Wood Anatomical Traits Reveal Different Structure of Peat Bog and Lowland Populations of Pinus sylvestris L. in the Carpathian Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites, Sampling and Tree Ring Analyses
2.2. Wood Anatomical Measurements
2.3. Statistical Analysis
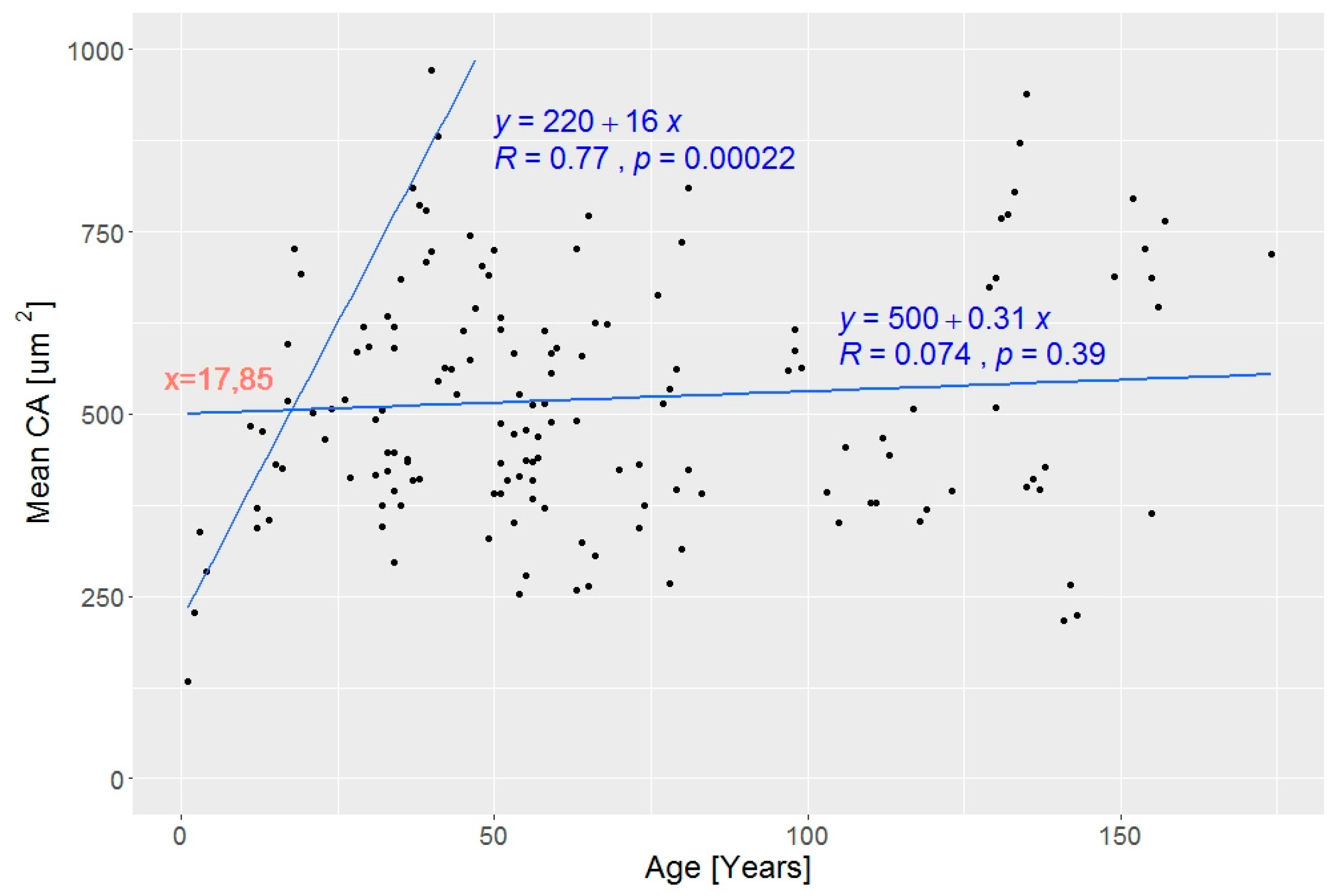
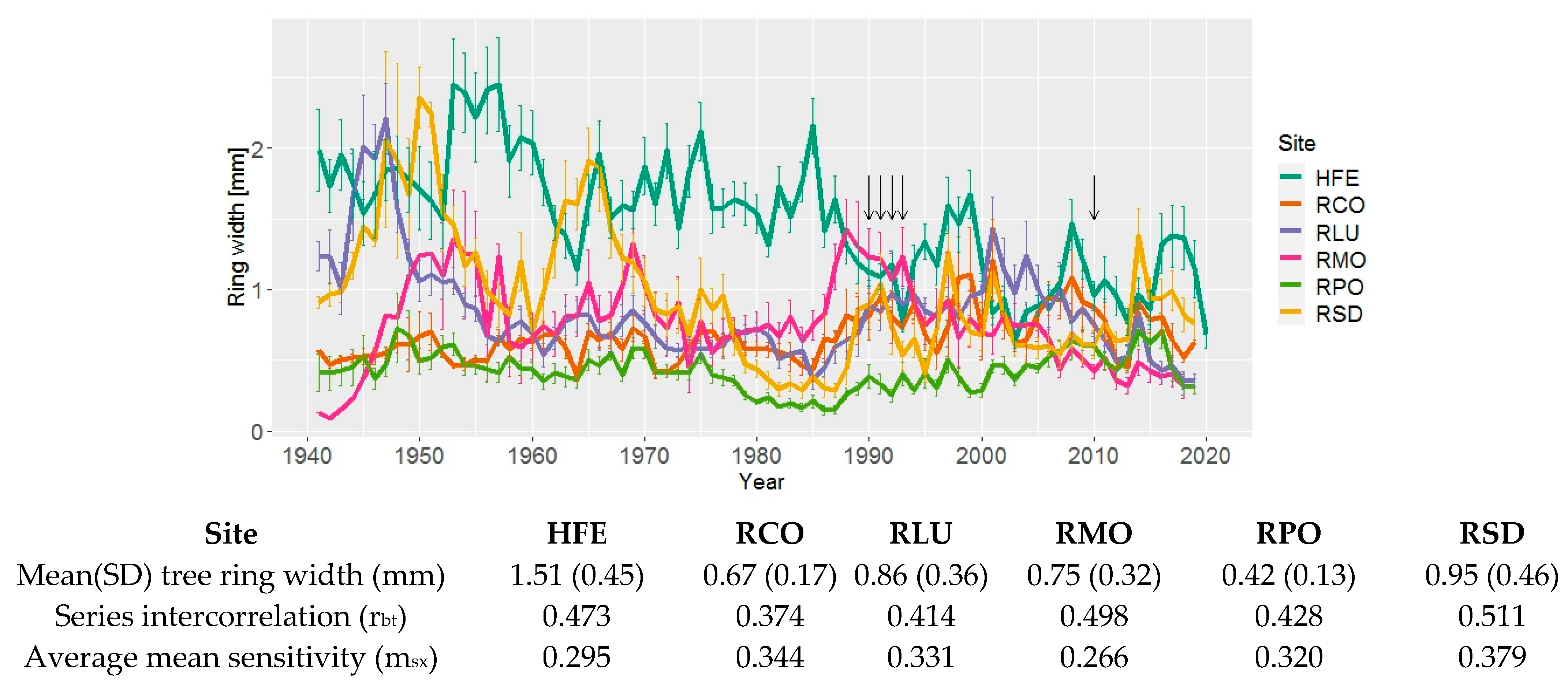
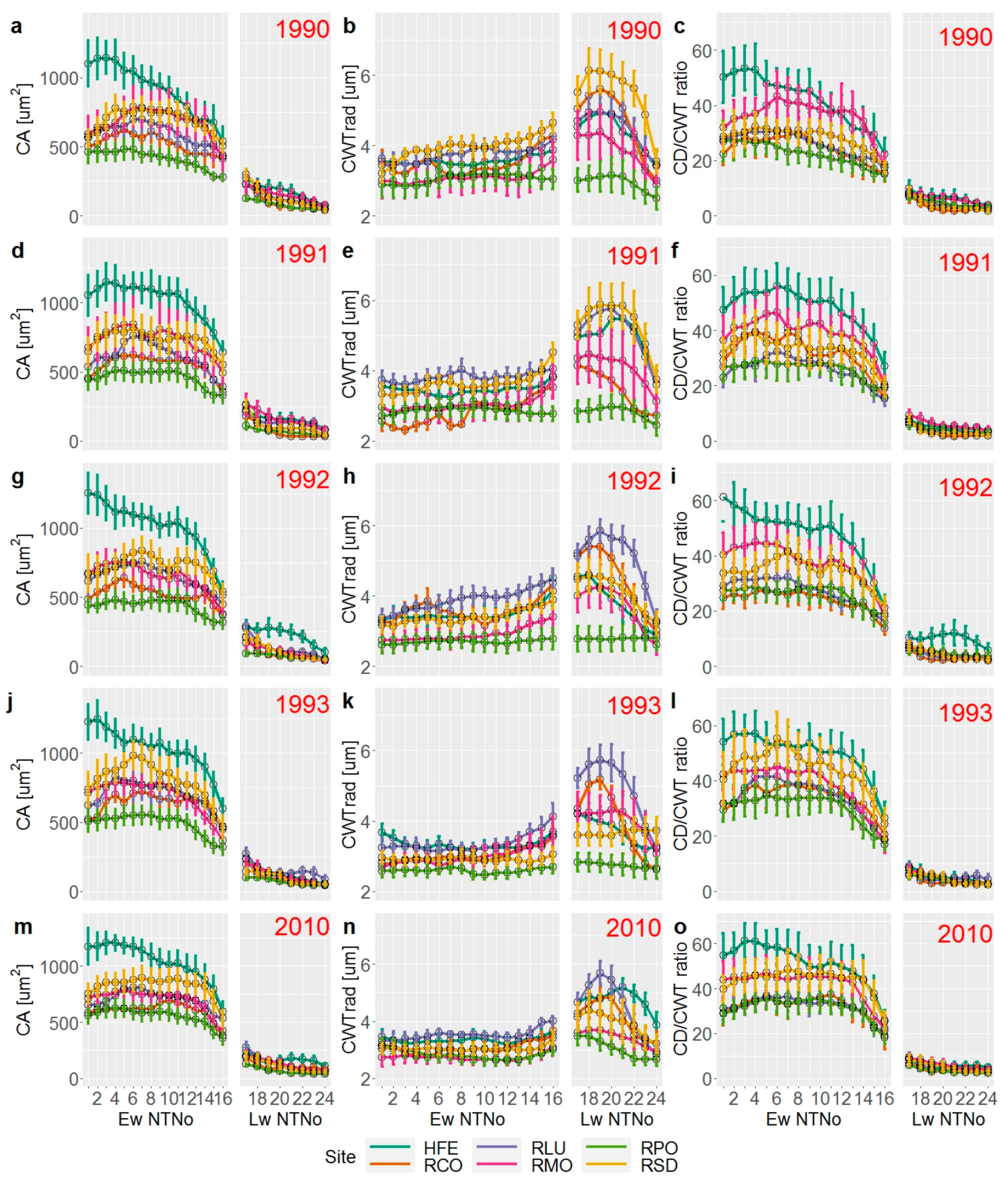
3. Results
3.1. Tree Ring width Chronologies
3.2. Wood Anatomical Differentiation by Habitat Types
3.3. Effect of Yearly and Monthly Climate on Wood Anatomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leuschner, C.; Ellenberg, H. Ecology of Central European Forests—Vegetation Ecology of Central Europe; Springer International Publishing: Cham, Switzerland, 2017; Volume I, ISBN 978-3-319-43040-9. [Google Scholar]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Sheth, S.N.; Angert, A.L. Demographic compensation does not rescue populations at a trailing range edge. Proc. Natl. Acad. Sci. USA 2018, 115, 201715899. [Google Scholar] [CrossRef] [PubMed]
- Fadrique, B.; Báez, S.; Duque, Á.; Malizia, A.; Blundo, C.; Carilla, J.; Osinaga-Acosta, O.; Malizia, L.; Silman, M.; Farfán-Ríos, W.; et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature 2018, 564, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.; Provan, J. What do we mean by ‘refugia’? Quat. Sci. Rev. 2008, 27, 2449–2455. [Google Scholar] [CrossRef]
- Keppel, G.; Van Niel, K.P.; Wardell-Johnson, G.W.; Yates, C.J.; Byrne, M.; Mucina, L.; Schut, A.G.T.; Hopper, S.D.; Franklin, S.E. Refugia: Identifying and understanding safe havens for biodiversity under climate change. Glob. Ecol. Biogeogr. 2012, 21, 393–404. [Google Scholar] [CrossRef]
- Rull, V. Microrefugia. J. Biogeogr. 2009, 36, 481–484. [Google Scholar] [CrossRef]
- Losos, J.B. Phylogenetic Perspectives on Community Ecology. Ecology 1996, 77, 1344–1354. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Anderson, J.T.; Song, B. Plant adaptation to climate change—Where are we? J. Syst. Evol. 2020, 58, 533–545. [Google Scholar] [CrossRef]
- Fonti, P.; Von Arx, G.; García-González, I.; Eilmann, B.; Sass-Klaassen, U.; Gärtner, H.; Eckstein, D. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 2010, 185, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Labra, M.; Grassi, F.; Sgorbati, S.; Ferrari, C. Distribution of genetic variability in southern populations of Scots pine (Pinus sylvestris L.) from the Alps to the Apennines. Flora Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 468–476. [Google Scholar] [CrossRef]
- Debreczy, Z.; Rácz, I.; Musial, K. Conifers around the World: Conifers of the Temperate Zones and Adjacent Regions; DendroPress: Budapest, Hungary, 2011; ISBN 978-9-632-19061-7. [Google Scholar]
- Tóth, E.G.; Köbölkuti, Z.A.; Pedryc, A.; Höhn, M. Evolutionary history and phylogeography of Scots pine (Pinus sylvestris L.) in Europe based on molecular markers. J. For. Res. 2017, 28, 637–651. [Google Scholar] [CrossRef]
