Composition and Specialization of the Lichen Functional Traits in a Primeval Forest—Does Ecosystem Organization Level Matter?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
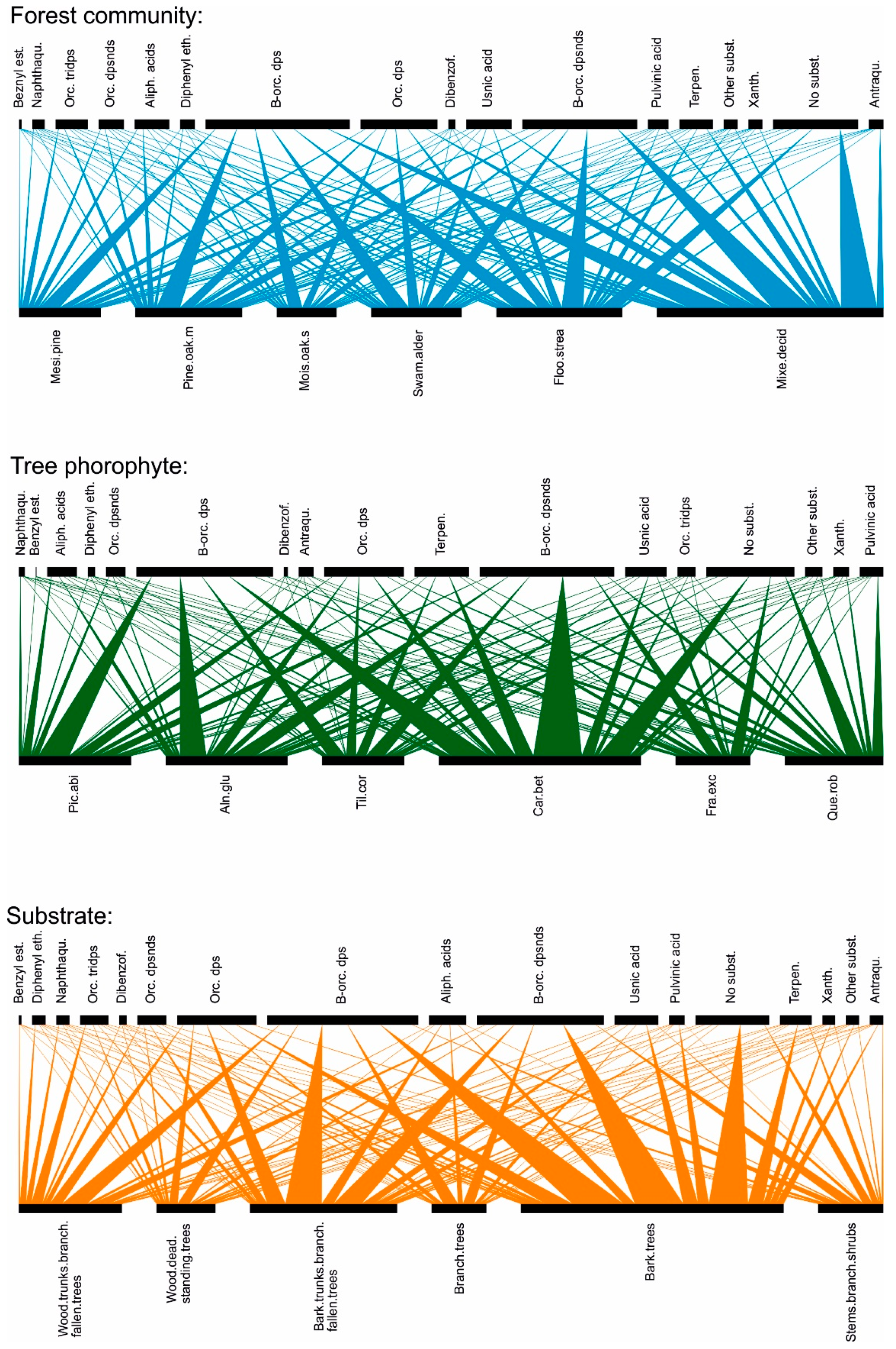
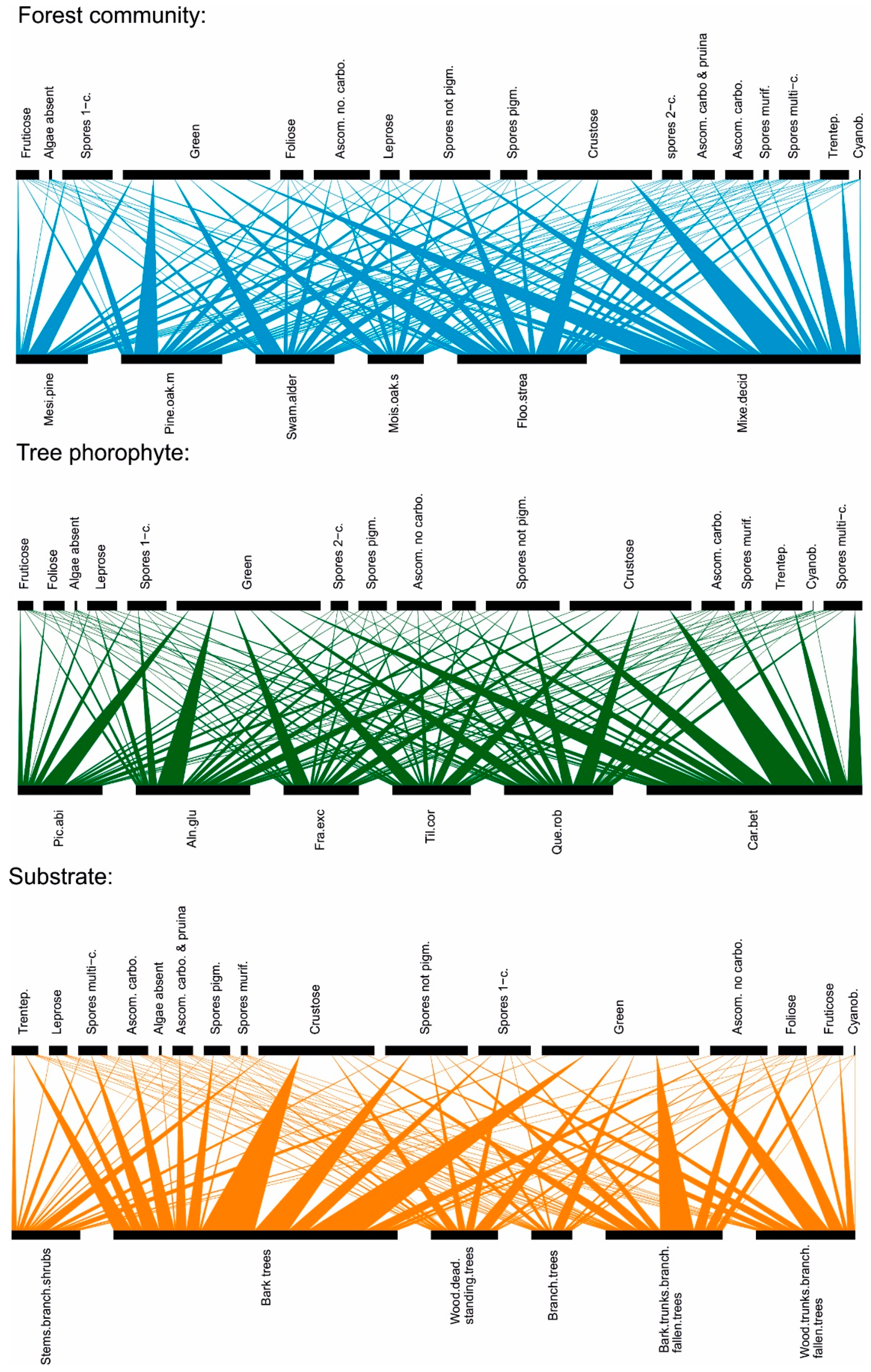
2.2. Functional Traits
2.2.1. Morphological and Anatomical Traits Considered:
- Thallus type (leprose, crustose, foliose, fruticose). Leprose and crustose lichen thalli (microlichens) and foliose and fruticose lichen thalli (macrolichens) were distinguished. Lichens with the placodioid thallus type were included in crustose lichens, and filamentous types (e.g., Usnea) in fruticose lichens. The morphological thallus types were used in determining the sensitivity of lichens to external factors and changes in the environment (bioindicator of environmental quality [2]), with crustose lichens being more resistant and fruticose lichens being the most sensitive [49].
- Photobiont type. Four groups of species were distinguished: lichens containing green algae (Asterochloris, Trebouxia, Stichococcus and others), lichens with Trentepohlia s.l., lichens with cyanobacteria (Nostoc) and non-lichenized taxa lacking photobionts commonly examined by lichenologists, i.e., species of Chaenothecopsis, Microcalicium and Mycocalicium. Trebouxia and other green algae are the most common photobionts in lichen symbioses [62,63], and they can colonize numerous environments and tolerate dry and insolated conditions [64]. Trentepohlia photobionts have a particular adaptation to environments with higher temperatures and humidity levels [65,66]. Cyanobacterial photobionts are desiccation-tolerant, but most of them require liquid water for rehydration [63,64].
- Ascospore dark pigmentation (ascospores that are dark-pigmented; ascospores that are not pigmented). The wall of ascospores can include pigments and melanins, which may relate to specific microhabitats [8], e.g., habitats of higher insolation to which species with dark ascospores are adapted [8,11].
- Ascospore septation (ascospores that are one-celled, two-celled, multi-celled with transverse septa only or muriform). This trait is probably connected with lichen dispersion and specialization to specific microhabitats [31], e.g., one-celled or two-celled ascospores are dominant in harsher environmental conditions such as higher insolation and temperature fluctuations, while multi-celled ascospores are dominant in milder environments [11].
- Ascomata texture and pigmentation (ascomata without carbonized structures, ascomata with carbonized structures and/or ascomata with pruina). Dark pigments and melanins present in fungal cells protect ascomata against high solar irradiation and photoinhibition, and dark fruitbodies probably occur more often in environments with high levels of abiotic stress [67]. The presence of pruina, mainly on apothecia, is a physical protection against excessive light or other extreme environmental conditions [68].
