Morphological and Physiological Responses of Pinus massoniana Seedlings to Different Light Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Treatments
2.2. Plant Growth Measurement
2.3. Spectral Reflectance
2.4. Chlorophyll Fluorescence Parameters
2.5. Pigment Analysis
2.6. Determination of Osmotic Regulation Substances and Lipid Peroxidation
2.7. Activities of Antioxidant Enzymes
2.8. Statistical Analysis
3. Results
3.1. Effects of Light Conditions on Morphological Characteristics
3.2. Effects of Light Conditions on Pigment Contents
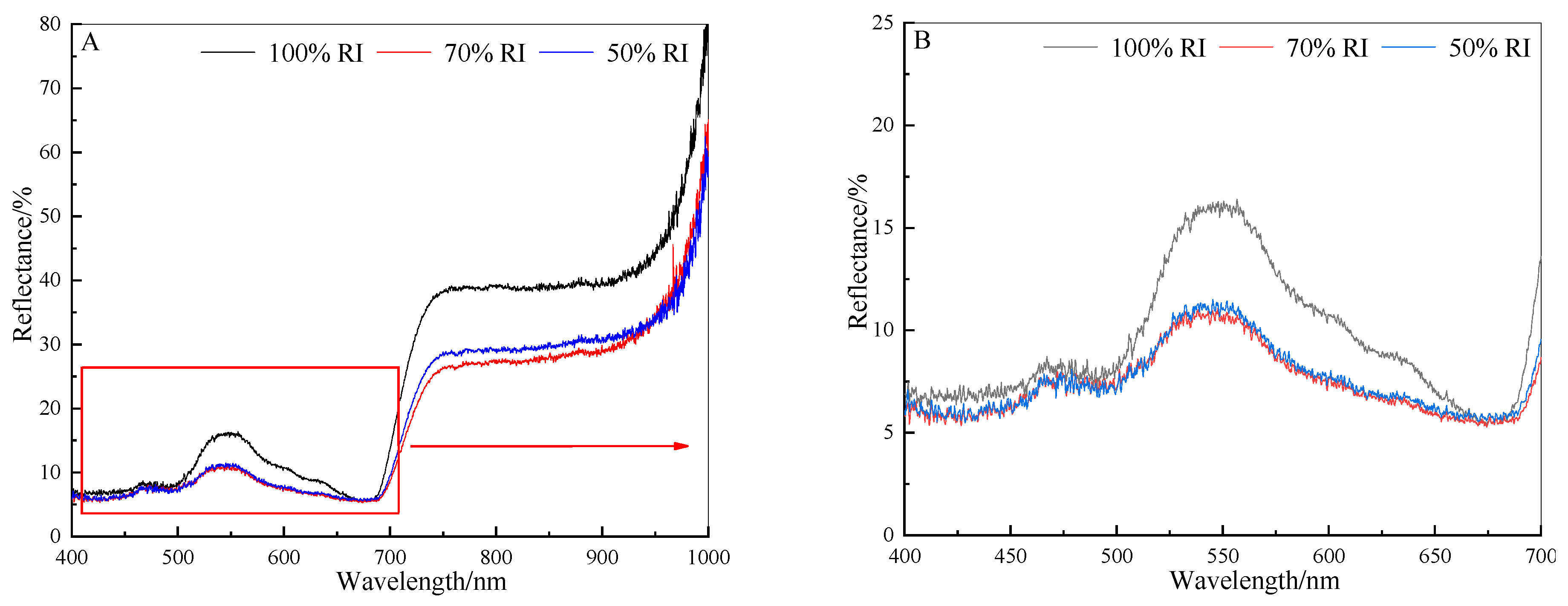
3.3. Spectral Reflectance Analyses
3.4. Effects of Light Conditions on Spectral Reflectance Indexes
3.5. Chlorophyll Fluorescence Parameters under Different Light Conditions
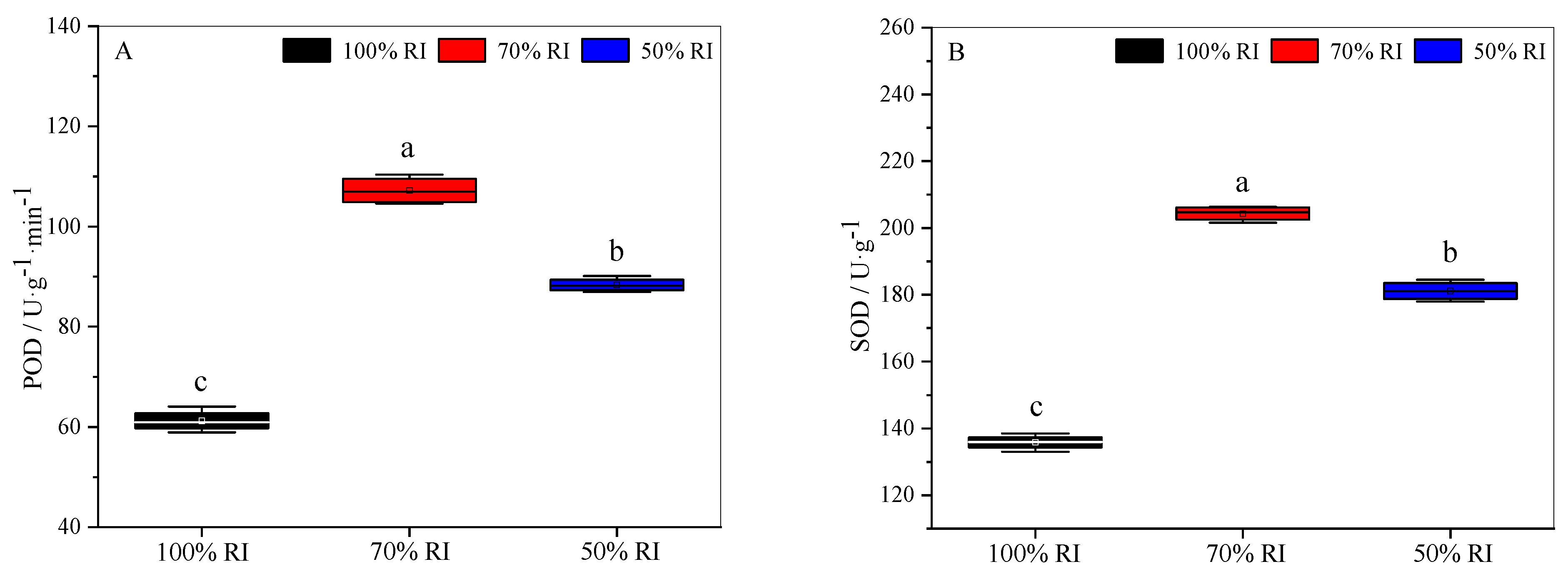
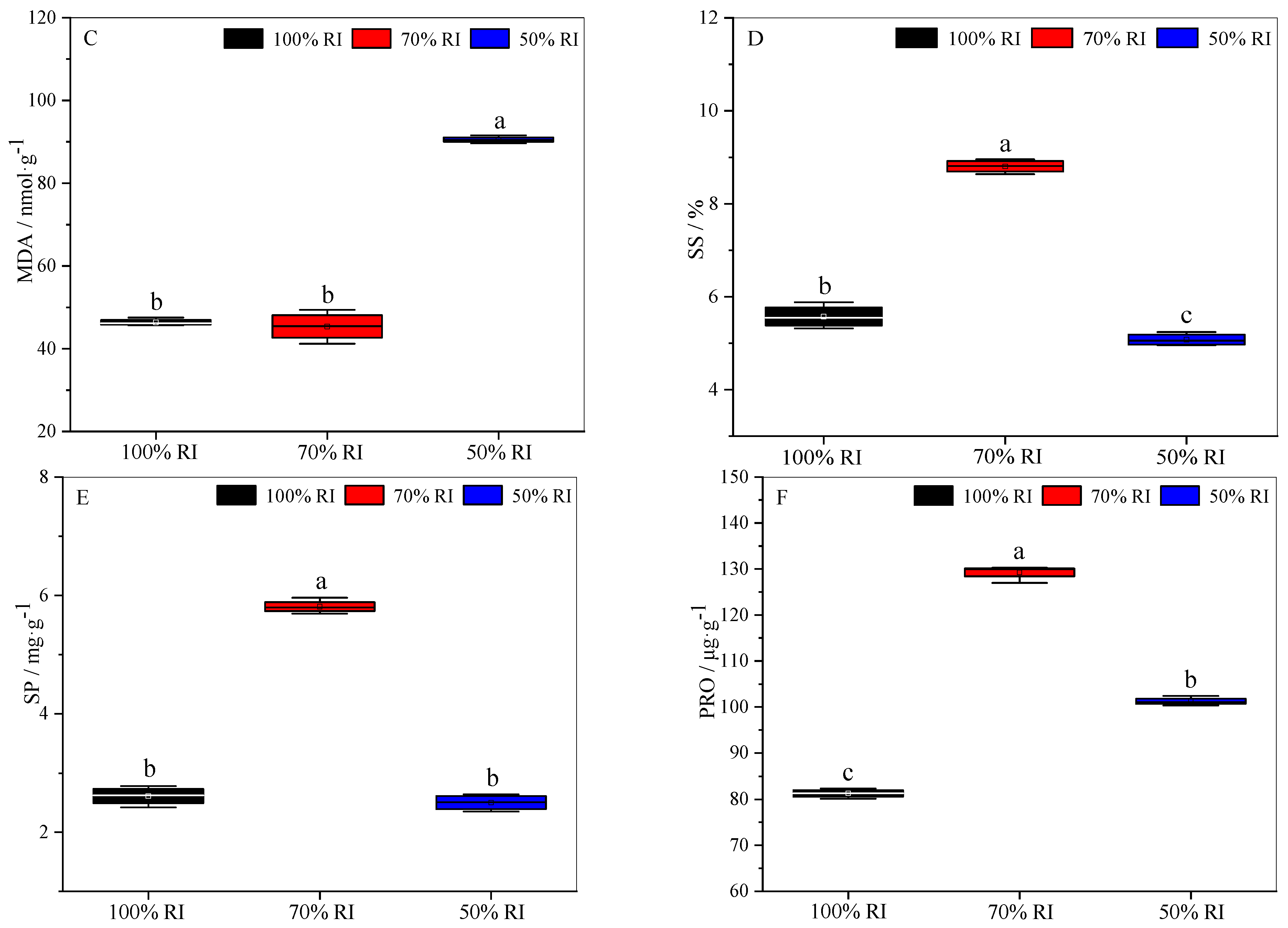
3.6. Physiological and Biochemical Properties at Different Light Intensities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, H.; Hu, Y.-Y.; Yu, W.-W.; Song, L.-L.; Wu, J.-S. Growth, photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments. Trees 2015, 29, 1011–1022. [Google Scholar] [CrossRef]
- Rozendaal, D.; Poorter, V. Plasticity in Leaf Traits of 38 Tropical Tree Species in Response to Light; Relationships with Light Demand and Adult Stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Shi, J.G.; Cui, H.Y.; Zhao, B.; Dong, S.T.; Liu, P.; Zhang, J.W. Effect of light on yield and characteristics of grain-filling of summer maize from flowering to maturity. Sci. Agric. Sin. 2013, 46, 4427–4434. [Google Scholar]
- Xie, X.; Yang, X.H.; Chen, X.Y. Effects of shading on leaf shape and photosynthetic characteristics of the transgenic lespedeza formosa with expressing BADH Gene. Sci. Silvae Sin. 2013, 49, 33–42. [Google Scholar]
- Liu, Z.; Cheng, R.; Xiao, W.; Guo, Q.; Wang, N. Effects of shading on growth and photosynthetic characteristics of distylium chinense seedlings. Sci. Silvae Sin. 2015, 51, 129–136. [Google Scholar]
- Pires, M.; Santos, H.A.; Santos, D.F.; Vasconcelos, A.S.; Aragão, C. Yield of muskmelon subjected to different water management with the use of polypropylene. Hortic. Bras. 2013, 31, 304–310. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Germino, M.J.; Johnson, D.M.; Reinhardt, K.; Smith, W.K.; Resler, L.M.; Bader, M.Y.; Sala, A.; Kueppers, L.M.; Broll, G. Seedling survival at timberline is critical to conifer mountain forest elevation and extent. Front. For. Glob. Chang. 2019, 2, 9. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Panigrahy, M.; Majeed, N.; Panigrahi, K.C.S. Low-light and its effects on crop yield: Genetic and genomic implications. J. Biosci. 2020, 45, 1–15. [Google Scholar] [CrossRef]