- Tóth, E.G.; Vendramin, G.G.; Bagnoli, F.; Cseke, K.; Höhn, M. High genetic diversity and distinct origin of recently fragmented Scots pine (Pinus sylvestris L.) populations along the Carpathians and the Pannonian Basin. Tree Genet. Genomes 2017, 13, 47. [Google Scholar] [CrossRef]
- Rubiales, J.M.; García-Amorena, I.; Álvarez, S.G.; Manzaneque, F.G. The Late Holocene extinction of Pinus sylvestris in the western Cantabrian Range (Spain). J. Biogeogr. 2008, 35, 1840–1850. [Google Scholar] [CrossRef]
- Cheddadi, R.; Vendramin, G.G.; Litt, T.; Francois, L.; Kageyama, M.; Lorentz, S.; Laurent, J.-M.; de Beaulieu, J.-L.; Sadori, L.; Jost, A.; et al. Imprints of glacial refugia in the modern genetic diversity of Pinus sylvestris. Glob. Ecol. Biogeogr. 2006, 15, 271–282. [Google Scholar] [CrossRef]
- Prus-Głowacki, W.; Urbaniak, L.; Bujas, E.; Curtu, A.L. Genetic variation of isolated and peripheral populations of Pinus sylvestris (L.) from glacial refugia. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 150–158. [Google Scholar] [CrossRef]
- Borhidi, A. Magyarország Növénytársulásai; Akadémiai Kiadó: Budapest, Hungary, 2003; ISBN 963-05-7983-9. [Google Scholar]
- Donita, N.; Popescu, A.; Pauca-Comanescu, M.; Mihailescu, S.; Biris, A. Habitatele din România; Editura Tehnicã Silvicã: Bukarest, Romania, 2005; ISBN 973-96001-4-X. [Google Scholar]
- Bölöni, J.; Molnár, Z.; Biró, M.; Horváth, F. Distribution of the (semi-)natural habitats in Hungary II. Woodlands and shrublands. Acta Bot. Hung. 2008, 50, 107–148. [Google Scholar] [CrossRef]
- Șofletea, N.; Mihai, G.; Ciocîrlan, E.; Curtu, A.L. Genetic Diversity and Spatial Genetic Structure in Isolated Scots Pine (Pinus sylvestris L.) Populations Native to Eastern and Southern Carpathians. Forests 2020, 11, 1047. [Google Scholar] [CrossRef]
- Köbölkuti, Z.A.; Tóth, E.G.; Ladányi, M.; Höhn, M. Morphological and anatomical differentiation in peripheral Pinus sylvestris L. populations from the Carpathian region. Dendrobiology 2017, 77, 105–117. [Google Scholar] [CrossRef]
- Köbölkuti, Z.A.; Höhn, M. Habitat type differentiation in peripheral Pinus sylvestris populations based on seed traits and germination data. Stud. Bot. Hung. 2018, 49, 97–119. [Google Scholar] [CrossRef]
- Ambroży, S.; Kapsa, M. Regeneration of Scots pine Pinus sylvestris L. in the Carpathians depends on site fertility. For. Res. Pap. 2019, 80, 189–200. [Google Scholar] [CrossRef]
- Bouriaud, O.; Popa, I. Comparative dendroclimatic study of Scots pine, Norway spruce, and silver fir in the Vrancea Range, Eastern Carpathian Mountains. Trees 2009, 23, 95–106. [Google Scholar] [CrossRef]
- Misi, D.; Náfrádi, K. Late Winter—Early Spring Thermal Conditions and Their Long-Term Effect on Tree-Ring Growth in Hungary. Balt. For. 2016, 22, 203–211. [Google Scholar]
- Nagavciuc, V.; Roibu, C.-C.; Ionita, M.; Mursa, A.; Cotos, M.-G.; Popa, I. Different climate response of three tree ring proxies of Pinus sylvestris from the Eastern Carpathians, Romania. Dendrochronologia 2019, 54, 56–63. [Google Scholar] [CrossRef]
