Abstract
Manor parks are characteristic objects in the agricultural landscape of Poland. Lack of proper management after World War II, however, led to their devastation from a cultural point of view, but may allow the regeneration of rare and endangered species. The aim of our study was to determine if the presence of forests in the vicinity of manor parks will work as an accelerator of the regeneration process of oak-hornbeam and ancient forest species. Phytosociological analyses were conducted in manor parks adjacent to forests and not adjacent to the forest as well as natural forests. The total number of plant species, number and percentage share of ancient forest species, and plant species consistent with oak-hornbeam habitat were analyzed using a GLM model. Characteristic species were identified using detrended correspondence analysis. Parks adjacent to forests and natural forests showed higher numbers of total species, ancient forest species, and oak-hornbeam species compared with parks not adjacent to forests, but there were no differences in percentage shares of ancient forest species and oak-hornbeam species. For all three types of studied objects, characteristic species could be identified. We conclude that adjacent forests allow greater regeneration of ancient forest species and oak-hornbeam forest species in manor parks.
1. Introduction
In Poland, manor parks are characteristic objects in the agricultural landscape. According to authors [1], the manor park is a landscape garden with a manor or palace outside the city. Manor parks, which were established on oak-hornbeam habitats, dominated in Poland during the 18th and 19th centuries. The owners of manor parks often attached fragments of existing natural forests to the park’s area. Nowadays, some manor parks have private owners, but most of them are state-owned [2]. Such historical manor parks, being very specific objects with restricted use, require appropriate species and forms of vegetation [3]. Therefore, the forests in the manor parks have been managed in such a way that species not consistent with these appropriate species and forms of vegetation are removed. As a result, manor parks are characterized by a more transformed habitat, vulnerable to the penetration of grasses and synanthropic species from surrounding areas. This may result in a significant higher number of non-forest species that are expansive, and displace forest species with their presence [4].
Slight negative anthropogenic impact on the vegetation of manor parks was already noticed during the pre-war period, but World War II was a period of serious degradation for these objects, and significant destruction of the plant cover. After World War II, however, manor parks underwent further transformation due to improper management, which in consequence led to the disappearance of characteristic compositional systems in individual objects. Lack of proper management, understood among others as the lack of mowing in woodland areas, significantly facilitated succession processes. In the 1970s and 1980s, the statement was formulated that manor parks were places with forest species, but in lower numbers than in forest communities [5].
The agricultural landscape is a mosaic of natural and anthropogenic components [6]. In order to maintain its biological diversity in Poland, measures have been taken to shape the landscape through preservation of trees, shrubs, reeds and peat vegetation [7]. The agricultural landscape requires the development and maintenance of the ecological structure at local, regional and national level. Despite Poland’s accession to the EU, the rank of protection of manor parks has not been strengthened, even if Poland undertook measures to preserve biological diversity not only in areas under legal protection, but also in other areas in order to strengthen the natural system by signing the Convention on Biological Diversity [8]. However, the manor parks with afforested parts can be very important objects, because they have the potential to preserve and shape biological diversity of agricultural areas [9]. In this context, the lack of proper management after World War II, as mentioned above, is a curse and a blessing at the same time. On the one hand, it has led to the devastation of manor parks from a cultural point of view. On the other hand, it may allow regeneration of rare and endangered species in these areas.
Based on the research carried out [10,11], it was found that herbaceous plant species are very important in woodlands. They are dynamic and provide appropriate indicators of habitat changes within a short time period. Among them, ancient forest species play a special role. According to studies [12,13], ancient forest species are diagnostic plants for ancient woodlands (more than 200 years old) and old woodlands (between 100–200 years old). They are good indicators of habitat continuity [14,15,16,17,18]. It should be underscored that most forest species are sensitive to habitat fragmentation [4].
According to researchers [19,20], forest complexes are important sources for the spreading and colonization of forest species as well as ancient ones. However, farmland located in the agricultural landscape hinders the migration of such species [21,22]. In this way, forest complexes located close to manor parks might have an impact on the composition of plant species in manor parks. In addition, the forest species have a positive impact on the regeneration of the undergrowth layer [23,24]. Researchers [25] also note that species acting as indicators play a significant role in the assessment of ecological corridors in fragmented agricultural landscape.