- Santos, D.; Daniela, V.S.; Letícia, D.; Cristina, S.A.; Carla, D.; Schramm, M.M. Relationships between reflectance and absorbance chlorophyll indices with RGB (Red, Green, Blue) image components in seedlings of tropical tree species at nursery stage. New For. 2019, 50, 377–388. [Google Scholar]
- Yamori, W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J. Plant Res. 2016, 129, 379–395. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Fang, S.; Zhou, M.; Qin, J. Responses of morphology, gas exchange, photochemical activity of photosystem ii, and antioxidant balance in cyclocarya paliurus to light spectra. Front. Plant Sci. 2018, 9, 1704. [Google Scholar] [CrossRef]
- Izaguirre, M.M.; Mazza, C.A.; Astigueta, M.; Ciarla, A.M.; Ballare, C.L. No time for candy: Passionfruit (Passiflora edulis) plants down-regulate damage-induced extra floral nectar production in response to light signals of competition. Oecologia 2013, 173, 213–221. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Liu, J. Comparison of the photosynthetic characteristics of two Isochrysis galbana strains under high light. Bot. Mar. 2014, 57, 477–481. [Google Scholar] [CrossRef]
- Torres, C.D.; Magnin, A.; Varela, S.A.; Stecconi, M.; Grosfeld, J.E.; Puntieri, J.G. Morpho-physiological responses of Nothofagus obliqua to light intensity and water status, with focus on primary growth dynamics. Trees 2018, 32, 1301–1314. [Google Scholar] [CrossRef]
- Parker, W.C.; Mohammed, G.H. Photosynthetic acclimation of shade-grown red pine (Pinus resinosa Ait.) seedlings to a high light environment. New For. 2000, 19, 1–11. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Stolárik, T.; Nožková, V.; Nosek, L.; Pavlovič, A. Dark chlorophyll synthesis may provide a potential for shade tolerance as shown by a comparative study with seedlings of European larch (Larix decidua) and Norway spruce (Picea abies). Trees 2018, 32, 951–965. [Google Scholar] [CrossRef]
- Alarcón, J.; Ortuno, M.; Nicolás, E.; Navarro, A.; Torrecillas, A. Improving water-use efficiency of young lemon trees by shading with aluminised-plastic nets. Agric. Water Manag. 2006, 82, 387–398. [Google Scholar] [CrossRef]