2.2.2. Reproduction Traits
- Ascomata type (generative structures formed by the fungus itself). Six types of ascomata, and their modifications, were distinguished: lecanoroid ascomata (i.e., with a thalline margin), lecideoid ascomata (with no algal cells in the margin), arthonioid ascomata, lirellate ascomata, stalked apothecia and perithecia [50,51]. The sexual reproduction of the fungal partner (mycobiont) ensures the retainment of population genetic variability, allowing adaptation to new and changing habitat conditions [49,63,69]. Ascopores are able to disperse over long distances [70].
- Asexual reproduction of mycobionts (pycnidia, hyphophores and sporodochia). Many species produce conidia or other structures, which are probably important for the effective distribution of mycobiont partners over long distances [10].
- Asexual reproduction type of both bionts (mycobiont and photobiont) by vegetative diaspores. Soredia and isidia were distinguished as the most common among lichens. Granules present in some lichens (e.g., Chrysothrix candelaris and Lepraria species) are included in the soredia category. Phyllidia (present only in Peltigera praetextata in our study) are included in the isidia category. Vegetative propagules are the fast and efficient mode of co-dispersal of compatible partners [49,63,71], and their production seems to be an adaptation to stable habitats [69,70,72,73].
2.2.3. Chemical (Lichen Secondary Metabolites) Traits
2.3. Data Analysis
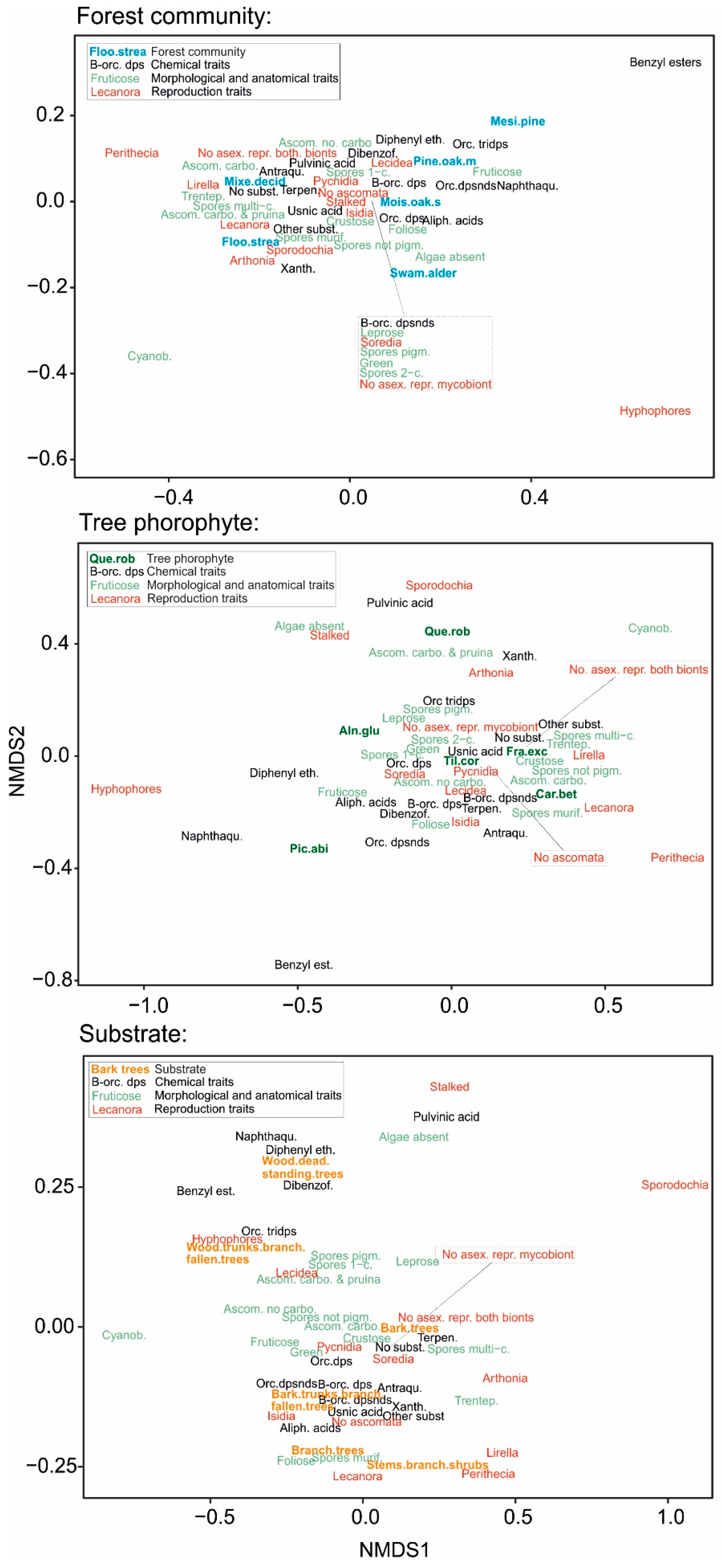
3. Results
4. Discussion
4.1. Possible Limitations of the Study
4.2. Specialization to Substrates
4.3. Specialization to Tree Species
4.4. Specialization to Forest Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Svoboda, D.; Peksa, O.; Veselá, J. Epiphytic lichen diversity in central European oak forests: Assessment of the influence of natural environmental factors and human influences. Environ. Pollut. 2010, 158, 812–819. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecol. Indic. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Lelli, C.; Bruun, H.H.; Chiarucci, A.; Donati, D.; Frascaroli, F.; Fritz, Ö.; Goldberg, I.; Nascimbene, J.; Tøttrup, A.P.; Rahbek, C.; et al. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manag. 2019, 432, 707–717. [Google Scholar] [CrossRef]
- Johansson, P.; Gustafsson, L. Red-listed and indicator lichens in woodland key habitats and production forests in Sweden. Can. J. For. Res. 2001, 31, 1617–1628. [Google Scholar] [CrossRef]
- Campbell, J.; Fredeen, A.L. Lobaria pulmonaria abundance as an indicator of macrolichen diversity in interior cedar-hemlock forests of east-central British Columbia. Can. J. Bot. 2004, 82, 970–982. [Google Scholar] [CrossRef]
- Nascimbene, J.; Brunialti, G.; Ravera, S.; Frati, L.; Caniglia, G. Testing Lobaria pulmonaria (L.) Hoffm. as an indicator of lichen conservation importance of Italian forests. Ecol. Indic. 2010, 10, 353–360. [Google Scholar] [CrossRef]
- Nascimbene, J.; Nimis, P.L.; Dainese, M. Epiphytic lichen conservation in the Italian Alps: The role of forest type. Fungal Ecol. 2014, 11, 164–172. [Google Scholar] [CrossRef]
- Bässler, C.; Cadotte, M.W.; Beudert, B.; Heibl, C.; Blaschke, M.; Bradtka, J.H.; Langbehn, T.; Werth, S.; Müller, J. Contrasting patterns of lichen functional diversity and species richness across an elevation gradient. Ecography 2016, 39, 689–698. [Google Scholar] [CrossRef]
- Benítez, A.; Aragón, G.; González, Y.; Prieto, M. Functional traits of epiphytic lichens in response to forest disturbance and as predictors of total richness and diversity. Ecol. Indic. 2018, 86, 18–26. [Google Scholar] [CrossRef]
