30-Year Changes in Oak-Hornbeam Forest after Windthrow
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Changes in the Density and Basal Area (BA) of Tree Stands
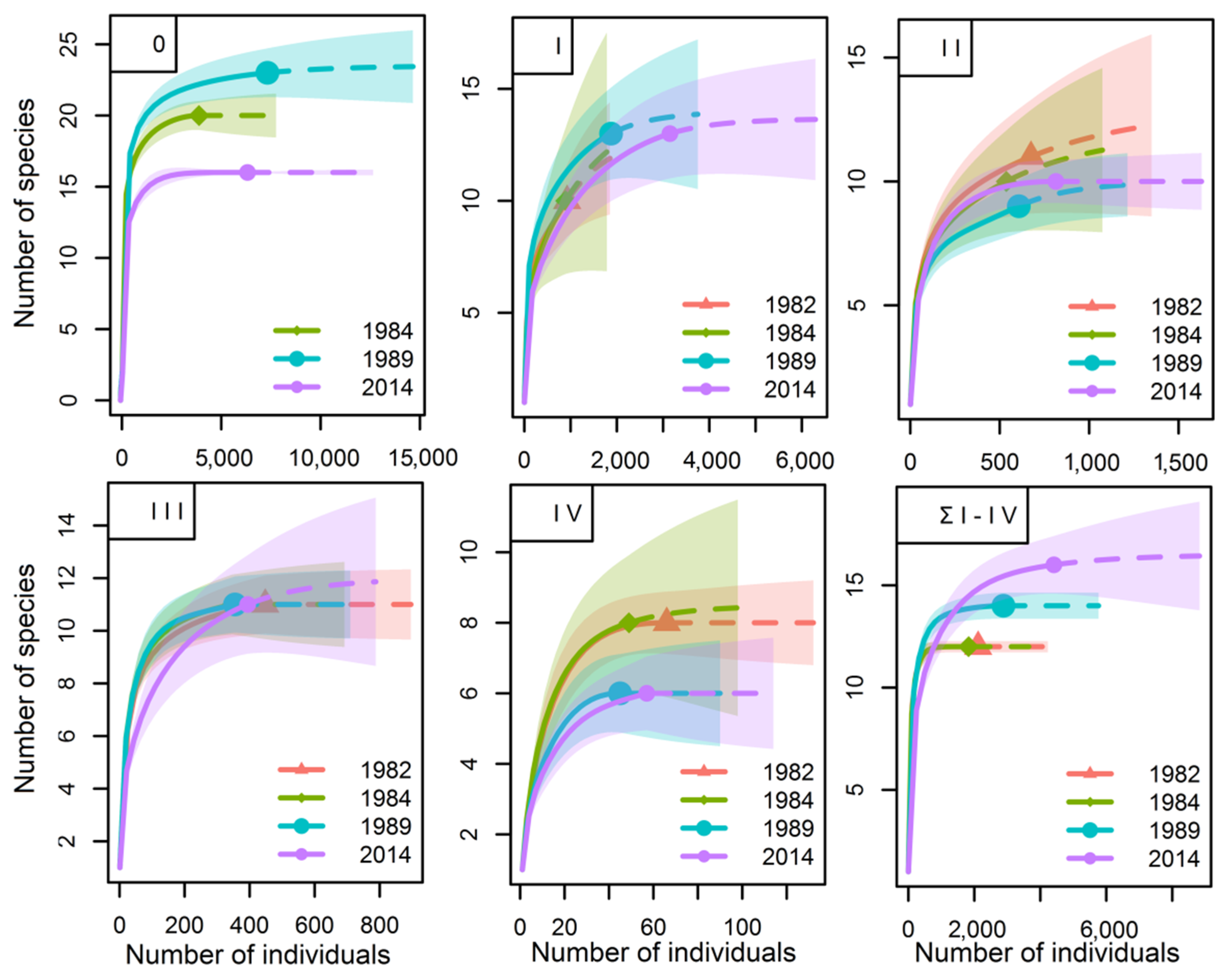
3.2. Species Richnessand Species Diversity
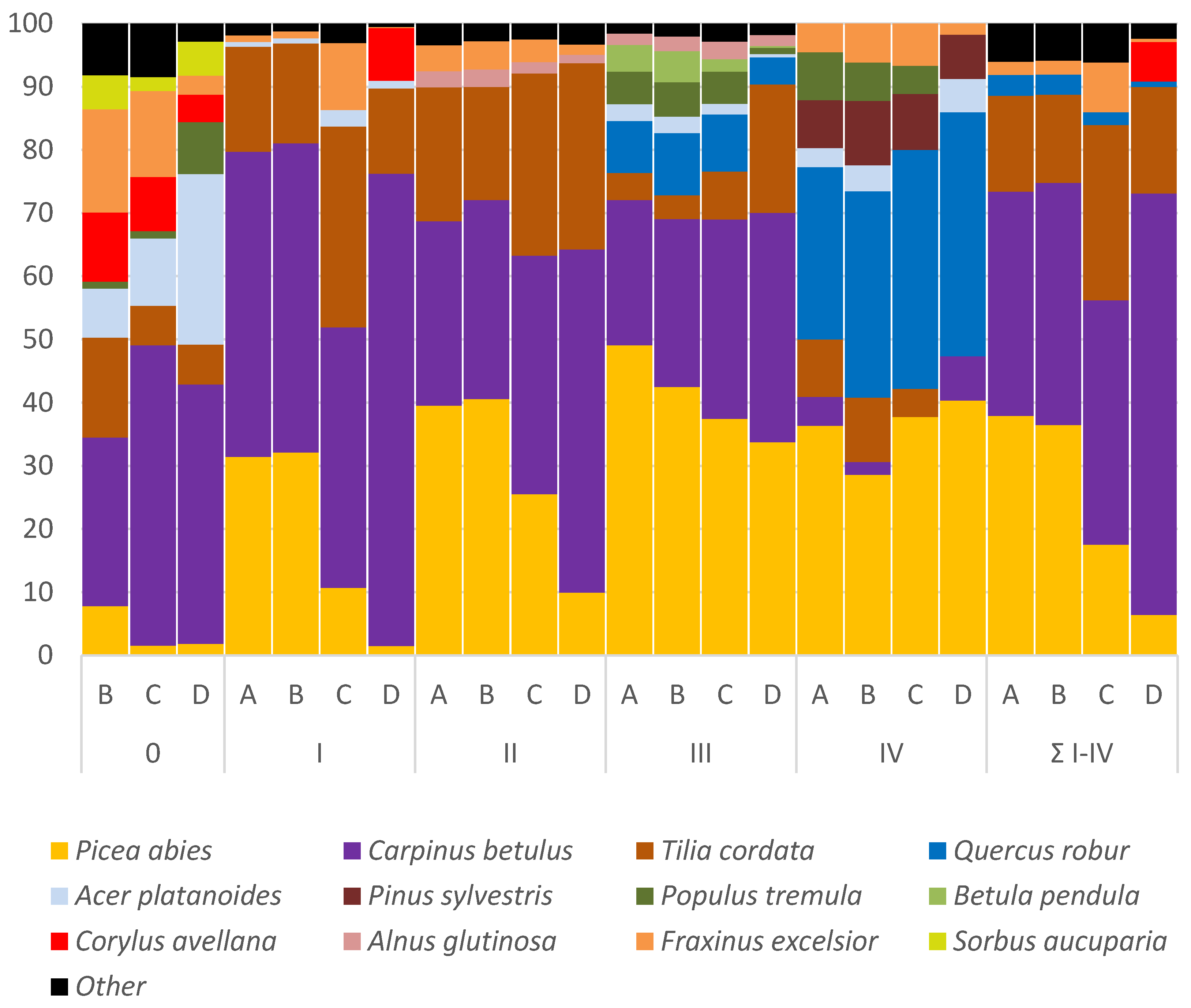
3.3. Species Composition
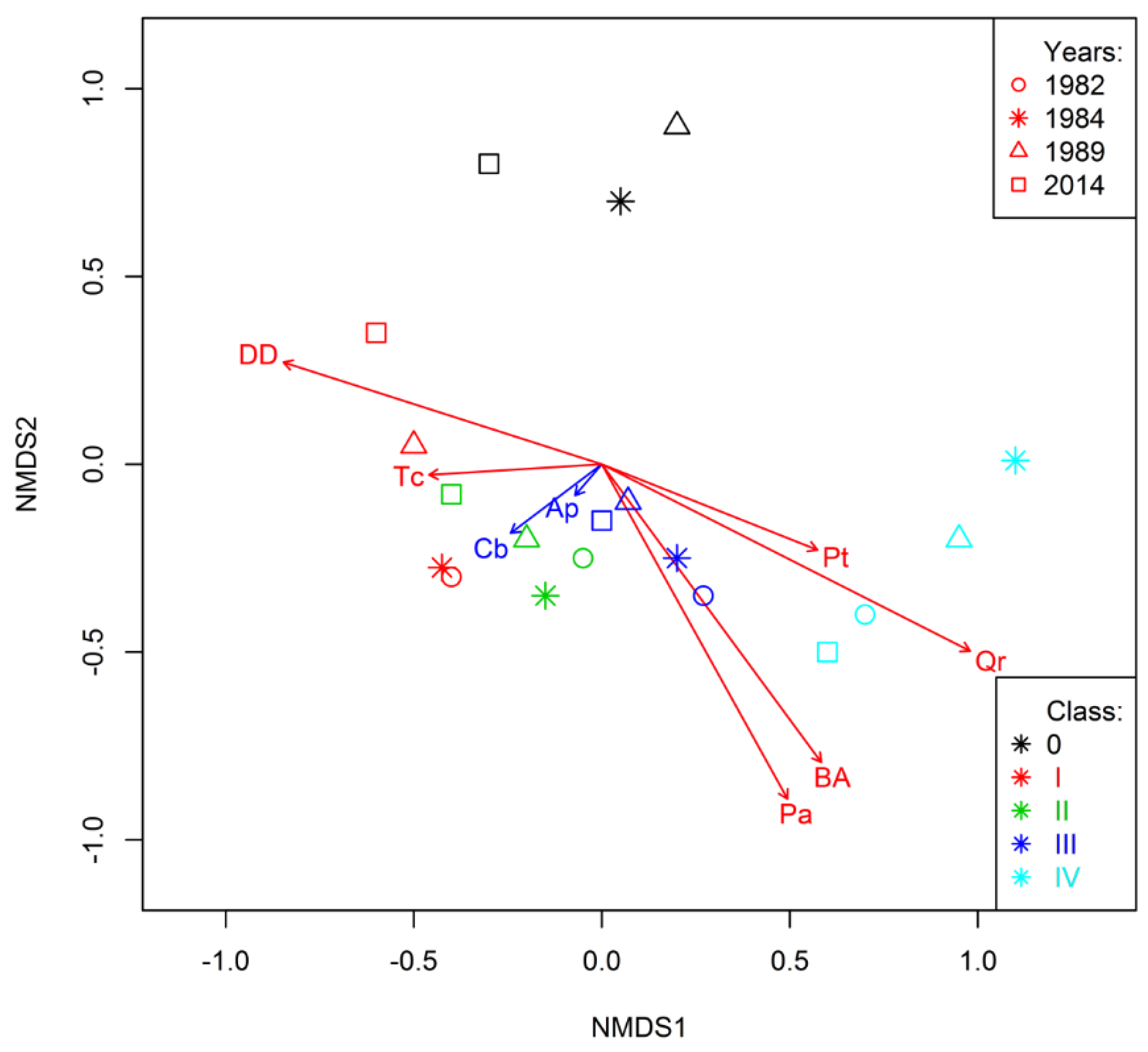
3.4. Trends Shaping Changes on Study Plots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meigs, G.W.; Keeton, W.S. Intermediate-severity wind disturbance in mature temperate forests: Legacy structure, carbon storage, and stand dynamics. Ecol. Appl. 2018, 28, 798–815. [Google Scholar] [CrossRef] [PubMed]
- Falťan, V.; Katina, S.; Minár, J.; Polčák, N.; Bánovský, M.; Maretta, M.; Zámečník, S.; Petrovič, F. Evaluation of abiotic controls on windthrow disturbance using a generalized additive model: A case study of the Tatra National Park, Slovakia. Forests 2020, 11, 1259. [Google Scholar] [CrossRef]
- Krišāns, O.; Matisons, R.; Kitenberga, M.; Donis, J.; Rust, S.; Elferts, D.; Jansons, Ā. Wind resistance of eastern baltic silver birch (Betula pendula Roth.) Suggests its suitability for periodically waterlogged sites. Forests 2021, 12, 21. [Google Scholar] [CrossRef]
- Christiansen, E.; Bakke, A. The spruce bark beetle of Euroasia. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A., Ed.; Springer: Boston, MA, USA, 1988; pp. 479–503. [Google Scholar] [CrossRef]
- Keczyński, A. Wpływ gradacji kornika drukarza na drzewostany obszaru ochrony ścisłej Białowieskiego Parku Narodowego. Kosmos 2002, 51, 471–474. [Google Scholar]