- Liang, W.; Heinrich, I.; Simard, S.; Helle, G.; Linan, I.D.; Heinken, T. Climate signals derived from cell anatomy of Scots pine in NE Germany. Tree Physiol. 2013, 33, 833–844. [Google Scholar] [CrossRef]
- Pritzkow, C.; Heinrich, I.; Grudd, H.; Helle, G. Relationship between wood anatomy, tree-ring widths and wood density of Pinus sylvestris L. and climate at high latitudes in northern Sweden. Dendrochronologia 2014, 32, 295–302. [Google Scholar] [CrossRef]
- Björklund, J.; Seftigen, K.; Fonti, P.; Nievergelt, D.; von Arx, G. Dendroclimatic potential of dendroanatomy in temperature-sensitive Pinus sylvestris. Dendrochronologia 2020, 60, 125673. [Google Scholar] [CrossRef]
- Ståhl, E.G. Transfer effects and variations in basic density and tracheid length of Pinus sylvestris L. populations. Stud. For. Suec. 1988, 180, 1–15. [Google Scholar]
- Schmitt, U.; Jalkanen, R.; Eckstein, D. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn. 2004, 38, 167–178. [Google Scholar] [CrossRef]
- Eilmann, B.; Weber, P.; Rigling, A.; Eckstein, D. Growth reactions of Pinus sylvestris L. and Quercus pubescens Willd. to drought years at a xeric site in Valais, Switzerland. Dendrochronologia 2006, 23, 121–132. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Anchukaitis, K.; Evans, M.; del Río, M.; Beeckman, H.; Cañellas, I. Effects of Drought on Xylem Anatomy and Water-Use Efficiency of Two Co-Occurring Pine Species. Forests 2017, 8, 332. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Beeckman, H.; Cañellas, I. Influence of drought on tree rings and tracheid features of Pinus nigra and Pinus sylvestris in a mesic Mediterranean forest. Eur. J. For. Res. 2013, 132, 33–45. [Google Scholar] [CrossRef]
- Fernández-de-Uña, L.; Rossi, S.; Aranda, I.; Fonti, P.; González-González, B.D.; Cañellas, I.; Gea-Izquierdo, G. Xylem and Leaf Functional Adjustments to Drought in Pinus sylvestris and Quercus pyrenaica at Their Elevational Boundary. Front. Plant Sci. 2017, 8, 1200. [Google Scholar] [CrossRef]
- Belokopytova, L.V.; Babushkina, E.A.; Zhirnova, D.F.; Panyushkina, I.P.; Vaganov, E.A. Pine and larch tracheids capture seasonal variations of climatic signal at moisture-limited sites. Trees 2019, 33, 227–242. [Google Scholar] [CrossRef]
- Swidrak, I.; Gruber, A.; Oberhuber, W. Cambial activity and xylem cell development in Pinus cembra and Pinus sylvestris at their climatic limits in the Eastern Alps in 2007. Phyton 2011, 51, 299–313. [Google Scholar]
- Panayotov, M.P.; Zafirov, N.; Cherubini, P. Fingerprints of extreme climate events in Pinus sylvestris tree rings from Bulgaria. Trees 2013, 27, 211–227. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Belokopytova, L.V.; Kostyakova, T.V.; Kokova, V.I. Earlywood and Latewood Features of Pinus sylvestris in Semiarid Natural Zones of South Siberia. Russ. J. Ecol. 2018, 49, 209–217. [Google Scholar] [CrossRef]