Owing to the significant importance of forest species and old forests (ancient species) in preserving the biological diversity of the rural landscape, the presented study was conducted. Manor parks have a potential to participate in contributing to the biological diversity, and this potential should also depend on the existence of adjacent forest stands. Thus, the research objective of our study was to determine the impact of the presence and absence of forest in the vicinity of manor parks on their composition of ancient forest species and oak-hornbeam forest species (Tilio-Carpinetum species) consistent with the natural habitat. We assume that adjacent forest stand will work as accelerators of the regeneration process of such species in the manor parks. In order to be able to assess the degree of regeneration, we also studied natural forest stands as reference stands.
We formulated the following research hypotheses.
(1) Adjacent forests are expected to have a positive impact on species numbers in manor parks, (2) occurrence of forests adjacent to manor parks impacts the number and percentage share of ancient forest species and those typical for oak-hornbeam forest (Tilio-Carpinetum species) in the studied objects, and (3) each type of studied objects (manor parks not adjacent to forests, manor parks adjacent to forests, natural forests) is characterized by groups of characteristic species.
2. Materials and Methods
2.1. Study Areas and Field Methods
The studied objects, manor parks, and natural forests, were located in the Podkraina Południowopodlaska Region in the Eastern part of Poland (Figure 1). According to literature [26] the Podkraina Południowopodlaska Region has an area of about 9000 km2. It is characterized by agricultural landscape with a mosaic of different types of forests, such as oak-hornbeam, mixed, oak and wet forests.
Figure 1.
Location of the Podkraina Południowopodlaska Region in the eastern part of Poland.
The following criteria for selecting manor parks was adopted in the paper: the same size of forest cover, homogeneous habitat, presence or lack of forest adjacent to a manor park, and lack of mowing of woodland areas after the World War II. The size of each woodland area was about 10 ha. Since the forests which were adjacent to the studied manor park had a size below 10 ha and they were not located on protected areas, they were not chosen for the study as reference areas for natural forests. Instead, we chose forests which were not adjacent to manor parks, but which fulfilled the criteria to be of appropriate size and location on protected areas (treated as “natural forests”). Based on this criteria, we selected 40 manor parks adjacent to forests, 40 manor parks not adjacent to forests, and 40 natural forests.
All studied objects were represented by oak-hornbeam habitat (Tilio-Carpinetum). The oak-hornbeam forests were about 100 years old. Floristic and phytosociological analyses were conducted in the year 2019. Phytosociological records were made inside the woodlots at a distance of 100 m from the ecotone inside the wooded area in order to capture the greatest diversity of plant species consistent with the natural habitat [27]. In each woodland, 10 research plots were designated. The research was conducted in duplicate, taking into account the spring and summer aspects. For each plot, these data were summed up and treated as one phytosociological record for further analysis. In each woodland, such phytosociological records were taken in the undergrowth layer (herbaceous, vascular plant species up to 30 cm height) on an area of 100 m2, according to the Braun-Blanquet method [28]. As a result, the number of 400 phytosociological records from each type of manor park and 400 phytosociological records from natural forests were obtained. Accordingly, the total number of phytosociological records was 1200. Nomenclature of plant species was done according to literature [29].
Among the registered plant species, ancient forest species and species typical for oak-hornbeam forests (Tilio-Carpinetum) were determined. The list of ancient forest plant species for Central and Western Europe [30] and complemented for Poland [31] was used. Forest plant species typical for oak-hornbeam forest (Tilio-Carpinetum) were distinguished according to literature [26]. We further distinguished associated species (indicator species occurring in more than one habitat), shrub species, grass species, and synanthropical species among the non-forest species [26].
2.2. Statistical Methods
In order to confirm the formulated research hypothesis in the manor parks and natural forests, the total number of plant species (hyp. 1), number, and percentage share of ancient forest species, as well as the number and percentage share of plant species consistent with Tilio-Carpinetum habitat (hyp. 2) per plot were analyzed.
The total species number, number, and share of species consistent with the habitat (Tilio-Carpinetum species), number, and share of ancient forest species were analyzed using a GLM model with two nested factors. The main factor was forest (park) type, and the nested factor was forest. As a model of dependent variables, we used a Poisson distribution, and for all shares a beta function [32]. That way, apart from the comparison of all types of forests, it was possible to assess the diversity among individual forests. Calculations were carried out using Dell Statistica version 13.
We carried out Detrended Correspondence Analysis (DCA) in order to detect characteristic species for the types of studied objects (hyp. 3) using PAST 4.03 [33]. The cover values were transformed to a scale (a numeric, categorical scale from 0 to 9) [34]. As traits (rows), single plots were used without assignation to specific forests. The difference in sample scores for the first and second axis between the types of studied objects were confirmed by one-way ANOVA.