- Sevillano, I.; Short, I.; Campion, J.; Grant, O.M.; Grant, J.; O’Reilly, C. Comparison of photosynthetic performance of Fagus sylvatica seedlings under natural and artificial shading. Environ. Exp. Bot. 2018, 152, 90–96. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci. Hortic. 2019, 256, 108556. [Google Scholar] [CrossRef]
- Wu, J.; Su, Y.; Wang, J.; He, Q.; Qiu, Q.; Ma, J.; Li, J. Morphological and physiological acclimation of Catalpa bungei plantlets to different light conditions. Photosynthetica 2018, 56, 537–548. [Google Scholar] [CrossRef]
- Chang, P.-T.; Hsieh, C.-C.; Jiang, Y.-L. Responses of ‘Shih Huo Chuan’pitaya (Hylocereus polyrhizus (Weber) Britt. & Rose) to different degrees of shading nets. Sci. Hortic. 2016, 198, 154–162. [Google Scholar]
- Holland, N.; Richardson, A.D. Stomatal length correlates with elevation of growth in four temperate species. J. Sustain. For. 2009, 28, 63–73. [Google Scholar] [CrossRef]
- He, Z.-s.; Tang, R.; Li, M.-j.; Jin, M.-r.; Xin, C.; Liu, J.-f.; Hong, W. Response of photosynthesis and chlorophyll fluorescence parameters of castanopsis kawakamii seedlings to forest gaps. Forests 2020, 11, 21. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.J.; Chow, W.S.; Xie, X.; Chen, Y.J.; Peng, C.L. Photosynthetic characteristics and light energy conversions under different light environments in five tree species occupying dominant status at different stages of subtropical forest succession. Funct. Plant Biol. 2015, 42, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-M.; Yu, H.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Xia, X.-J. Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci. 2010, 179, 202–208. [Google Scholar] [CrossRef]
- Deng, Y.; Li, C.; Shao, Q.; Ye, X.; She, J. Differential responses of double petal and multi petal jasmine to shading: I. Photosynthetic characteristics and chloroplast ultrastructure. Plant Physiol. Biochem. 2012, 55, 93–102. [Google Scholar] [CrossRef]
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-l. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Maruta, T.; Tanouchi, A.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis chloroplastic ascorbate peroxidase isoenzymes play a dual role in photoprotection and gene regulation under photooxidative stress. Plant Cell Physiol. 2010, 51, 190–200. [Google Scholar] [CrossRef]
- Kong, D.-X.; Li, Y.-Q.; Wang, M.-L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant. 2016, 38, 120. [Google Scholar] [CrossRef]