- Del Castillo, E.M.; Longares, L.A.; Gričar, J.; Prislan, P.; Gil-Pelegrín, E.; Čufar, K.; de Luis, M. Living on the Edge: Contrasted Wood-Formation Dynamics in Fagus sylvatica and Pinus sylvestris under Mediterranean Conditions. Front. Plant Sci. 2016, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Prislan, P.; Gričar, J.; de Luis, M.; Novak, K.; Martinez del Castillo, E.; Schmitt, U.; Koch, G.; Štrus, J.; Mrak, P.; Žnidarič, M.T.; et al. Annual Cambial Rhythm in Pinus halepensis and Pinus sylvestris as Indicator for Climate Adaptation. Front. Plant Sci. 2016, 7, 1923. [Google Scholar] [CrossRef]
- Hereş, A.-M.; Camarero, J.J.; López, B.C.; Martínez-Vilalta, J. Declining hydraulic performances and low carbon investments in tree rings predate Scots pine drought-induced mortality. Trees 2014, 28, 1737–1750. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Graf Pannatier, E.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Fonti, P.; Rigling, A. Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol. 2009, 29, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hengl, T.; Mendes de Jesus, J.; Heuvelink, G.B.M.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [PubMed]
- Szalai, S.; Auer, I.; Hiebl, J.; Milkovich, J.; Radim, T.; Stepanek, P.; Zahradnicek, P.; Bihari, Z.; Lakatos, M.; Szentimrey, T.; et al. Climate of the Greater Carpathian Region; Final Technical Report; European Commission, JRC: Brussels, Belgium, 2013. [Google Scholar]
- Tóth, E.G.; Bede-Fazekas, Á.; Vendramin, G.G.; Bagnoli, F.; Höhn, M. Mid-Pleistocene and Holocene demographic fluctuation of Scots pine (Pinus sylvestris L.) in the Carpathian Mountains and the Pannonian Basin: Signs of historical expansions and contractions. Quat. Int. 2017, 1–12. [Google Scholar] [CrossRef]
- Carrer, M.; Unterholzner, L.; Castagneri, D. Wood anatomical traits highlight complex temperature influence on Pinus cembra at high elevation in the Eastern Alps. Int. J. Biometeorol. 2018, 62, 1745–1753. [Google Scholar] [CrossRef]
- Carrer, M.; von Arx, G.; Castagneri, D.; Petit, G. Distilling allometric and environmental information from time series of conduit size: The standardization issue and its relationship to tree hydraulic architecture. Tree Physiol. 2015, 35, 27–33. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. (Eds.) Methods of Dendrochronology—Applications in the Environmental Sciences; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; ISBN 0792305868. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree Ring Res. 2001, 57, 205–211. [Google Scholar]
- Van der Werf, G.W.; Sass-Klaassen, U.G.W.; Mohren, G.M.J. The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 2007, 25, 103–112. [Google Scholar] [CrossRef]
- Von Arx, G.; Carrer, M. ROXAS—A new tool to build centuries-long tracheid-lumen chronologies in conifers. Dendrochronologia 2014, 32, 290–293. [Google Scholar] [CrossRef]
- Yang, K.C.; Benson, C.A.; Wong, J.K. Distribution of juvenile wood in two stems of Larix laricina. Can. J. For. Res. 1986, 16, 1041–1049. [Google Scholar] [CrossRef]
- Vaganov, E.A. The tracheidogram method in tree-ring analysis and its application. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; pp. 63–76. ISBN 0792305868. [Google Scholar]