- Malíček, J.; Palice, Z.; Vondrák, J.; Kostovčík, M.; Lenzová, V.; Hofmeister, J. Lichens in old-growth and managed mountain spruce forests in the Czech Republic: Assessment of biodiversity, functional traits and bioindicators. Biodivers. Conserv. 2019, 28, 3497–3528. [Google Scholar] [CrossRef]
- Soto-Medina, E.; Lücking, R.; Silverstone-Sopkin, P.A.; Torres, A.M. Changes in functional and taxonomic diversity and composition of corticolous lichens in an altitudinal gradient in Colombia. Cryptogam. Mycol. 2019, 40, 97–115. [Google Scholar] [CrossRef]
- Koch, N.M.; Martins, S.M.A.; Lucheta, F.; Müller, S.C. Functional diversity and traits assembly patterns of lichens as indicators of successional stages in a tropical rainforest. Ecol. Indic. 2013, 34, 22–30. [Google Scholar] [CrossRef]
- Hurtado, P.; Prieto, M.; Martínez-Vilalta, J.; Giordani, P.; Aragón, G.; López-Angulo, J.; Košuthová, A.; Merinero, S.; Díaz-Peña, E.M.; Rosas, T.; et al. Disentangling functional trait variation and covariation in epiphytic lichens along a continent-wide latitudinal gradient. Proc. R. Soc. B 2020, 287, 20192862. [Google Scholar] [CrossRef]
- Stofer, S.; Bergamini, A.; Aragón, G.; Carvalho, P.; Coppins, B.J.; Davey, S.; Dietrich, M.; Farkas, E.; Kärkkäinen, K.; Keller, C.; et al. Species richness of lichen functional groups in relation to land use intensity. Lichenologist 2006, 38, 331–353. [Google Scholar] [CrossRef]
- Pinho, P.; Bergamini, A.; Carvalho, P.; Branquinho, C.; Stofer, S.; Scheidegger, C.; Maguas, C. Lichen functional groups as ecological indicators of the effects of land-use in Mediterranean ecosystems. Ecol. Indic. 2012, 15, 36–42. [Google Scholar] [CrossRef]
- Giordani, P.; Rizzi, G.; Caselli, A.; Modenesi, P.; Malaspina, P.; Mariotti, M.G. Fire affects the functional diversity of epilithic lichen communities. Fungal Ecol. 2016, 20, 49–55. [Google Scholar] [CrossRef]
- Zarabska-Bożejewicz, D.; Kujawa, K. The effect of land use on taxonomical and functional diversity of lichens in an agricultural landscape. Fungal Ecol. 2018, 33, 72–79. [Google Scholar] [CrossRef]
- Fritz, O.; Gustafsson, L.; Larsson, K. Does forest continuity matter in conservation? A study of epiphytic lichens and bryophytes in beech forests of southern Sweden. Biol. Conserv. 2008, 141, 655–668. [Google Scholar] [CrossRef]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe–A review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Wolseley, P.; Sanderson, N.; Thüs, H.; Carpenter, D.; Eggleton, P. Patterns and drivers of lichen species composition in a NW-European lowland deciduous woodland complex. Biodivers. Conserv. 2017, 26, 401–419. [Google Scholar] [CrossRef]
- Hofmeister, J.; Hošek, J.; Brabec, M.; Hermy, M.; Dvořák, D.; Fellner, R.; Malíček, J.; Palice, Z.; Tenčík, A.; Holá, E.; et al. Shared affinity of various forest-dwelling taxa point to the continuity of temperate forests. Ecol. Indic. 2019, 101, 904–912. [Google Scholar] [CrossRef]
- Rounsevell, M.; Fischer, M.; Torre-Marin Rando, A.; Mader, A. The IPBES Regional Assessment Report on Biodiversity and Ecosystem Services for Europe and Central Asia; Bonn, Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES): Bonn, Germany, 2018. [Google Scholar]
- Aragón, G.; Martínez, I.; García, A. Loss of epiphytic diversity along a latitudinal gradient in southern Europe. Sci. Total Environ. 2012, 426, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jüriado, I.; Paal, J. Epiphytic lichen synusiae and functional trait groups in boreo-nemoral deciduous forests are influenced by host tree and environmental factors. Nord. J. Bot. 2019, 37, 01939. [Google Scholar] [CrossRef]