- Marini, L.; Lindelöw, A.; Jönsson, A.M.; Wulff, S.; Schroeder, L.M. Population dynamics of the spruce bark beetle: A long-term study. OIKOS 2013, 122, 1768–1776. [Google Scholar] [CrossRef]
- Hroššo, B.; Mezei, P.; Potterf, M.; Majdák, A.; Blaženec, M.; Korolyova, N.; Jakuš, R. Drivers of spruce bark beetle (Ips typographus) infestations on downed trees after severe windthrow. Forests 2020, 11, 1290. [Google Scholar] [CrossRef]
- Kawecka, A. Zmiany roślinności i odnowienie naturalne w drzewostanach Puszczy Białowieskiej uszkodzonych przez huragany. Parki Nar. I Rezerw. Przyr. 1992, 10, 45–67. [Google Scholar]
- Szwagrzyk, J. Rozległe naturalne zaburzenia w ekosystemach leśnych: Ich zasięg, charakter i znaczenie dla dynamiki lasu. Wiadomości Ekol. 2000, 1, 3–19. [Google Scholar]
- Mitchell, S.J. Wind as a natural disturbance agent in forests: A synthesis. Forestry 2013, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.J.; Pickett, S.T. Forest reorganization: A case study in an old-growth forest catastrophic blowdown. Ecology 1995, 76, 763–774. [Google Scholar] [CrossRef]
- Peterson, C.J. Catastrophic wind damage to North American forests and the potential impact of climate change. Sci. Total Environ. 2000, 262, 287–311. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Goodrick, S.L.; Outcalt, K.W. Disturbance and coastal forests: A strategic approach to forest management in hurricane impact zones. For. Ecol. Manag. 2007, 250, 119–135. [Google Scholar] [CrossRef]
- Rich, R.L.; Frelich, L.E.; Reich, P.B. Wind-throw mortality in the southern boreal forest: Effects of species, diameter and stand age. J. Ecol. 2007, 95, 1261–1273. [Google Scholar] [CrossRef]