- Denne, M.P. Definition of latewood according to Mork (1928). IAWA Bull. New. Ser. 1988, 10, 59–62. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Campelo, F.; De Luis, M.; Bräuning, A.; Grabner, M.; Battipaglia, G.; Cherubini, P. Intra-annual Density Fluctuations in Tree Rings: How, When, Where, and Why? IAWA J. 2016, 37, 232–259. [Google Scholar] [CrossRef]
- Sperry, J.S. Evolution of Water Transport and Xylem Structure. Int. J. Plant Sci. 2003, 164, S115–S127. [Google Scholar] [CrossRef]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and function in conifer tracheids and angiosperm vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; DeLucia, E.H. Xylem conductivity and vulnerability to cavitation of ponderosa pine growing in contrasting climates. Tree Physiol. 2000, 20, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Majer, A. Fenyves a Bakonyalján; Akadémiai Kiadó: Budapest, Hungary, 1988; ISBN 963-05-4461-x. [Google Scholar]
- Kalliokoski, T.; Makinen, H.; Jyske, T.; Nojd, P.; Linder, S. Effects of nutrient optimization on intra-annual wood formation in Norway spruce. Tree Physiol. 2013, 33, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Jolly, W.M.; Dobbertin, M.; Zimmermann, N.E.; Reichstein, M. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys. Res. Lett. 2005, 32, L18409. [Google Scholar] [CrossRef]
- Piermattei, A.; Campelo, F.; Büntgen, U.; Crivellaro, A.; Garbarino, M.; Urbinati, C. Intra-annual density fluctuations (IADFs) in Pinus nigra (J. F. Arnold) at high-elevation in the central Apennines (Italy). Trees 2020, 34, 771–781. [Google Scholar] [CrossRef]
- Theodorou, C. Soil moisture and the mycorrhizal association of Pinus radiata D. don. Soil Biol. Biochem. 1978, 10, 33–37. [Google Scholar] [CrossRef]
- Lieffers, V.J.; Rothwell, R.L. Rooting of peatland black spruce and tamarack in relation to depth of water table. Can. J. Bot. 1987, 65, 817–821. [Google Scholar] [CrossRef]
- Rigling, A.; Waldner, P.O.; Forster, T.; Bräker, O.U.; Pouttu, A. Ecological interpretation of tree-ring width and intraannual density fluctuations in Pinus sylvestris on dry sites in the central Alps and Siberia. Can. J. For. Res. 2001, 31, 18–31. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Tyree, M.T.; Davis, S.D.; Cochard, H. Biophysical Perspectives of Xylem Evolution: Is there a Tradeoff of Hydraulic Efficiency for Vulnerability to Dysfunction? IAWA J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J. Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol. 2003, 23, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J. Impacts of Freezing on Long Distance Transport in Woody Plants. In Vascular Transport in Plants; Elsevier: Amsterdam, The Netherlands, 2005; pp. 401–424. ISBN 9780120884575. [Google Scholar]
- Mihalca, I.A. The landscape of marshes in the Land of Dorna. Analele Univ. Oradea Ser. Geogr. 2012, 22, 66–71. [Google Scholar]
- Aurelia, D.M.; Toader, C. Contributions to the Study of Vegetation in Tinovul Mare (Poiana Stampei—District of Suceava). Bul. Grădinii Bot. Iaşi 2007, 14, 75–80. [Google Scholar]