- Kobayashi, K.; Amore, T.; Lazaro, M. Light-emitting diodes (LEDs) for miniature hydroponic lettuce. Sci. Res. 2013, 3, 74–77. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wu, L.; Chen, J.; Dong, L. Morphological plasticity, photosynthesis and chlorophyll fluorescence of Athyrium pachyphlebium at different shade levels. Photosynthetica 2011, 49, 611–618. [Google Scholar] [CrossRef]
- Tozzi, E.S.; Easlon, H.M.; Richards, J.H. Interactive effects of water, light and heat stress on photosynthesis in F remont cottonwood. Plant Cell Environ. 2013, 36, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, Y.; Song, L.; Jacobs, D.F.; Du, X.; Ying, Y.; Shao, Q.; Wu, J. Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. J. Plant Growth Regul. 2017, 36, 148–160. [Google Scholar] [CrossRef]
- Basave Villalobos, E.; Alcalá Cetina, V.M.; Lopez Lopez, M.A.; Aldrete, A.; Del Valle Paniagua, D.H. Nursery practices increase seedling performance on nutrient-poor soils in Swietenia humilis. iFor. Biogeosci. For. 2014, 8, 552. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Quevedo-Rojas, A.; García-Núñez, C.; Jerez-Rico, M.; Jaimez, R.; Schwarzkopf, T. Leaf acclimation strategies to contrasting light conditions in saplings of different shade tolerance in a tropical cloud forest. Funct. Plant Biol. 2018, 45, 968–982. [Google Scholar] [CrossRef]
- Casale, M.; Bagnasco, L.; Giordani, P.; Mariotti, M.G.; Malaspina, P. NIR spectroscopy as a tool for discriminating between lichens exposed to air pollution. Chemosphere 2015, 134, 355–360. [Google Scholar] [CrossRef]
- Couture, J.J.; Serbin, S.P.; Townsend, P.A. Spectroscopic sensitivity of real-time, rapidly induced phytochemical change in response to damage. New Phytol. 2013, 198, 311–319. [Google Scholar] [CrossRef]
- Kumar, L.; Schmidt, K.; Dury, S.; Skidmore, A. Imaging spectrometry and vegetation science. In Imaging Spectrometry; Springer: Heideiberg, The Netherlands, 2002; pp. 111–155. [Google Scholar]
- Kolhe, S.; Deshmukh, R. Detection of acid rain stress effect on plants using spectroradiometer—A review. Int. J. Innov. Res. Comput. Sci. Technol. 2016, 4, 13095–13100. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Letts, M.G.; Phelan, C.A.; Johnson, D.R.; Rood, S.B. Seasonal photosynthetic gas exchange and leaf reflectance characteristics of male and female cottonwoods in a riparian woodland. Tree Physiol. 2008, 28, 1037–1048. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Townsend, P.A.; Pellegrini, E.; Nali, C.; Couture, J.J. Reflectance spectroscopy: A novel approach to better understand and monitor the impact of air pollution on Mediterranean plants. Environ. Sci. Pollut. Res. 2018, 25, 8249–8267. [Google Scholar] [CrossRef]