- Sławski, M. Changes in species composition and cover of understory plants in stands disturbed by a hurricane in Piska Forest in 2002. Sylwan 2014, 158, 661–668. [Google Scholar]
- Skłodowski, J.W.; Garbalińska, P. Ground beetle assemblages (Coleoptera, Carabidae) in the third year of regeneration after a hurricane in the Puszcza Piska pine forests. Balt. J. Coleopt. 2007, 7, 17–36. [Google Scholar]
- Skłodowski, J.W.; Buszyniewicz, J.; Domański, M. Spontaneous regeneration of a stand disturbed by a hurricane in July 2002. Sylwan 2014, 158, 499–508. [Google Scholar]
- Dobrowolska, D. Forest regeneration in northeastern Poland following a catastrophic blowdown. Can. J. For. Res. 2015, 45, 1172–1182. [Google Scholar] [CrossRef]
- Holeksa, J.; Jaloviar, P.; Kucbel, S.; Saniga, M.; Svoboda, M.; Szewczyk, J.; Szwagrzyk, J.; Zielonka, T.; Żywiec, M. Models of disturbance driven dynamics in the West Carpathian spruce forests. For. Ecol. Manag. 2017, 388, 79–89. [Google Scholar] [CrossRef]
- Szwagrzyk, J.; Gazda, A.; Dobrowolska, D.; Chećko, E.; Zaremba, J.; Tomski, A. Natural regeneration following wind disturbance increases the diversity of managed lowland forests in NE Poland. J. Veg. Sci. 2018, 29, 898–906. [Google Scholar] [CrossRef]
- Kujawa, A.; Orczewska, A.; Falkowski, M.; Blicharska, M.; Bohdan, A.; Buchholz, L.; Chylarecki, P.; Gutowski, J.M.; Latałowa, M.; Mysłajek, R.W.; et al. The Białowieża Forest—A UNESCO Natural Heritage Site—Protection priorities. Leśne Pr. Badaw. For. Res. Pap. 2016, 77, 302–323. [Google Scholar] [CrossRef] [Green Version]