Selection of characteristic species was done based on defined criteria for the species scores for the first and second axis with a species score <−10 for the first axis for natural forests; species scores >200 for the first axis, and >170 for the second axis for manor parks adjacent to forests; and species scores >200 for the first axis and <0 for the second axis for manor parks not adjacent to forests.
3. Results
Based on the 1200 phytosociological records (Supplementary Material Table S1), parks not adjacent to forests showed a significantly lower (p < 10−6) total species number than forests and parks adjacent to forests. In natural forests, we observed on average 28.0, in parks adjacent to forests 30.4, and in parks not adjacent to forest only 18.1 species per plot. A large, statistically significant (p < 10−6) differentiation between individual objects of the same type, especially for parks adjacent to forests, should be noticed (Figure 2).
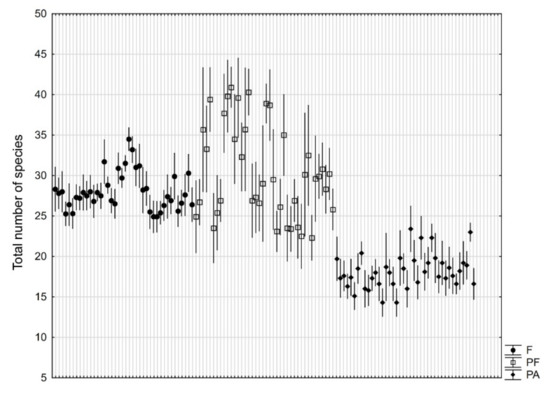
Figure 2.
Total number (mean ± 95% confidence interval based on 10 research plots of 100 m2) of plant species in natural forests (F), manor parks adjacent to forests (PF), and manor parks not adjacent to forests (PA).
The average number of ancient forest species was similar for parks adjacent to forests (11.1) and natural forests (11.8). Numbers of ancient forest species were significantly higher (p < 10−6) in these two types than in parks not adjacent to forests (7.8) (Figure 3).
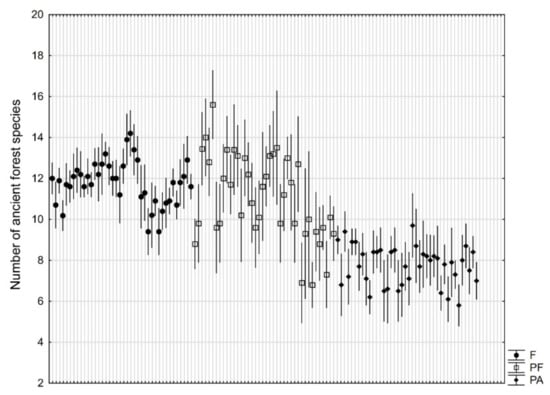
Figure 3.
Number (mean ± 95% confidence interval based on 10 research plots of 100 m2) of ancient forest species in natural forests (F), manor parks adjacent to forests (PF), and manor parks not adjacent to forests (PA).
The difference in percentage share of ancient forest species was close to being significant (p = 0.054). It was similar for nature reserves (42%) and parks not adjacent to forests (43%), and lower for parks adjacent to forest (38%). A significant variability (p < 10−6) between individual objects is worth noting, especially for parks adjacent to forest (Figure 4).
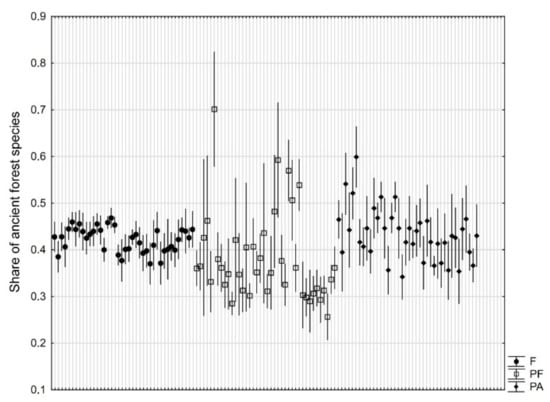
Figure 4.
Share (mean ± 95% confidence interval based on 10 research plots of 100 m2) of ancient forest species in natural forests (F), manor parks adjacent to forests (PF), and manor parks not adjacent to forests (PA).
The number of species consistent with the Tilio-Carpinetum habitat showed a different pattern. The difference between the types of objects was significant (p = 0.0008). The species number was similar for natural forest (18.6) and manor parks adjacent to forests (18.7), but lower for parks not adjacent to forests (12.5) (Figure 5).