- Barker, J.E. Growth and Wood Properties of Pinus radiata in Relation to Applied Ethylene. N. Z. J. For. Sci. 1979, 9, 15–19. [Google Scholar]
- Yamamoto, F.; Kozlovwski, T.T. Effect of ethrel on growth and stem anatomy of Pinus halepensis seedlings. IAWA Bull. New Ser. 1987, 8, 11–19. [Google Scholar] [CrossRef]
- Yamamoto, F.; Kozlowski, T.T. Effects of Flooding of Soll on Growth, Stem Anatomy, and Ethylene Production of Thuja Orientalis Seedlings. IAWA J. 1987, 8, 21–29. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Gindl, W.; Grabner, M.; Wimmer, R. Effects of altitude on tracheid differentiation and lignification of Norway spruce. Can. J. Bot. 2001, 79, 815–821. [Google Scholar] [CrossRef]
- Hevia, A.; Campelo, F.; Chambel, R.; Vieira, J.; Alía, R.; Majada, J.; Sánchez-Salguero, R. Which matters more for wood traits in Pinus halepensis Mill., provenance or climate? Ann. For. Sci. 2020, 77, 55. [Google Scholar] [CrossRef]
- Borbély, E.; Indreica, A. Conservation status of the rare species Betula nana in the peatbog Tinovul Luci (the Harghita Mountains, Romania). Stud. Univ. Babeş-Bolyai Biol. 2019, 64, 5–12. [Google Scholar] [CrossRef]
- Rosner, S.; Světlík, J.; Andreassen, K.; Børja, I.; Dalsgaard, L.; Evans, R.; Luss, S.; Tveito, O.E.; Solberg, S. Novel Hydraulic Vulnerability Proxies for a Boreal Conifer Species Reveal That Opportunists May Have Lower Survival Prospects under Extreme Climatic Events. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Diaconu, A.-C.; Tanţău, I.; Knorr, K.-H.; Borken, W.; Feurdean, A.; Panait, A.; Gałka, M. A multi-proxy analysis of hydroclimate trends in an ombrotrophic bog over the last millennium in the Eastern Carpathians of Romania. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 538, 109390. [Google Scholar] [CrossRef]
- Jelonek, T.; Tomczak, A.; Remlein, A.; Katrusiak, A.; Arasimowicz-Jelonek, M.; Gzyl, J. The novel relationship between the morphological characteristics of trees and ultrastructure of wood tissue in scots pine (Pinus sylvestris L.). Wood Res. 2015, 60, 519–530. [Google Scholar]
- Tóth, E.G. Phylogeography and Adaptive Genetic Variation of Scots Pine (Pinus sylvestris L.) Populations from the Carpathians and the Pannonian Basin. Ph.D. Thesis, Szent István University, Budapest, Hungary, 2017. [Google Scholar]
- Gruber, A.; Strobl, S.; Veit, B.; Oberhuber, W. Impact of drought on the temporal dynamics of wood formation in Pinus sylvestris. Tree Physiol. 2010, 30, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.; de Luís, M.; Raventós, J.; Čufar, K. Climatic signals in tree-ring widths and wood structure of Pinus halepensis in contrasted environmental conditions. Trees 2013, 27, 927–936. [Google Scholar] [CrossRef]
- Carvalho, A.; Nabais, C.; Vieira, J.; Rossi, S.; Campelo, F. Plastic Response of Tracheids in Pinus pinaster in a Water-Limited Environment: Adjusting Lumen Size instead of Wall Thickness. PLoS ONE 2015, 10, e0136305. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Camarero, J.J.; Carrer, M. Linking wood anatomy and xylogenesis allows pinpointing of climate and drought influences on growth of coexisting conifers in continental Mediterranean climate. Tree Physiol. 2016, 36, 502–512. [Google Scholar] [CrossRef] [PubMed]