- Cholewińska, O.; Adamowski, W.; Jaroszewicz, B. Homogenization of Temperate Mixed Deciduous Forests in Białowieża Forest: Similar Communities Are Becoming More Similar. Forests 2020, 11, 545. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.M.; Samojlik, T.; Zimny, M.; Latałowa, M. Białowieża Forest—A relic of the high naturalness of european forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef] [Green Version]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef] [Green Version]
- Bernadzki, E.; Bolibok, L.; Brzeziecki, B.; Zajączkowski, J.; Żybura, H. Rozwój Drzewostanów Naturalnych Białowieskiego Parku Narodowego w Okresie 1936 do 1996; Fundacja Rozwój SGGW: Warszawa, Poland, 1998; p. 271. [Google Scholar]
- Więcko, E. Puszcza Białowieska; PWN: Warszawa, Poland, 1972; p. 193. [Google Scholar]
- Niklasson, M.; Zin, E.; Zielonka, T.; Feijen, M.; Korczyk, A.F.; Churski, M.; Samojlik, T.; Jędrzejewska, B.; Gutowski, J.M.; Brzeziecki, B. A 350-year tree-ring fire record from Białowieża Primeval Forest, Poland: Implications for Central European lowland fire history. J. Ecol. 2010, 98, 1319–1329. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Bielak, K.; Bolibok, L.; Drozdowski, S.; Zajączkowski, J.; Żybura, H. Structural and compositional dynamics of strictly protected woodland communities with silvicultural implications, using Białowieża Forest as an example. Ann. Sci. 2018, 75, 89. [Google Scholar] [CrossRef] [Green Version]
- Miścicki, S. Structure and dynamics of temperate lowland natural forest in the Białowieża National Park, Poland. Forestry 2012, 85, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Gazda, A.; Miścicki, S. Forecast of the development of natural forest resources using a size–class growth model. Sylwan 2016, 160, 207–218. [Google Scholar]