- Giordani, P.; Incerti, G.; Rizzi, G.; Rellini, I.; Nimis, P.L.; Modenesi, P. Functional traits of cryptogams in Mediterranean ecosystems are driven by water, light and substrate interactions. J. Veg. Sci. 2014, 25, 778–792. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L. Epiphytic lichen diversity along elevational gradients: Biological traits reveal a complex response to water and energy. J. Biogeogr. 2015, 42, 1222–1232. [Google Scholar] [CrossRef]
- Keczyński, A. The Forests of the Strict Reserve of Białowieża National Park; Białowieski Park Narodowy: Białowieża, Poland, 2017; pp. 1–304. [Google Scholar]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.M.; Samojlik, T.; Zimny, M.; Latałowa, M. Białowieża Forest–A relic of the high naturalness of European forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef]
- Łubek, A.; Kukwa, M.; Jaroszewicz, B.; Czortek, P. Identifying mechanisms shaping lichen functional diversity in a primeval forest. For. Ecol. Manag. 2020, 475, 118434. [Google Scholar] [CrossRef]
- Pentecost, A. Some observations on the size and shape of lichen ascospores in relation to ecology and taxonomy. New Phytol. 1981, 89, 667–678. [Google Scholar] [CrossRef]
- Dietrich, M.; Scheidegger, C. Frequency, diversity and ecological strategies of epiphytic lichens in the Swiss Central Plateau and the Pre-Alps. Lichenologist 1997, 29, 237–258. [Google Scholar] [CrossRef]
- Ellis, C.J.; Coppins, B.J. Contrasting functional traits maintain lichen epiphyte diversity in response to climate and autogenic succession. J. Biogeogr. 2006, 33, 1643–1656. [Google Scholar] [CrossRef]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits; John Wiley and Sons, Ltd.: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Żarnowiecki, G. Związki pomiędzy pokrywą śnieżną a roślinnością Białowieskiego Parku Narodowego. Prace Geogr. 2008, 126, 67–87. [Google Scholar]
- Parviainen, J. Virgin and natural forests in the temperate zone of Europe. For. Snow Landsc. Res. 2005, 79, 9–18. [Google Scholar]
- Łubek, A.; Kukwa, M.; Jaroszewicz, B.; Czortek, P. Changes in the epiphytic lichen biota of Białowieża Primeval Forest are not explained by climate warming. Sci. Total Environ. 2018, 643, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Łubek, A.; Kukwa, M.; Czortek, P.; Jaroszewicz, B. Lichenicolous fungi are more specialized than their lichen hosts in primeval forest ecosystems, Białowieża Forest, northeast Poland. Fungal Ecol. 2019, 42, 100866. [Google Scholar] [CrossRef]
- Łubek, A.; Kukwa, M.; Czortek, P.; Jaroszewicz, B. Impact of Fraxinus excelsior dieback on biota of ash-associated lichen epiphytes at the landscape and community level. Biodivers. Conserv. 2020, 29, 431–450. [Google Scholar] [CrossRef]
- Cieśliński, S.; Czyżewska, K.; Faliński, J.B.; Klama, H.; Mułenko, W.; Żarnowiec, J. Relicts of the primeval (virgin) forest. Relict phenomena. In Cryptogamous Plants in the Forest Communities of Białowieża National Park (Project CRYPTO 3); Faliński, J.B., Mułenko, W., Eds.; Polish Botanical Society: Warsaw, Poland, 1996; Volume 6, pp. 197–216. [Google Scholar]
- Faliński, J.B. Phytophenological atlas of the forest communities and species of Bałowieża National Park. Phytocoen. Arch. Geobot. 2001, 8, 1–160. [Google Scholar]
- Barkman, J.J. Phytosociology and Ecology of Cryptogamic Epiphytes; Van Gorcum and Company: Assen, The Netherlands, 1958. [Google Scholar]
- Ratyńska, H.; Wojterska, M.; Brzeg, A.; Kołacz, M. Multimedialna Encyklopedia Zbiorowisk Roślinnych Polski; Instytut Edukacyjnych Technologii Informatycznych: Bydgoszcz, Poland, 2010. [Google Scholar]
- Fałtynowicz, W.; Kossowska, M. The lichens of Poland. A fourth checklist. Acta Bot. Sil. Monogr. 2016, 8, 3–122. [Google Scholar]
- Sérusiaux, E.; Brand, A.M.; Motiejūnaitė, J.; Orange, A.; Coppins, B.J. Lecidea doliiformis belongs to Micarea, Catillaria alba to Biatora, and Biatora ligni-mollis occurs in Western Europe. Bryologist 2010, 113, 333–344. [Google Scholar] [CrossRef]