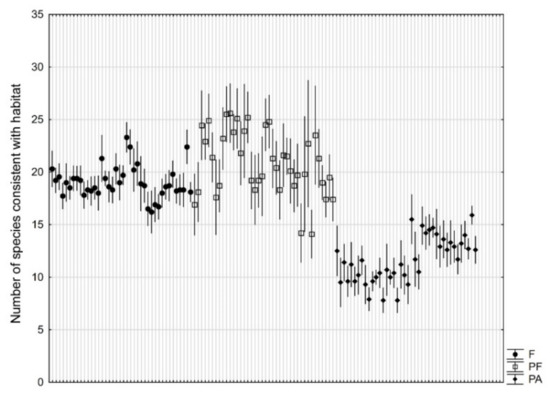
Figure 5.
Number (mean ± 95% confidence interval based on 10 research plots of 100 m2) of plant species consistent with Tilio-Carpinetum habitat in natural forests (F), manor parks adjacent to forests (PF), and manor parks not adjacent to forests (PA).
The percentage share of species consistent with the Tilio-Carpinetum habitat did not differ significantly (p = 0.08) between types of studied objects. However, there was a significant variation between individual objects (p < 10−6), especially for parks adjacent to forests (Figure 6).
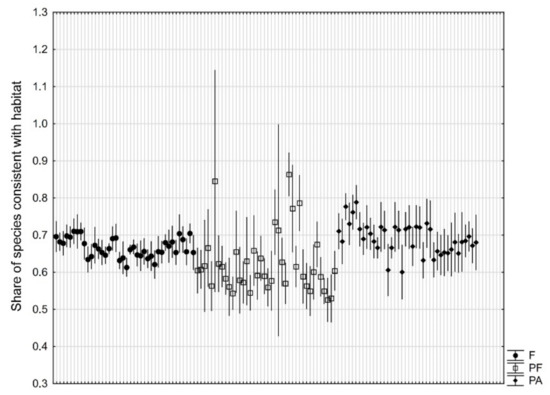
Figure 6.
Share (mean ± 95% confidence interval based on 10 research plots of 100 m2) of plant species consistent with Tilio-Carpinetum habitat in natural forests (F), manor parks adjacent to forests (PF), and manor parks not adjacent to forests (PA).
Two gradients detected by the DCA (Figure 7) separated distinctly all types of studied objects. The first axis, which explained 34% of variation, split natural forests from both types of manor parks. The second axis (20% explained variation), however, separated the two types of parks. ANOVA confirmed the division of the types of objects with a significance of p < 10−6 for the first axis and p = 0.0003 for the second axis.
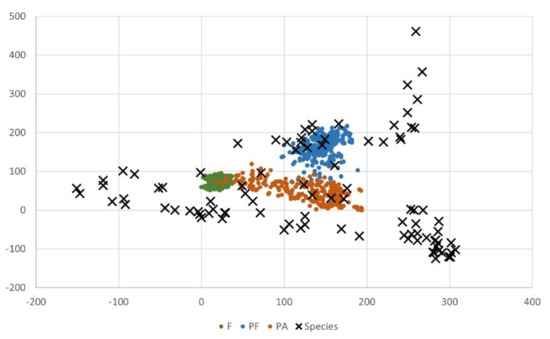
Figure 7.
Ordination plot based on detrended correspondence analysis (DCA) of the species of the undergrowth layer and the plots (F: natural forests; PF: manor parks adjacent to forests; PA: manor parks not adjacent to forests).
Indicator species of natural forests were represented by forest species. In contrast, indicator species identified for the manor parks were principally synanthropical species, grasses, and associated species (Table 1).

Table 1.
Characteristic species for the types of studied objects, their ecological characteristics, and species scores for first and second ordination axis.
4. Discussion
Our studies on the composition of plant species in manor parks showed that the forests adjacent to manor parks have impact on the composition of plant species. In agreement with our assumption, the largest number of species was detected in manor parks adjacent to forests and in the natural forests (hyp. 1). All of the examined sites contained species of ancient forests and species consistent with Tilio-Carpinetum habitat, but their largest number was in forests and in manor parks adjacent to forests. However, we could not detect a significant difference in the share of ancient forest species and the share of species consistent with Tilio-Carpinetum habitat between the types of studied objects (hyp. 2).