- Paczoski, J. Lasy Białowieży; Państwowa Rada Ochrony Przyrody, Monografie Naukowe: Warszawa, Poland, 1930; Volume 1, p. 575. [Google Scholar]
- Kawecka, A.; Gutowski, J.M. Skutki huraganowych wiatrów z zimy 1982/1983r. w Puszczy Białowieskiej. Pr. Inst. Badaw. Leśnictwa 1988, 677, 113–124. [Google Scholar]
- Gutowski, J.M.; Kubisz, D. Entomofauna drzewostanów pohuraganowych w Puszczy Białowieskiej. Pr. Inst. Badaw. Leśnictwa Ser. A 1995, 788, 91–129. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 18 February 2021).
- PMCMR: Calculate Pairwise Multiple Comparisons of Mean Rank Sums. Available online: https://CRAN.R-project.org/package=PMCMR (accessed on 13 February 2021).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 15 February 2021).
- iNEXT: iNterpolation and EXTrapolation for Species Diversity. R package version 2.0.19. Available online: http://chao.stat.nthu.edu.tw/blog/software-download/ (accessed on 20 February 2021).
- Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 February 2021).
- Minchin, P.R. An evaluation of relative robustness of techniques for ecological ordinations. Vegetatio 1987, 69, 89–107. [Google Scholar] [CrossRef]
- Paliy, O.; Shankar, V. Application of multivariate statistical techniques in microbial ecology. Mol. Ecol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J. Vegan: An Introduction to Ordination. 2020. Available online: https://cran.r-project.org/web/packages/vegan/vignettes/intro-vegan.pdf (accessed on 9 May 2021).