- Durako, M.J.; Howarth, J.F. Leaf spectral reflectance shows Thalassia testudinum seedlings more sensitive to hypersalinity than hyposalinity. Front. Plant Sci. 2017, 8, 1127. [Google Scholar] [CrossRef]
- Deng, X.; Xiao, W.; Shi, Z.; Zeng, L.; Lei, L. Combined Effects of Drought and Shading on Growth and Non-Structural Carbohydrates in Pinus massoniana Lamb. Seedlings. Forests 2020, 11, 18. [Google Scholar] [CrossRef]
- Climent, J.; e Silva, F.C.; Chambel, M.R.; Pardos, M.; Almeida, M.H. Freezing injury in primary and secondary needles of Mediterranean pine species of contrasting ecological niches. Ann. For. Sci. 2009, 66, 407. [Google Scholar] [CrossRef]
- Kuusk, V.; Niinemets, Ü.; Valladares, F. Structural controls on photosynthetic capacity through juvenile-to-adult transition and needle ageing in Mediterranean pines. Funct. Ecol. 2018, 32, 1479–1491. [Google Scholar] [CrossRef]
- Wang, H.; Wu, F.; Wu, C.; Yu, S.; Zhu, X.; Xie, W. Growth and physiological responses of seedlings with different leaf shapes to drought and re-watering in Pinus massoniana. J. Northeast For. Univ. 2018, 46, 1–6. [Google Scholar]
- Wang, H.; Wu, F.; Zhu, X.; Xie, W. Effects of leaf types on growth and chlorophyll fluorescence characteristics in Pinus massoniana seedlings. Sci. Silv. Sin. 2019, 55, 183–192. [Google Scholar]
- Liu, Z.; Jiang, F.; Li, F.; Jin, G. Coordination of intra and inter-species leaf traits according to leaf phenology and plant age for three temperate broadleaf species with different shade tolerances. For. Ecol. Manag. 2019, 434, 63–75. [Google Scholar] [CrossRef]
- Alsiņa, I.; Dūma, M.; Dubova, L.; Šenberga, A.; Daģis, S. Comparison of different chlorophylls determination methods for leafy vegetables. Agron. Res. 2016, 14, 309–316. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Guo, Y.-P.; Guo, D.-P.; Zhou, H.-F.; Hu, M.-J.; Shen, Y.-G. Photoinhibition and xanthophyll cycle activity in bayberry (Myrica rubra) leaves induced by high irradiance. Photosynthetica 2006, 44, 439. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Miyake, C.; Miyata, M.; Shinzaki, Y.; Tomizawa, K.-i. CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—Relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol. 2005, 46, 629–637. [Google Scholar] [CrossRef]