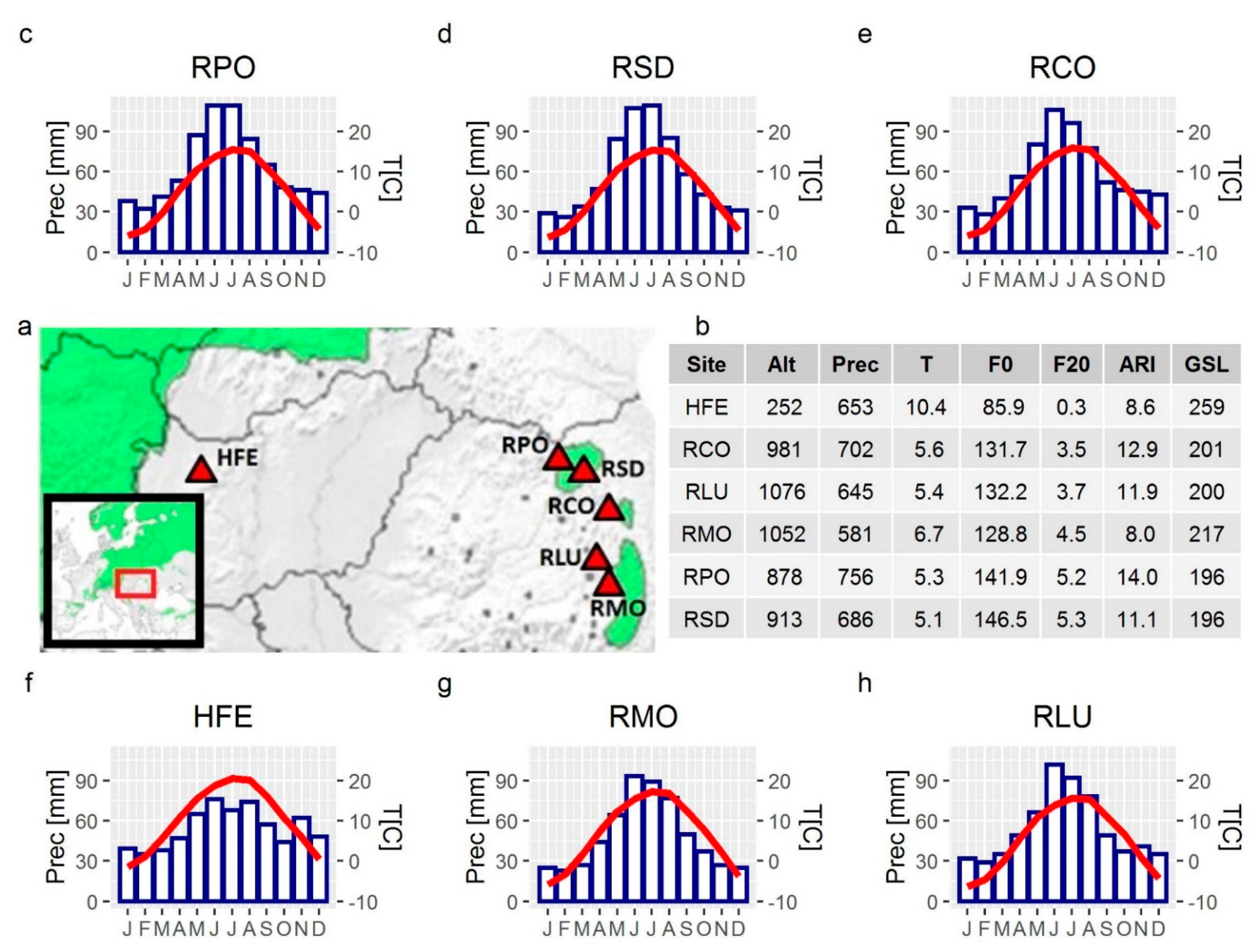

| Code | Country | Residential Area | Habitat Type | Community Type | Latitude, Longitude | Altitude (m) | Slope Aspect | Area (km2) | Sampled Trees | Selected Trees for Wood Anatomy | Mean Age (Year) (SD) | DBH (cm) (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFE | HU | Fenyőfő | Mixed forest | Festuco vaginatae-Pinetum sylvestris | 47°21′26.0″ N 17°46′08.4″ E | 252 | SE | 4.49 | 10 | 8 | 115 (47.0) | 55.1 (8.9) |
| RCO | RO | Suhardul Mic | Rocky substrate | Seslerio-rigidae-Pinetum sylvestris | 46°48′06.0″ N 25°47′31.4″ E | 981 | NW | 0.04 | 3 | 3 | 101 (49.0) | 19.2 (3.3) |
| RLU | RO | Tinovul Luci | Peat bog | Sphagnion magellanici, Vaccinio uliginosi-Pinetea | 46°17′51.6″ N 25°43′39.2″ E | 1076 | - | 2.73 | 12 | 7 | 77 (6.6) | 17.4 (2.8) |
| RMO | RO | Băile Tusnad | Peat bog | Sphagnion magellanici, Vaccinio uliginosi-Pinetea | 46°08′07.9″ N 25°54′11.0″ E | 1052 | - | 0.58 | 9 | 8 | 59 (12.0) | 12.5 (3.3) |
| RPO | RO | Poiana Stampei | Peat bog | Sphagnion magellanici, Vaccinio uliginosi-Pinetea | 47°17′56.5″ N 25°06′59.8″ E | 878 | - | 1.43 | 11 | 5 | 95 (37.7) | 13.6 (2.8) |
| RSD | RO | Saru Dornei | Peat bog | Sphagnion magellanici, Vaccinio uliginosi-Pinetea | 47°15′28.8″ N 25°21′24.3″ E | 913 | - | 0.41 | 12 | 6 | 61 (17.1) | 16.2 (6.1) |
| Dependent Variable | Site | Year | Climate Variables | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 06 | ARI 04 | ARI 06 | ARI 07 | pPPT_0508 | |||
| CA | Ew | Coef | 1045.59 | −606.72 | −505.64 | −291.49 | −712.27 | −412.49 | 1.48 | 62.75 | 32.61 | 95.25 | −5.54 | 57.07 | 619.59 | 62.40 | −2.54 |
| χ2 | 2575.65 *** | 87.13 *** | 40.03 *** | 69.73 *** | 39.47 *** | 35.21 *** | 32.48 *** | ||||||||||
| Lw | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 05 | PPT 10 | ARI 04 | ARI 05 | pARI_0508 | ||
| Coef | 337.20 | −71.17 | −37.42 | −10.74 | −126.87 | −81.41 | −99.81 | 65.23 | −44.78 | 6.45 | −1.80 | −0.76 | 14.21 | 142.02 | −175.64 | ||
| χ2 | 649.76 *** | 340.49 *** | 53.13 *** | 60.79 *** | 9.17 ** | 47.81 *** | 196.55 *** | ||||||||||
| CWTrad | Ew | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 04 | PPT 06 | ARI 06 | ARI 08 | pARI_0508 | |
| Coef | 4.23 | −0.442 | −0.467 | −0.648 | −1.493 | −0.929 | −0.486 | 0.037 | −0.838 | −0.610 | −0.003 | −0.029 | 2.773 | 0.971 | −0.302 | ||
| χ2 | 1776.65 *** | 784.54 *** | 36.54 *** | 84.26 *** | 67.15 *** | 212.96 *** | 6.37 * | ||||||||||
| Lw | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 07 | PPT 08 | ARI 06 | ARI 07 | pARI_0508 | ||
| Coef | 3.25 | −0.12 | 0.28 | −0.43 | −1.93 | −0.46 | −0.06 | −0.78 | −0.55 | −1.59 | 0.04 | 0.01 | −0.28 | −5.31 | 1.69 | ||
| χ2 | 1376.55 *** | 372.15 *** | 38.52 *** | 120.78 *** | 6.74 ** | 39.48 *** | 62.72 *** | ||||||||||
| CD/CWT ratio | Ew | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 05 | ARI 04 | ARI 07 | -PPT_0508 | ||