- Clarke, K. Nonparametric Multivariate Analyses of Changes in Community Structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Peterson, C.J. First-decade biomass and carbon accumulation, and woody community change after severe wind damage in a hemlock-white pine forest remnant. Forests 2021, 12, 231. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Czerepko, J.; et al. Light availability and land-use history drive biodiversity and functional changes in forest herb layer communities. J. Ecol. 2019, 108, 1411–1425. [Google Scholar] [CrossRef]
- Sutinen, R.; Teirilä, A.; Pänttäjä, M.; Sutinen, M.L. Distribution and diversity of tree species with respect to soil electrical characteristics in Finnish Lapland. Can. J. Res. 2002, 32, 1158–1170. [Google Scholar] [CrossRef]
- Spinu, A.P.; Niklasson, M.; Zin, E. Mesophication in temperate Europe: A dendrochronological reconstruction of tree succession and fires in a mixed deciduous stand in Białowieża Forest. Ecol. Evol. 2020, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Sokołowski, A.W. Fitosocjologiczna charakterystyka zbiorowisk leśnych Białowieskiego Parku Narodowego. Parki Nar. I Rezerw. Przyr. 1993, 12, 5–190. [Google Scholar]
- Brzeziecki, B.; Pommerening, A.; Miścicki, S.; Drozdowski, D.; Żybura, H. A common lack of demographic equilibrium among tree species in Białowieża National Park (NE Poland): Evidence from long-term plots. J. Veg. Sci. 2016, 27, 460–469. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Woods, K.; Bolibok, L.; Zajączkowski, J.; Drozdowski, S.; Bielak, K.; Żybura, H. Over 80 years without major disturbance, late-successional Białowieża woodlands exhibit complex dynamism, with coherent compositional shifts towards true old-growth conditions. J. Ecol. 2020, 108, 1138–1154. [Google Scholar] [CrossRef]
- Zajączkowski, J. Natural forest regeneration on the example of research area in Białowieża National Park. Sylwan 1999, 143, 5–14. [Google Scholar]
- Bobiec, A.; Bobiec, M. Influence of spruce decline in stands of the Białowieża National Park on natural oak regeneration. Sylwan 2012, 156, 243–251. [Google Scholar] [CrossRef]
- Bobiec, A. Białowieża Primeval Forest as a remnant of culturally modified ancient forest. Eur J For. Res. 2012, 131, 1269–1285. [Google Scholar] [CrossRef] [Green Version]
- Bobiec, A.; Buchholz, L.; Churski, M.; Chylarecki, P.; Fałtynowicz, W.; Gutowski, J.M.; Jaroszewicz, B.; Kuijper, D.P.J.; Kujawa, A.; Mikusek, R.; et al. Dlaczego martwe świerki są potrzebne w Puszczy Białowieskiej? Tech. Rap. 2016, 7, 14–16. [Google Scholar] [CrossRef]
- Faliński, J.B.; Pawlaczyk, P. Zarys ekologii. In Grab Zwyczajny Carpinus betulus L.; Bugała, W., Ed.; Sorus: Poznań-Kórnik, Poland, 1993; pp. 157–263. [Google Scholar]
- Bernadzki, E.; Bolibok, L.; Brzeziecki, B.; Zajączkowski, J.; Żybura, H. Compositional dynamics of natural forests in the Białowieża National Park, northeastern Poland. J. Veg. Sci. 1998, 9, 229–238. [Google Scholar] [CrossRef]
- Brzeziecki, B. Long-term dynamics of natural tree stands on the example of two forest communities of the Białowieża National Park: Pino-Quercetum Tilio-Carpinetum. Studia Nat. 2008, 54, 9–22. [Google Scholar]
- Brzeziecki, B.; Bernadzki, E. Langfristige Entwicklung von zwei Waldgesellschaften im Białowieża-Urwald. Schweizezerische Z. Forstwes. 2008, 159, 80–90. [Google Scholar] [CrossRef]