- Sukhova, E.; Mudrilov, M.; Vodeneev, V.; Sukhov, V. Influence of the variation potential on photosynthetic flows of light energy and electrons in pea. Photosynth. Res. 2018, 136, 215–228. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Dere, S.; Gunes, T.; Sivaci, R. Spectrophotometric Determination of Chlorophyll-A, B and Total Carotenoid Contents of Some Algae Species Using Different Solvents. Turk. J. Bot. 1998, 22, 13–17. [Google Scholar]
- Sánchez, F.J.; Manzanares, M.a.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Miller, G.; Honig, A.; Stein, H.; Suzuki, N.; Mittler, R.; Zilberstein, A. Unraveling Δ1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J. Biol. Chem. 2009, 284, 26482–26492. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Shao, Q.; Li, C.; Ye, X.; Tang, R. Differential responses of double petal and multi petal jasmine to shading: II. Morphology, anatomy and physiology. Sci. Hortic. 2012, 144, 19–28. [Google Scholar] [CrossRef]
- Yuan-Bing, L.A.-R.Z.; Deng-Ke, C. Effects of salt stress on the growth and the antioxidant enzyme activity of Thellungiella halophila. Bull. Bot. Res. 2006, 26, 216–221. [Google Scholar]
- Favaretto, V.F.; Martinez, C.A.; Soriani, H.H.; Furriel, R.P. Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environ. Exp. Bot. 2011, 70, 20–28. [Google Scholar] [CrossRef]
- Riikonen, J. Pre-cultivation of Scots pine and Norway spruce transplant seedlings under four different light spectra did not affect their field performance. New For. 2016, 47, 607–619. [Google Scholar] [CrossRef]
- Yao, X.-y.; Liu, X.-y.; Xu, Z.-g.; Jiao, X.-l. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, R.; Hu, Y.; Song, Y.; Hänninen, H.; Wu, J. Interactive effects of drought and shading on Torreya grandis seedlings: Physiological and growth responses. Trees 2019, 33, 951–961. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.; Oleksyn, J.; Westoby, M.; Walters, M. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, M.; Wang, G.; Wu, T.; Zhang, C. Growth, morphology and biomass allocation in response to light gradient in five subtropical evergreen broadleaved tree seedlings. J. Trop. For. Sci. 2013, 537–546. [Google Scholar]
- Zhang, Y.; Liu, A.; Huang, S. Effects of shading on some morphological and physiological characteristics of Begonia semperflorens. Pak. J. Bot. 2018, 50, 2173–2179. [Google Scholar]