According to researchers [35], the studied objects are patches of vegetation that can be classified as larger islands due to the probability of occurrence of valuable forest species consistent with the natural habitat. In the context of preserving ancient forest species, authors [35,36]) drew attention to the size of the “island”. The larger the “island”, the more ancient forest species, forest species consistent with the natural habitat, and a higher species diversity can be expected. Researchers [37] emphasized that large forest patches are important for maintaining population stability. Accordingly, in our study the neighbourhood of forests had a positive influence on the total species number in manor parks, lifting it to the level of natural forests.
The presence of adjacent forests also caused an increase of the number of ancient forest species and species consistent with Tilio-Carpinetum habitat to the level of natural forests. However, adjacent forests did not influence the percentage share of ancient forest species and species consistent with Tilio-Carpinetum habitat. Interestingly, regarding the percentage shares, we could not discover significant differences between all three types of studied objects. In another study [38], the size of adjacent forests also did not influence the percentage share of such species in manor parks. These results might be due to the fact that many aspects decide the presence of species in manor parks and forests, such as, for example, the spreading abilities of the species, their ability to adapt to new fragmented forests, and the location of the forests. Open space around manor parks not adjacent to forests promotes the spreading of seeds by the wind as well as by animals. Open habitats, such as grasslands and pastures, affect wind dynamics (redirection and promotion of airflow), thereby increasing the likelihood of wind-dispersed seed uplift. Closed habitats, such as forests, are known to impede wind dispersal [39]. According to our results, areas such as fields, villages, meadows and pastures surrounding the manor parks not adjacent to the forests had an impact on the appearance of non-forest plants such as grasses and synanthropical species, which are, the majority of time, transported by wind. Yet, animals and birds may have a significant contribution to the synanthropisation of more natural habitats, too [40].
Because they are adjacent to fields and meadows, among the colonizers will be only a low number of forest plants, for forest species have slow dispersal mechanisms [18]. However, many species of plants characteristic in the undergrowth layer of old forests have the ability to adapt to new, fragmented, small, and isolated forests in the agricultural landscape [41]. Moreover, researchers [19,20] stated that the location of the forest patch is crucial, especially in the immediate vicinity of old forests, because it has a positive effect on the natural regeneration of the undergrowth layer. The latter factors may counteract the hypothesized displacement of forest species by expansive non-forest species. Accordingly, the distance between the manor parks studied and the natural forests can be a worthy subject of future studies.
Besides the positive effects on species numbers observed by us, the manor parks and their adjacent forests may provide other beneficial functions. They may support the diversity not only of vascular plants [9,42], but also of animals such as insects [43] and bats [44]. They can also have positive effects on abiotic factors such as soil conditions [45].
For all types of studied objects, characteristic species could be identified (hyp. 3). However, they differed between types. Natural reserves showed characteristic forest plant species consistent with the oak-hornbeam habitat, indicating a lack of anthropogenic impact. Regarding the manor parks, probably the types of surroundings areas such as agricultural areas with fields, pastures, and meadows had an impact on the set of non-forest plants such as characteristic species of manor parks not adjacent to forests. They were characterized by a more transformed habitat, which probably influenced the intrusion of synanthropical species from the surrounding areas [4]. They were represented by Geum urbanum, Chelidonium majus, Plantago major, and Impatiens parviflora, species which prefer a less dense canopy of trees and occur mostly on areas with anthropogenic impact [46,47]. Manor parks adjacent to forests were characterized by non-forest species, too, but the synanthropical species and grasses, for example Urtica dioica or Sambucus nigra, were mostly typical for transformed forests relating to human tourism activities [47].
5. Conclusions
Owing to the rarity of oak-hornbeam forests in Poland [46], the habitats of manor parks can become refuges of “forest islands”, increasing the biological diversity of the agricultural landscape [9,48,49]. In the agricultural landscape, most species consistent with Tilio-Carpinetum habitat only survive due to the presence of small remains of former large forest complexes since these species are sensitive to anthropogenic pressure [50,51]. It is important that manor parks “collect” ancient forest species and plants consistent with Tilio-Carpinetum habitat. It should be noted that the manor parks adjacent to forests “create” “bigger forest islands” with higher species numbers and a characteristic species composition. There is no “typical” ecotone zone in these woodlands because they adhere to each other. It can be stated here that habitat continuity occurs, which allows greater regeneration and stabilization in the composition of ancient forest species and oak-hornbeam forest species in the undergrowth layer [52,53].
