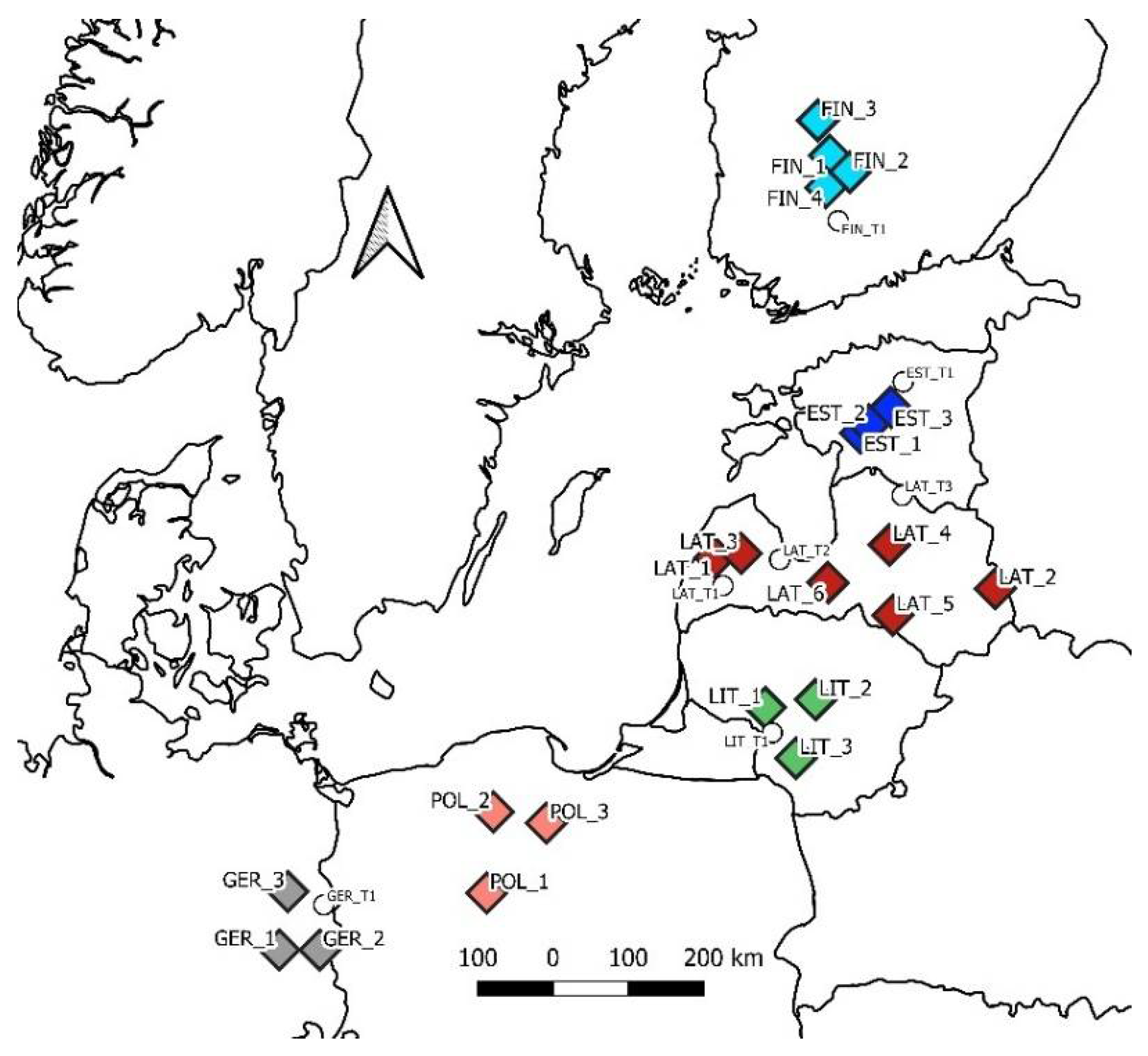

| Site | Latitude, ° N | Longitude, ° E | Soil | Admixture | Stand Age |
|---|---|---|---|---|---|
| FIN_1 | 61.81 | 24.31 | Silty | Pine, 20% | 95 |
| FIN_2 | 61.61 | 24.81 | Silty | Pine, 10% | 101 |
| FIN_3 | 62.23 | 24.01 | Sandy | 121 | |
| FIN_4 | 61.43 | 24.21 | Silty | 99 | |
| EST_1 | 58.49 | 24.98 | Silty | Pine, 10% | 92 |
| EST_2 | 58.59 | 25.18 | Sandy | Pine, 20% | 101 |
| EST_3 | 58.79 | 25.69 | Silty | Birch, 30% | 121 |
| LAT_1 | 56.99 | 21.76 | Silty | Pine, birch, 30% | 108 |
| LAT_2 | 56.58 | 27.85 | Sandy | Pine, 10% | 79 |
| LAT_3 | 57.05 | 22.33 | Silty | Birch, 30% | 112 |
| LAT_4 | 57.15 | 25.58 | Silty | 97 | |
| LAT_5 | 56.31 | 25.62 | Sandy | Birch, 10% | 110 |
| LAT_6 | 56.71 | 24.23 | Silty | 111 | |
| LIT_1 | 55.21 | 22.91 | Silty | 75 | |
| LIT_2 | 55.31 | 23.96 | Sandy | Pine, 20% | 91 |
| LIT_3 | 54.61 | 23.56 | Sandy | Birch, pine, 30% | 85 |
| POL_1 | 52.82 | 17.47 | Silty | 82 | |
| POL_2 | 53.79 | 17.46 | Sandy | Pine, 20% | 112 |
| POL_3 | 53.71 | 18.54 | Silty | Birch, 10% | 108 |
| GER_1 | 51.86 | 13.60 | Silty | 83 | |
| GER_2 | 51.92 | 14.37 | Silty | Birch, 10% | 72 |
| GER_3 | 52.56 | 13.60 | Silty | 79 |
| FIN (4) | EST (3) | LAT (6) | LIT (3) | POL (3) | GER (3) | |
|---|---|---|---|---|---|---|
| Mean annual temperature, °C | 4.2–4.7 | 6.2–6.7 | 6.1–7.4 | 7.5–7.6 | 8.4–9.2 | 9.8–10.2 |
| Annual temperature st. dev, °C | 0.73–0.74 | 0.68–0.69 | 0.64–0.67 | 0.65–0.66 | 0.7–0.73 | 0.71–0.72 |
| Mean minimum January temperature, °C | −19.9–−19.3 | −16.2–−15.2 | −15.3–−10.1 | −12–−11.2 | −7.9–−7.5 | −6.5–−6.1 |
| Mean maximum January temperature, °C | 1.9–2.4 | 4.1–4.5 | 3.9–5.1 | 5.3–5.7 | 8.2–9 | 10.2–10.4 |
| Mean minimum July temperature, °C | 11.5–12.1 | 13.0–13.7 | 12.5–13.7 | 13.2–13.4 | 13.3–13.8 | 14.1–14.4 |
| Mean maximum July temperature, °C | 22.1–22.6 | 22.6–22.8 | 21.4–23.6 | 23.6–23.8 | 22.6–24.4 | 24.5–25.3 |
| Mean minimum May–September temperature, °C | 7.9–8.5 | 9.7–10.5 | 9.4–10.8 | 10.5–10.7 | 10.8–11.2 | 11.8–12.1 |