- Fernandes, A.M.; Fortini, E.A.; de Carvalho Müller, L.A.; Batista, D.S.; Vieira, L.M.; Silva, P.O.; do Amaral, C.H.; Poethig, R.S.; Otoni, W.C. Leaf development stages and ontogenetic changes in passionfruit (Passiflora edulis Sims.) are detected by narrowband spectral signal. J. Photochem. Photobiol. B Biol. 2020, 209, 111931. [Google Scholar] [CrossRef] [PubMed]
- Viera Silva, D.; Dos Anjos, L.; Brito-Rocha, E.; Dalmolin, A.C.; Mielke, M.S. Calibration of a multi-species model for chlorophyll estimation in seedlings of Neotropical tree species using hand-held leaf absorbance meters and spectral reflectance. iFor. Biogeosci. For. 2016, 9, 829. [Google Scholar] [CrossRef]
- Kelly, J.; Landhäusser, S.; Chow, P. The impact of light quality and quantity on root-to-shoot ratio and root carbon reserves in aspen seedling stock. New For. 2015, 46, 527–545. [Google Scholar] [CrossRef]
- Gao, Z.; Khalid, M.; Jan, F.; Jiang, X.; Yu, X. Effects of light-regulation and intensity on the growth, physiological and biochemical properties of Aralia elata (miq.) seedlings. S. Afr. J. Bot. 2019, 121, 456–462. [Google Scholar] [CrossRef]
- Schmitt, F.-J.; Renger, G.; Friedrich, T.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 835–848. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Daryanto, S.; Guo, S.; Huang, Z.; Wang, Z.; Wang, L.; Ma, X. Responses of Chinese fir and Schima superba seedlings to light gradients: Implications for the restoration of mixed broadleaf-conifer forests from Chinese fir monocultures. For. Ecol. Manag. 2018, 419, 51–57. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Scopel, A.L.; Sanchez, R.A. Far-red radiation reflected from adjacent leaves: An early signal of competition in plant canopies. Science 1990, 247, 329–332. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. Quantifying succulence: A rapid, physiologically meaningful metric of plant water storage. Plant Cell Environ. 2012, 35, 1533–1542. [Google Scholar] [CrossRef]
- Xie, H.; Yu, M.; Cheng, X. Leaf non-structural carbohydrate allocation and C: N: P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Biochem. 2018, 124, 146–154. [Google Scholar] [CrossRef] [PubMed]


| Spectrum Parameters | Definition | Reference |
|---|---|---|
| Carotenoid Reflectance Index | CRI = (1/R510) − (1/R550) | [56] |
| Structure Intensive Pigment Index | SIPI = (R800 − R445)/(R800 + R680) | [45] |
| Red Edge Normalized Difference Vegetation Index | RENDVI = (R750 − R705)/(R750 + R705) | [57] |