Nature protection and management cannot be limited only to protected areas, i.e., nature reserves, but must also take into account manor parks, which are associated, among others, with improved species migration between isolated habitat fragments. However, a better understanding of regional differences in biological diversity patterns and the underlying causes and consequences for nature conservation strategies require much greater research efforts in the rural landscape ecology of Poland.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12050538/s1, Table S1: Data of the phytosociological records (Braun-Blanquet) in natural forests (records 1–240), Table S2: Data of the phytosociological records (Braun-Blanquet) in natural forests (records 241–400), Table S3: Data of the phytosociological records (Braun-Blanquet) in manor parks adjacent to forests (records 1–240), Table S4: Data of the phytosociological records (Braun-Blanquet) in manor parks adjacent to forests (records 241–400), Table S5: Data of the phytosociological records (Braun-Blanquet) in manor parks not adjacent to forests (records 1–150), Table S6: Data of the phytosociological records (Braun-Blanquet) in manor parks not adjacent to forests (records 151–300), Table S7: Data of the phytosociological records (Braun-Blanquet) in manor parks not adjacent to forests (records 301–400).
Author Contributions
Conceptualization, B.F.-P., M.O. and A.S.; methodology, B.F.-P.; validation, B.F.-P., M.O. and A.S.; investigation, B.F.-P.; data curation, B.F.-P. and M.O.; writing—original draft preparation, B.F.-P., M.O. and A.S.; writing—review and editing, B.F.-P., M.O. and A.S., visualization, B.F.-P. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Acknowledgments
We thank Czesław Wysocki for valuable methodical remarks. This paper is communication no. 536 in the tradition of the Laboratory of Evaluation and Assessment of Natural Resources, Warsaw University of Life Sciences—SGGW.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Latowski, K.; Zieliński, J. Parki wiejskie—wybrane zagadnienia geobotaniczne i kulturowe. In Szata Roślinna Wielkopolski i Pojezierza Południowopomorskiego, Przewodnik Sesji Terenowej 52. Zjazdu PTB, 24−28 Września 2001; Wojterska, M., Ed.; Bogucki Wyd. Naukowe: Poznań, Poland, 2001; pp. 291–304. [Google Scholar]
- Fabiańska, P. Rola parków podworskich w krajobrazie wsi. In Krajobraz i Ogród Wiejski, t. 1, Nowe Idee i Metody w Architekturze Krajobrazu; Janecki, J., Borkowski, Z., Eds.; Wydawnictwo KUL: Lublin, Poland, 2004; pp. 165–175. [Google Scholar]
- International Council on Monuments and Sites. Historic gardens (The Florence Charter 1981). Available online: https://www.icomos.org/charters/gardens_e.pdf (accessed on 17 January 2020).
- Brunet, J.; Valtinat, K.; Mayr, M.L.; Felton, A.; Lindbladh, M.; Bruun, H.H. Understory succession in post-agricultural oak forests: Habitat fragmentation affects forest specialists and generalists differently. For. Ecol. Manag. 2011, 262, 1863–1871. [Google Scholar] [CrossRef]
- Fijałkowski, D.; Kseniak, M. Parki Wiejskie Lubelszczyzny. Stan, Ochrona i Rewaloryzacja Biocenotyczna; PWNL: Warsaw, Poland, 1982. [Google Scholar]
- Vellend, M.; Baeten, L.; Myers-Smith, I.H.; Elmendorf, S.C.; Beauséjour, R.; Brown, C.D. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl. Acad. Sci. USA 2013, 110, 19456–19459. [Google Scholar] [CrossRef]
- Chmielewski, T.J. Pojezierze Łęczycko-Włodawskie: Przekształcenia Struktury Ekologicznej Krajobrazu i Uwarunkowania Zagospodarowania Przestrzennego; Monografie Komitetu Inżynierii Środowiska PAN: Lublin, Poland, 2001. [Google Scholar]
- Convention on Biological Diversity. Available online: https://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 12 March 2021).