- Czarnota, P.; Guzow-Krzemińska, B. Bacidina mendax sp. nov., a new widespread species in Central Europe, together with a new combination within the genus Bacidina. Lichenologist 2018, 50, 43–57. [Google Scholar] [CrossRef]
- Ertz, D.; Sanderson, N.; Łubek, A.; Kukwa, M. Two new species of Arthoniaceae from old-growth European forests: Arthonia thoriana and Inoderma sorediatum, and a new genus for Schismatomma niveum. Lichenologist 2018, 50, 161–172. [Google Scholar] [CrossRef]
- Boluda, C.G.; Rico, V.J.; Divakar, P.K.; Nadyeina, O.; Myllys, L.; McMullin, R.T.; Zamora, J.C.; Scheidegger, C.; Hawksworth, D.L. Evaluating methodologies for species delimitation: The mismatch between phenotypes and genotypes in lichenized fungi (Bryoria sect. Implexae, Parmeliaceae). Persoonia 2019, 42, 75–100. [Google Scholar] [CrossRef]
- Purvis, W. Lichens; Smithsonian Institute Press: Washington, DC, USA, 2000; pp. 1–112. [Google Scholar]
- Smith, C.W.; Aptroot, A.; Coppins, R.J.; Fletcher, A.; Gilbert, O.L.; Lames, P.W.; Wolseley, P.A. The Lichens of Great Britain and Ireland; The British Lichen Society: London, UK, 2009; pp. 1–1046. [Google Scholar]
- Wirth, V.; Hauck, M.; Schultz, M. Die Flechten Deutschlands; Ulmer: Stuttgart, Germany, 2013; Volume 1, pp. 1–1244. [Google Scholar]
- Aptroot, A.; Diederich, P.; van Herk, C.M.; Spier, L.; Wirth, V. Protoparmelia hypotremella, a new sterile corticolous species from Europe, and its lichenicolous fungi. Lichenologist 1997, 29, 415–424. [Google Scholar] [CrossRef]
- Divakar, P.K.; Molina, M.C.; Lumbsch, H.T.; Crespo, A. Parmelia barrenoae, a new lichen species related to Parmelia sulcata (Parmeliaceae) based on molecular and morphological data. Lichenologist 2005, 37, 37–46. [Google Scholar] [CrossRef][Green Version]
- Czarnota, P. The Lichen Genus Micarea (Lecanorales, Ascomycota) In Poland. Pol. Bot. Stud. 2007, 23, 1–199. [Google Scholar]
- Czarnota, P.; Guzow-Krzemińska, B. A phylogenetic study of the Micarea prasina group shows that Micarea micrococca includes three distinct lineages. Lichenologist 2010, 42, 7–21. [Google Scholar] [CrossRef]
- Kukwa, M. The Lichen Genus Ochrolechia in Europe; Fundacja Rozwoju Uniwersytetu Gdańskiego, Gdańsk: Gdansk, Poland, 2011; pp. 1–308. ISBN 978-83-7531-170-9. [Google Scholar]
- Palice, Z.; Printzen, C.; Spribille, T.; Elix, J.A. Notes on the synonyms of Lecanora filamentosa. Graph. Scr. 2011, 23, 1–7. [Google Scholar]
- Guzow-Krzemińska, B.; Czarnota, P.; Łubek, A.; Kukwa, M. Micarea soralifera sp. nov., a new sorediate species in the M. prasina group. Lichenologist 2016, 48, 161–169. [Google Scholar] [CrossRef]
- Guzow-Krzemińska, B.; Łubek, A.; Malíček, J.; Tønsberg, T.; Oset, M.; Kukwa, M. Lecanora stanislai, a new, sterile, usnic acid containing lichen species from Eurasia and North America. Phytotaxa 2017, 329, 201–211. [Google Scholar] [CrossRef]
- Malíček, J.; Palice, Z.; Vondrák, J.; Łubek, A.; Kukwa, M. Bacidia albogranulosa (Ramalinaceae, lichenized Ascomycota), a new sorediate lichen from European old-growth forests. MycoKeys 2018, 44, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Launis, A.; Malíček, J.; Svensson, M.; Tsurykau, A.; Sérusiaux, E.; Myllys, L. Sharpening species boundaries in the Micarea prasina group, with a new circumscription of the type species M. prasina. Mycologia 2019, 11, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Friedl, T.; Büdel, B. Photobionts. In Lichen Biology; Nash, T.H., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 9–26. [Google Scholar]
- Honegger, R. The symbiotic phenotype of lichen-forming Ascomycetes and their endo–and epibionts. In The Mycota Fungal Associations, 2nd ed.; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 288–339. [Google Scholar]