- Brzeziecki, B.; Keczyński, A.; Zajączkowski, J.; Drozdowski, S.; Gawron, L.; Buraczyk, W.; Bielak, K.; Szeligowski, H.; Dzwonkowski, M. Threatened tree species of the Białowieża National Park (the Strict Reserve). Sylwan 2012, 156, 252–261. [Google Scholar] [CrossRef]
- Paluch, R. Long-term changes of tree species composition in natural stands of the Białowieża Forest. Sylwan 2015, 159, 278–288. [Google Scholar] [CrossRef]
- Paluch, R.; Gabrysiak, K.A. Long-term changes of tree species composition in high protective regime stands in the Augustów and Knyszyn Forests. Sylwan 2019, 163, 846–854. [Google Scholar] [CrossRef]
- Macek, M.; Wild, J.; Kopecký, M.; Červenka, J.; Svoboda, M.; Zenáhlíková, J.; Brůna, J.; Mosandl, R.; Fischer, A. Life and death of Picea abies after bark-beetle outbreak: Ecological processes driving seedling recruitment. Ecol. Appl. 2017, 27, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Number of Study Plot | Location | Volume of Trees in 1982 (Before Windthrow) [m3/ha] | Degree of Stand Damage (DD) [%] |
|---|---|---|---|
| 3 | N52.65331 E23.65169 | 115.8 | 10.7 |
| 4 | N52.65212 E23.65171 | 88.5 | 25.9 |
| 6 | N52.60593 E23.62461 | 132.7 | 7.6 |
| 7 | N52.60362 E23.62299 | 141.8 | 7.6 |
| 8 | N52.72302 E23.86908 | 117.4 | 49.4 |
| 9 | N52.72244 E23.85999 | 77.9 | 77.4 |
| 10 | N52.72334 E23.85963 | 62.3 | 59.3 |
| 11 | N52.73280 E23.82935 | 108.1 | 25.8 |
| 15 | N52.74294 E23.86752 | 153.2 | 17.7 |
| Class | 1982 | 1984 | 1989 | 2014 | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1724.4 ± 256.7 | a | 3256.0 ± 481.2 | b | 2809.3 ± 640.3 | b | ||
| I | 411.6 ± 84.1 | ab | 396.9 ± 80.5 | a | 833.0 ± 256.8 | a | 1398.7 ± 204.1 | b |
| II | 300.0 ± 41.4 | a | 238.7 ± 41.9 | a | 270.0 ± 30.9 | a | 361.8 ± 53.5 | a |
| III | 199.1 ± 22.4 | a | 153.8 ± 24.5 | b | 158.0 ± 21.7 | ab | 175.1 ± 11.9 | ab |
| IV | 29.3 ± 6.1 | a | 21.8 ± 6.2 | b | 20.0 ± 5.7 | b | 25.3 ± 7.6 | ab |
| Ʃ I-IV | 940.0 ± 89.7 | ab | 811.1 ± 94.7 | a | 1281.0 ± 236.5 | a | 1960.9 ± 210.7 | b |
| Class | 1982 | 1984 | 1989 | 2014 | ||||
|---|---|---|---|---|---|---|---|---|
| I | 0.5 ± 0.1 | a | 0.5 ± 0.1 | a | 0.5 ± 0.1 | a | 1.0 ± 0.2 | a |
| II | 3.8 ± 0.6 | ab | 3.0 ± 0.6 | a | 3.3 ± 0.5 | ab | 4.6 ± 0.7 | b |
| III | 19.9 ± 2.1 | a | 15.0 ± 2.4 | b | 15.0 ± 2.3 | ab | 16.8 ± 1.1 | ab |
| IV | 10.8 ± 2.5 | a 1 | 8.1 ± 2.4 | b 1 | 7.4 ± 2.2 | ab 1 | 10.1 ± 3.3 | ab 1 |
| Ʃ I-IV | 34.9 ± 2.7 | a | 26.5 ± 3.8 | b | 26.3 ± 3.5 | b | 32.5 ± 3.0 | ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrysiak, K.A.; Paluch, R.; Gawryś, R. 30-Year Changes in Oak-Hornbeam Forest after Windthrow. Forests 2021, 12, 600. https://doi.org/10.3390/f12050600
Gabrysiak KA, Paluch R, Gawryś R. 30-Year Changes in Oak-Hornbeam Forest after Windthrow. Forests. 2021; 12(5):600. https://doi.org/10.3390/f12050600
Chicago/Turabian StyleGabrysiak, Karolina Anna, Rafał Paluch, and Radosław Gawryś. 2021. "30-Year Changes in Oak-Hornbeam Forest after Windthrow" Forests 12, no. 5: 600. https://doi.org/10.3390/f12050600
APA StyleGabrysiak, K. A., Paluch, R., & Gawryś, R. (2021). 30-Year Changes in Oak-Hornbeam Forest after Windthrow. Forests, 12(5), 600. https://doi.org/10.3390/f12050600