- Liira, J.; Lõhmus, K.; Tuisk, E. Old manor parks as potential habitats for forest flora in agricultural landscape of Estonia. Biol. Conserv. 2012, 146, 144–154. [Google Scholar] [CrossRef]
- Alberdi, I.S.; Condés, S.; Martínez-Millán, J. Review of monitoring and assessing ground vegetation biodiversity in national forest inventories. Environ. Monit. Assess. 2010, 164, 649–676. [Google Scholar] [CrossRef]
- Del Vecchio, S.; Slaviero, A.; Fantinato, E.; Buffa, G. The use of plant community attributes to detect habitat quality in coastal environments. AoB Plants 2016, 8, plw040. [Google Scholar] [CrossRef]
- Wulf, M. Plant species as indicators as ancient woodland in north-western Germany. J. Veg. Sci. 1997, 8, 635–642. [Google Scholar] [CrossRef]
- Wulf, M. Preference of plant species for woodlands with differing habitat continuities. Flora 2003, 198, 444–460. [Google Scholar] [CrossRef]
- Verheyen, K.; Bossuyt, B.; Honnay, O.; Hermy, M. Herbaceous plant community structure of ancient and recent forest in two contrasting forest types. Basic Appl. Ecol. 2003, 4, 537–546. [Google Scholar] [CrossRef]
- Kolb, A.; Diekmann, M. Effects of environment, habitat configuration, and forest continuity on the distribution of forest plant species. J. Veg. Sci. 2004, 15, 199–208. [Google Scholar] [CrossRef]
- Stefańska-Krzaczek, E.; Kacki, Z.; Szypuła, B. Coexistence of ancient forest species as an indicator of high species richness. For. Ecol. Manag. 2016, 365, 12–21. [Google Scholar] [CrossRef]
- Lalechère, E.; Jabot, F.; Archaux, F.; Deffuant, G. Non-equilibrium Plant metapopulation dynamics challenge the concept of ancient/recent forest species. Ecol. Model. 2017, 366, 48–57. [Google Scholar] [CrossRef]
- Webb, J.C.; Goodenough, A.E. Questioning the reliability of “ancient” woodland indicators: Resilience to interruptions and persistence following deforestation. Ecol. Indic. 2018, 84, 354–363. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Gawroński, S. The role woodlands fragments, soil types, and dominant species in secondary succession on the western Carpatian foothills. Vegetatio 1994, 111, 149–160. [Google Scholar] [CrossRef]
- Bossuyt, B. Plant species and soil dynamics across ancient-recent forest ecotones: Consequences for ecological restoration. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2001. [Google Scholar]
- Mortelliti, A.; Amori, G.L.; Boitani, L. The role of habitat quality in fragmented landscapes: A conceptual overview and prospectus for future research. Oecologia 2010, 163, 535–1547. [Google Scholar] [CrossRef] [PubMed]
- Aggemyr, E.; Cousins, S.A.O. Landscape structure and land use history influence changes in island plant composition after 100 years. J. Biogeogr. 2010, 39, 1645–1656. [Google Scholar] [CrossRef]
- Hill, J.L.; Curran, P.J. Species composition in fragmented forest: Conservation implications of changing forest area. Appl. Geogr. 2001, 21, 157–174. [Google Scholar] [CrossRef]
- Hill, J.L.; Curran, P.J. Area, shape, and isolation of tropical forest fragments: Effects on tree species diversity and implications for conservations. J. Biogeogr. 2003, 30, 1391–1403. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Wasof, S.; Decocq, G. Using process-based indicator species to evaluate ecological corridors in fragmented landscape. Biol. Conserv. 2016, 201, 152–159. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisko Roślinnych Polski; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2012. [Google Scholar]
- Żarska, B. Zmiany roślinności w strefie brzegowej lasów. Ochr. Śr. Zasobów Nat. 1997, 12, 105–115. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, 2nd ed.; Springer: Wien, Austria, 1951. [Google Scholar]
- Mirek, Z.; Zając, M.; Zając, A.; Piękoś-Mirkowa, H. Vascular Plants of Poland: A Checklist; Instytut Botaniki im. Władysława Szafera Polskiej Akademii Nauk, Komitet Badań Naukowych: Cracow, Poland, 1995. [Google Scholar]
- Hermy, M.; Honnay, O.; Firbank, L.; Grashof-Bokdam, C.; Lawesson, J.E. An ecological comparison between ancient and other forest plant species of Europe and the implications for conservation. Biol. Conserv. 1999, 91, 9–22. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S. Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. Typologia zbiorowisk i kartografia roślinności w Polsce. Pr. Geogr. 2001, 178, 119–132. [Google Scholar]