| Coef | 41.01 | −26.45 | −22.17 | −9.42 | −24.55 | −13.90 | 3.12 | 3.35 | 6.06 | 8.70 | −0.018 | 3.12 | 4.21 | −0.03 | |||
| χ2 | 2147.10 *** | 447.95 *** | 15.42 *** | 63.80 *** | 57.11 *** | 5.18 * | |||||||||||
| Lw | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 08 | ARI 05 | ARI 07 | ARI 10 | |||
| Coef | 5.67 | −3.47 | −1.43 | −0.02 | −2.82 | −2.61 | −0.97 | 0.01 | 0.48 | 0.85 | −0.02 | 0.48 | 0.71 | 0.53 | |||
| χ2 | 401.48 *** | 78.73 *** | 31.40 *** | 11.40 *** | 13.60 ** | 33.19 *** | |||||||||||
| TNo indices | Ew | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 05 | PPT 08 | ARI 04 | ARI 07 | ARI_08 | |
| Coef | 1.40 | −1.203 | −0.937 | −0.541 | −1.340 | −1.042 | −0.339 | −0.055 | −0.410 | −0.428 | −0.001 | −0.014 | 0.122 | 0.317 | 1.882 | ||
| χ2 | 273.81 *** | 53.08 *** | 3.91 * | 8.41 ** | 7.85 ** | 21.53 *** | 13.04 *** | ||||||||||
| Lw | Int | RCO | RLU | RMO | RPO | RSD | 1991 | 1992 | 1993 | 2010 | PPT 05 | PPT 08 | ARI 05 | ARI 09 | pARI_0912 | ||
| Coef | 2.01 | −0.05 | −0.31 | −0.99 | −0.90 | −0.73 | 0.35 | −0.04 | −0.26 | −0.41 | 0.02 | 0.005 | −1.13 | −0.45 | −0.03 | ||
| χ2 | 156.40 *** | 12.70 * | 15.62 *** | 5.62 * | 10.36 ** | 6.12 * | 13.43 *** | ||||||||||
| Parameter | HFE | RCO | RLU | RMO | RPO | RSD | |
|---|---|---|---|---|---|---|---|
| Earlywood | mean(SE) CA (μm2) | 999.27 (5.47) | 570.06 (6.45) | 645.86 (4.54) | 688.37 (5.13) | 470.44 (3.76) | 758.53 (5.40) |
| mean(SE) CWTrad (μm) | 3.45 (0.01) | 3.25 (0.02) | 3.70 (0.01) | 2.95 (0.02) | 2.81 (0.01) | 3.38 (0.01) | |
| mean(SE) CD/CWT | 47.60 (0.30) | 29.01 (0.38) | 28.61 (0.24) | 38.58 (0.27) | 27.36 (0.23) | 37.04 (0.31) | |
| Latewood | mean(SE) CA (μm2) | 168.30 (2.66) | 95.92 (3.78) | 135.67 (2.67) | 129.18 (2.53) | 71.54 (1.34) | 111.07 (2.61) |
| mean(SE) CWTrad (μm) | 4.33 (0.03) | 4.21 (0.06) | 4.84 (0.04) | 3.78 (0.04) | 2.87 (0.02) | 4.46 (0.05) | |
| mean(SE) CD/CWT | 6.51 (0.11) | 3.62 (0.13) | 4.48 (0.09) | 5.41 (0.08) | 4.17 (0.08) | 4.16 (0.10) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palla, B.; Ladányi, M.; Cseke, K.; Buczkó, K.; Höhn, M. Wood Anatomical Traits Reveal Different Structure of Peat Bog and Lowland Populations of Pinus sylvestris L. in the Carpathian Region. Forests 2021, 12, 494. https://doi.org/10.3390/f12040494
Palla B, Ladányi M, Cseke K, Buczkó K, Höhn M. Wood Anatomical Traits Reveal Different Structure of Peat Bog and Lowland Populations of Pinus sylvestris L. in the Carpathian Region. Forests. 2021; 12(4):494. https://doi.org/10.3390/f12040494
Chicago/Turabian StylePalla, Balázs, Márta Ladányi, Klára Cseke, Krisztina Buczkó, and Mária Höhn. 2021. "Wood Anatomical Traits Reveal Different Structure of Peat Bog and Lowland Populations of Pinus sylvestris L. in the Carpathian Region" Forests 12, no. 4: 494. https://doi.org/10.3390/f12040494
APA StylePalla, B., Ladányi, M., Cseke, K., Buczkó, K., & Höhn, M. (2021). Wood Anatomical Traits Reveal Different Structure of Peat Bog and Lowland Populations of Pinus sylvestris L. in the Carpathian Region. Forests, 12(4), 494. https://doi.org/10.3390/f12040494