- Saini, K.C.; Nayaka, S.; Bast, F. Diversity of lichen photobionts: Their coevolution and bioprospecting potential. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Satyanarayana, T., Johri, B.N., Das, S.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 307–323. [Google Scholar] [CrossRef]
- Hametner, C.; Stocker-Wörgötter, E.; Grube, M. New insights into diversity and selectivity of Trentepohlialean lichen photobionts from the extratropics. Symbiosis 2014, 63, 31–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kosecka, M.; Jabłońska, A.; Flakus, A.; Rodriguez-Flakus, P.; Kukwa, M.; Guzow-Krzemińska, B. Trentepohlialean algae (Trentepohliales, Ulvophyceae) show preference to selected mycobiont lineages in lichen symbioses. J. Phycol. 2020, 56, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Mafole, T.C.; Solhaug, K.A.; Minibayeva, F.V.; Beckett, R.P. Occurrence and possible roles of melanic pigments in lichenized ascomycetes. Fungal Biol. Rev. 2019, 33, 159–165. [Google Scholar] [CrossRef]
- Koch, N.M.; Matos, P.; Branquinho, C.; Pinho, P.; Lucheta, F.; Martins, S.M.A.; Vargas, V.M.F. Selecting lichen functional traits as ecological indicators of the effects of urban environment. Sci. Total Environ. 2019, 654, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Buschbom, J.; Mueller, G.M. Testing “species pair” hypotheses: Evolutionary processes in the lichen-forming species complex Porpidia flavocoerulescens and Porpidia melinodes. Mol. Biol. Evol. 2006, 23, 574–586. [Google Scholar] [CrossRef]
- Ronnås, C.; Werth, S.; Ovaskainen, O.; Várkonyi, G.; Scheidegger, C.; Snäll, T. Discovery of long-distance gamete dispersal in a lichen-forming ascomycete. New Phytol. 2017, 216, 216–226. [Google Scholar] [CrossRef]
- Nimis, P.L.; Martellos, S. On the ecology of sorediate lichens in Italy. Bibl. Lichenol. 2003, 86, 393–406. [Google Scholar]
- Ellis, C.J.; Coppins, B.J. Reproductive strategy and the compositional dynamics of crustose lichen communities on aspen (Populus tremula L.) in Scotland. Lichenologist 2007, 39, 377–391. [Google Scholar] [CrossRef]
- Ertz, D.; Guzow-Krzemińska, B.; Thor, G.; Łubek, A.; Kukwa, M. Photobiont switching causes changes in the reproduction strategy and phenotypic dimorphism in the Arthoniomycetes. Sci. Rep. 2018, 8, 4952. [Google Scholar] [CrossRef]
- Rundel, P.W. The ecological role of secondary lichen substances. Biochem. Syst. Ecol. 1978, 6, 157–170. [Google Scholar] [CrossRef]
- Orange, A.; James, P.W.; White, F.J. Microchemical Methods for the Identification of Lichens; British Lichen Society: London, UK, 2001; pp. 1–101. [Google Scholar]
- Elix, J.A. A Catalogue of Standardized Chromatographic Data and Biosynthetic Relationships for Lichen Substance. Available online: https://www.anbg.gov.au/abrs/lichenlist/Chem%20Cat%203.pdf (accessed on 19 January 2020).
- Molnár, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z. Nat. C 2010, 65, 3–4. [Google Scholar] [CrossRef]
- Galun, M.; Shomer-Ilan, A. Secondary metabolic products. In CRC Handbook of Lichenology; Galun, M., Ed.; CRC Press: Boca Raton, FL, USA, 1988; Volume 3, pp. 1–148. [Google Scholar]
- Rikkinen, J. What’s behind the pretty colours? A study on the photobiology of lichens. Bryobrothera 1995, 4, 8–227. [Google Scholar]
- Luo, H.; Yamamoto, Y.; Kim, J.A.; Jung, J.S.; Koh, Y.J.; Hur, J.-S. Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Polar Biol. 2009, 32, 1033–1040. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fruend, J.; Gruber, B. Package ‘bipartite’ – Visualising Bipartite Networks and Calculating Some (Ecological) Indices. 2020. Available online: https://cran.r-project.org/web/packages/bipartite/bipartite.pdf (accessed on 19 January 2020).