| Mean maximum May–September temperature, °C | 18.1–18.6 | 19.2–19.3 | 18.6–20.5 | 20.7–20.9 | 20.1–21.7 | 22–22.8 |
| Mean annual precipitation, mm | 538–587 | 696–699 | 641–772 | 631–696 | 535–646 | 551–585 |
| Annual precipitation st. dev., mm | 59–66 | 82–84 | 75–91 | 71–78 | 71–81 | 75–79 |
| Mean May–September precipitation, mm | 291–310 | 324–336 | 310–356 | 328–344 | 296–338 | 284–297 |
| May–September precipitation st. dev., mm | 56–61 | 63–69 | 59–71 | 64–67 | 62–70 | 62–66 |
| Site | Timespan | Mean ± St. Dev, mm | SENS | AC1 | N | r-Bar | EPS | SNR |
|---|---|---|---|---|---|---|---|---|
| FIN_1 | 1923–2017 | 2.16 ± 0.79 | 0.17 | 0.76 | 30 | 0.44 | 0.94 | 15.50 |
| FIN_2 | 1917–2017 | 1.88 ± 0.71 | 0.16 | 0.81 | 22 | 0.36 | 0.90 | 8.71 |
| FIN_3 | 1897–2017 | 1.52 ± 0.54 | 0.18 | 0.75 | 28 | 0.34 | 0.91 | 10.08 |
| FIN_4 | 1919–2017 | 1.50 ± 0.66 | 0.19 | 0.81 | 26 | 0.39 | 0.93 | 13.87 |
| EST_1 | 1927–2018 | 1.95 ± 0.80 | 0.20 | 0.78 | 30 | 0.51 | 0.96 | 22.75 |
| EST_2 | 1918–2018 | 2.02 ± 0.73 | 0.20 | 0.68 | 25 | 0.35 | 0.91 | 10.32 |
| EST_3 | 1898–2018 | 1.69 ± 0.68 | 0.20 | 0.73 | 28 | 0.44 | 0.94 | 14.82 |
| LAT_1 | 1900–2017 | 1.46 ± 0.76 | 0.24 | 0.77 | 20 | 0.54 | 0.96 | 21.79 |
| LAT_2 | 1931–2017 | 2.80 ± 1.53 | 0.27 | 0.73 | 18 | 0.43 | 0.92 | 11.70 |
| LAT_3 | 1900–2017 | 1.21 ± 0.47 | 0.20 | 0.73 | 19 | 0.39 | 0.91 | 10.35 |
| LAT_4 | 1913–2017 | 2.26 ± 1.45 | 0.21 | 0.86 | 20 | 0.60 | 0.96 | 26.92 |
| LAT_5 | 1900–2017 | 2.07 ± 0.87 | 0.24 | 0.65 | 19 | 0.40 | 0.91 | 10.29 |
| LAT_6 | 1900–2017 | 1.56 ± 0.79 | 0.22 | 0.80 | 24 | 0.57 | 0.96 | 27.15 |
| LIT_1 | 1933–2017 | 2.15 ± 1.02 | 0.23 | 0.76 | 27 | 0.49 | 0.96 | 22.57 |
| LIT_2 | 1935–2017 | 2.35 ± 1.15 | 0.21 | 0.79 | 27 | 0.53 | 0.96 | 24.85 |
| LIT_3 | 1943–2017 | 2.73 ± 1.15 | 0.22 | 0.74 | 28 | 0.46 | 0.95 | 20.53 |
| POL_1 | 1937–2018 | 1.73 ± 0.82 | 0.26 | 0.71 | 29 | 0.63 | 0.98 | 43.57 |
| POL_2 | 1907–2018 | 1.72 ± 0.93 | 0.31 | 0.66 | 26 | 0.49 | 0.96 | 23.65 |