- Xue-Kun Song, P.; Tan, M. Marginal models for longitudinal continuous proportional data. Biometrics 2000, 56, 496–502. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Graae, B.J.; Oakland, R.H. Influence of historical, geographical and environmental variables on understory composition and richness in Danish forest. J. Veg. Sci. 2004, 15, 465–474. [Google Scholar] [CrossRef]
- Mac Arthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Naff, T.; Kolk, J. Colonization credit of post-agricultural forest patches in NE Germany. Biol. Conserv. 2015, 182, 155–163. [Google Scholar] [CrossRef]
- Fornal-Pieniak, B.; Ollik, M.; Schwerk, A. Impact of surroundings landscape structure on formation of plant species. Appl. Ecol. Environ. Res. 2018, 16, 6483–6497. [Google Scholar] [CrossRef]
- Damschen, E.I.; Baker, D.V.; Bohrer, G.; Nathan, R.; Orrock, J.L.; Turner, J.R.; Brudvig, L.A.; Haddad, N.M.; Levey, D.J.; Tewksbury, J.J. How fragmentation and corridors affect wind dynamics and seed dispersal in open habitats. Proc. Natl. Acad. Sci. USA 2014, 111, 3484–3489. [Google Scholar] [CrossRef] [PubMed]
- Król, S. Problemy synantropizacji lasów a penetracja antropofitów dendroflory. Sylwan 2002, 146, 75–89. [Google Scholar] [CrossRef]
- Eriksson, O. Regional dynamics of plants: A review of evidence of remnant, source-sink, and metapopulations. Oikos 1996, 77, 248–258. [Google Scholar] [CrossRef]
- Liira, J.; Sepp, T.; Parrest, O. The forest structure and ecosystem quality in conditions of anthropogenic disturbance along productivity gradient. For. Ecol. Manag. 2007, 250, 34–46. [Google Scholar] [CrossRef]
- Desender, K.; Ervynck, A.; Tack, G. Beetle diversity and historical ecology of woodlands in Flanders. Belg. J. Zool. 1999, 129, 139–155. [Google Scholar]
- Kalda, R.; Kalda, O.; Lõhmus, K.; Liira, J. Multi-scale ecology of woodland bat the role of species pool, landscape complexity and stand structure. Biodivers. Conserv. 2014, 24, 337–353. [Google Scholar] [CrossRef]
- De Keersmaeker, L.; Martens, L.; Verheyen, K.; Hermy, M.; de Schrijver, A.; Lust, N. Impact of soil fertility and isolation on diversity of herbaceosus woodland species colonizig afforestations in Muizen forest (Belgium). For. Ecol. Manag. 2004, 188, 291–297. [Google Scholar] [CrossRef]
- Matuszkiewicz, J.M.; Kowalska, A.; Solon, J.; Degórski, M.; Kozłowska, A.; Roo-Zielińska, E.; Zawiska, I.; Wolski, J. Long-term evolution models of post-agricultural forests. Pr. Geogr. 2013, 240, 1–318. [Google Scholar]
- Łaska, G. Ecological consequences of deforestation and afforestation on a post-arable land: Changes in the composition and structure of plant communities and transformations of oak-hornbeam habitats and soil. Ecol. Quest. 2014, 20, 9–21. [Google Scholar] [CrossRef][Green Version]
- Higgs, E.; Falk, D.A.; Guerrini, A.; Hall, M.; Harris, J.; Hobbs, R.J.; Jackson, S.T.; Rhemtulla, J.M.; Throop, W. The changing role of history in restoration ecology. Front. Ecol. Environ. 2014, 12, 499–506. [Google Scholar] [CrossRef]
- Evers, C.R.; Wardropper, C.B.; Branoff, B.; Granek, E.F.; Hirsch, S.L.; Link, T.E.; Olivero-Lora, S.; Wilson, C. The ecosystem services and biodiversity of novel ecosystems: A literature review. Glob. Ecol. Conserv. 2017, 13, e00362. [Google Scholar] [CrossRef]
- Andersen, J.; Rowcliffe, J.M.; Cowlishaw, G. Does the matrix matter? A forest primate in a complex agricultural landscape. Biol. Conserv. 2007, 135, 212–222. [Google Scholar] [CrossRef]
- Jamoneau, A.; Chabrerie, O.; Closset-Kopp, D.; Decocq, G. Fragmentation alters beta-diversity patterns of habitat specialists within forest metacommunities. Ecography 2012, 35, 124–133. [Google Scholar] [CrossRef]
- Hermy, M.; Verheyen, K. Legacies of the past in the present-day forest biodiversity: A review of past land-use effects on forest plant species composition and diversity. Ecol. Res. 2007, 22, 361–371. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Hattab, T.; Decocq, G. Do drivers of forestry vehicles also drive herb layer changes (1970–2015) in a temperate forest with contrasting habitat and management conditions? J. Ecol. 2019, 107, 1439–1456. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).