| Normalized Difference Vegetation Index | NDVI = (R800 − R680)/(R800 + R680) | [45] |
| Modified Red Edge Simple Ratio Index | MRESRI = (R705 − R445)/(R705/R455) | [56] |
| Photochemical Reflectance Index | PRI = (R531 − R570)/(R531 + R570) | [58] |
| Water Band Index | WBI = (R970/R900) | [58] |
| Treatment | Seedling Height/cm | Ground Diameter/mm | Height/Diameter Ratio | Seedlings with Secondary Needles/% |
|---|---|---|---|---|
| 100% RI | 12.41 ± 0.82 b | 1.46 ± 0.11 a | 8.50 ± 0.56 b | 24.33 ± 3.78 b |
| 70% RI | 13.56 ± 0.88 a | 1.28 ± 0.06 b | 10.59 ± 0.74 a | 37.33 ± 4.62 a |
| 50% RI | 10.49 ± 0.49 c | 0.94 ± 0.06 c | 11.16 ± 0.37 a | 2.00 ± 0.01 c |
| 20% RI | − | − | − | − |
| Treatment | 400–500 nm | 500–600 nm | 600–700 nm | 700–800 nm | 660–680 nm/720–740 nm |
|---|---|---|---|---|---|
| Blue | Green/Yellow-Orange | Red | Far-Red | Red/Far-Red | |
| 100% RI | 32.41 | 22.48 | 20.87 | 24.24 | 0.899 |
| 70% RI | 32.41 | 22.48 | 20.87 | 24.24 | 0.899 |
| 50% RI | 32.39 | 22.46 | 20.89 | 24.26 | 0.899 |
| Treatment | CRI | SIPI | RENDVI | NDVI | MRESRI | PRI | WBI |
|---|---|---|---|---|---|---|---|
| 100% RI | 0.048 ± 0.003 c | 0.787 ± 0.012 c | 0.434 ± 0.018 b | 0.815 ± 0.019 b | 4.362 ± 0.625 c | 0.045 ± 0.002 b | 1.026 ± 0.035 c |
| 70% RI | 0.081 ± 0.006 a | 0.854 ± 0.009 a | 0.589 ± 0.028 a | 0.942 ± 0.056 a | 5.269 ± 1.158 a | 0.071 ± 0.009 a | 1.317 ± 0.076 a |
| 50% RI | 0.064 ± 0.007 b | 0.819 ± 0.008 b | 0.594 ± 0.035 a | 0.723 ± 0.041 c | 4.859 ± 0.985 b | 0.038 ± 0.011 c | 1.172 ± 0.102 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wu, F.; Li, M.; Zhu, X.; Shi, C.; Ding, G. Morphological and Physiological Responses of Pinus massoniana Seedlings to Different Light Gradients. Forests 2021, 12, 523. https://doi.org/10.3390/f12050523
Wang H, Wu F, Li M, Zhu X, Shi C, Ding G. Morphological and Physiological Responses of Pinus massoniana Seedlings to Different Light Gradients. Forests. 2021; 12(5):523. https://doi.org/10.3390/f12050523
Chicago/Turabian StyleWang, Haoyun, Feng Wu, Min Li, Xiaokun Zhu, Changshuang Shi, and Guijie Ding. 2021. "Morphological and Physiological Responses of Pinus massoniana Seedlings to Different Light Gradients" Forests 12, no. 5: 523. https://doi.org/10.3390/f12050523
APA StyleWang, H., Wu, F., Li, M., Zhu, X., Shi, C., & Ding, G. (2021). Morphological and Physiological Responses of Pinus massoniana Seedlings to Different Light Gradients. Forests, 12(5), 523. https://doi.org/10.3390/f12050523