| POL_3 | 1911–2018 | 2.16 ± 1.04 | 0.27 | 0.70 | 28 | 0.41 | 0.94 | 14.90 |
| GER_1 | 1935–2017 | 3.19 ± 1.44 | 0.32 | 0.55 | 29 | 0.58 | 0.96 | 23.97 |
| GER_2 | 1946–2017 | 2.42 ± 1.23 | 0.30 | 0.65 | 18 | 0.51 | 0.95 | 17.61 |
| GER_3 | 1939–2017 | 1.79 ± 0.97 | 0.34 | 0.64 | 16 | 0.58 | 0.95 | 20.35 |
| Fixed Effects | |||
| Smoothening Term | Effective Degree of Freedom | F-Value | p-Value |
| Previous September temperature | 2.72 | 18.9 | <0.001 |
| February temperature | 2.22 | 14.2 | <0.001 |
| June temperature | 1.00 | 15.4 | <0.001 |
| June precipitation | 2.80 | 7.6 | <0.001 |
| July SPEI | 2.87 | 26.8 | <0.001 |
| Random Effects | |||
| Term | Variance | ||
| Year | 0.0162 | ||
| Stand | 0.0039 | ||
| Residual (scale) | 0.0155 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matisons, R.; Elferts, D.; Krišāns, O.; Schneck, V.; Gärtner, H.; Wojda, T.; Kowalczyk, J.; Jansons, Ā. Nonlinear Weather–Growth Relationships Suggest Disproportional Growth Changes of Norway Spruce in the Eastern Baltic Region. Forests 2021, 12, 661. https://doi.org/10.3390/f12060661
Matisons R, Elferts D, Krišāns O, Schneck V, Gärtner H, Wojda T, Kowalczyk J, Jansons Ā. Nonlinear Weather–Growth Relationships Suggest Disproportional Growth Changes of Norway Spruce in the Eastern Baltic Region. Forests. 2021; 12(6):661. https://doi.org/10.3390/f12060661
Chicago/Turabian StyleMatisons, Roberts, Didzis Elferts, Oskars Krišāns, Volker Schneck, Holger Gärtner, Tomasz Wojda, Jan Kowalczyk, and Āris Jansons. 2021. "Nonlinear Weather–Growth Relationships Suggest Disproportional Growth Changes of Norway Spruce in the Eastern Baltic Region" Forests 12, no. 6: 661. https://doi.org/10.3390/f12060661
APA StyleMatisons, R., Elferts, D., Krišāns, O., Schneck, V., Gärtner, H., Wojda, T., Kowalczyk, J., & Jansons, Ā. (2021). Nonlinear Weather–Growth Relationships Suggest Disproportional Growth Changes of Norway Spruce in the Eastern Baltic Region. Forests, 12(6), 661. https://doi.org/10.3390/f12060661