- Poisot, T.; Lepennetier, G.; Martinez, E.; Ramsayer, J.; Hochberg, M.E. Resource availability affects the structure of a natural bacteria-bacteriophage community. Biol. Lett. 2011, 7, 201–204. [Google Scholar] [CrossRef]
- Poisot, T.; Bever, J.D.; Nemri, A.; Thrall, P.H.; Hochberg, M.E. A conceptual framework for the evolution of ecological specialisation. Ecol. Lett. 2011, 14, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Poisot, T.; Canard, E.; Mouquet, N.; Hochberg, M.E. A comparative study of ecological specialization estimators. Methods Ecol. Evol. 2012, 3, 537–544. [Google Scholar] [CrossRef]
- Schoener, T.W. Food webs from the small to the large. Ecology 1989, 70, 1559–1589. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Julliard, R.; Clavel, J.; Devictor, V.; Jiguet, F.; Couvet, D. Spatial segregation of specialists and generalists in bird communities. Ecol. Lett. 2006, 9, 1237–1244. [Google Scholar] [CrossRef]
- De Mendiburu, F. Package ‘agricolae’. Statistical Procedures for Agricultural Research. 2020. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 19 January 2020).
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.D. Arguments for rejecting the sequential bonferroni in ecological studies. Oikos 2003, 100, 403–405. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘vegan’—Community Ecology Package. 2018. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 19 January 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Winemiller, K.O.; Fitzgerald, D.B.; Bower, L.M.; Pianka, E.R. Functional traits, convergent evolution, and periodic tables of niches. Ecol. Lett. 2015, 18, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.M.; Mason, C.M.; Medeiros, J.S. The evolution of functional traits in plants: Is the giant still sleeping? Int. J. Plant. Sci. 2020, 181, 1–8. [Google Scholar] [CrossRef]
- Garty, J.; Galun, M. Selectivity in lichen-substrate relationships. Flora 1971, 163, 530–534. [Google Scholar] [CrossRef]
- Zúñiga, C.; Leiva, D.; Carú, M.; Orlando, J. Substrates of Peltigera lichens as a potential source of Cyanobionts. Microbial Ecol. 2017, 74, 561–569. [Google Scholar] [CrossRef]
- Resl, P.; Fernández-Mendoza, F.; Mayrhofer, H.; Spribille, T. The evolution of fungal substrate specificity in a widespread group of crustose lichens. Proc. R. Soc. B 2018, 285, 20180640. [Google Scholar] [CrossRef]
- Cieśliński, S.; Czyżewska, K.; Fabiszewski, J. Red list of the lichens in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szeląg, Z., Eds.; W. Szafer Institute of Botany, PASc: Kraków, Poland, 2006; pp. 71–90. [Google Scholar]
- Motiejūnaitė, J.; Czyżewska, K.; Cieśliński, S. Lichens–Indicators of old-growth forests in biocentres of Lithuania and north-east Poland. Bot. Lith. 2004, 10, 59–74. [Google Scholar]
- Tibell, L. Crustose lichens as indicators of forest continuity in boreal coniferous forests. Nord. J. Bot. 1992, 12, 427–450. [Google Scholar] [CrossRef]
- Holien, H. Influence of site and stand factors on the distribution of crustose lichens of the Caliciales in a suboceanic spruce forest area. Lichenologist 1996, 28, 315–330. [Google Scholar] [CrossRef]
- Spribille, T.; Bunnell, F.L.; Thor, G.; Goward, T.; Björk, C.R. Lichens on dead wood: Species-substrate relationships in the epiphytic lichen floras of the Pacific Northwest and Fennoscandia. Ecography 2008, 31, 741–750. [Google Scholar] [CrossRef]
- Johansson, V.; Snäll, T.; Ranius, T. Epiphyte metapopulation dynamics are explained by species traits, connectivity and patch dynamics. Ecology 2012, 93, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Sanders, W.B.; Lücking, R. Reproductive strategies, relichenization and thallus development observed in situ in leaf-dwelling lichen communities. New Phytol. 2002, 155, 425–435. [Google Scholar] [CrossRef]

| Functional Trait | Trait Id | Forest Community | Tree Phorophyte | Substrate |
|---|---|---|---|---|
| Chemical traits (lichen secondary metabolites) | ||||
| Aliphatic acids | Aliph. acids | 0.384 | 0.491 | 0.568 |
| Antraquinoses | Antraqu. | 0.754 | 0.744 | 0.848 |
| Beta-orcinol depsides | B-orc.dps | 0.542 | 0.481 | 0.606 |
| Beta-orcinol depsinodes | B-orc.dpsnds | 0.611 | 0.643 | 0.674 |
| Benzyl esters | Benzyl est. | 0.585 | 0.840 | 0.742 |
| Dibenzofurans | Dibenzof. | 0.587 | 0.506 | 0.708 |
| Diphenyl ethers | Diphenyl eth. | 0.561 | 0.600 | 0.748 |
| Naphthaquinones | Naphthaqu. | 0.379 | 0.797 | 0.709 |
| No substances | No subst. | 0.725 | 0.634 | 0.823 |
| Orcinol depsides | Orc. dps | 0.522 | 0.368 | 0.612 |
| Orcinol depsinodes | Orc. dpsnds | 0.464 | 0.540 | 0.537 |
| Orcinol tridepsides | Orc. tridps | 0.473 | 0.384 | 0.679 |
| Other substances | Other subst. | 0.669 | 0.672 | 0.803 |
| Pulvinic acid deriverates | Pulvinic acid | 0.634 | 0.768 | 0.911 |
| Terpenoids | Terpen. | 0.661 | 0.568 | 0.782 |
| Usnic acid deriverates | Usnic acid | 0.560 | 0.500 | 0.608 |
| Xanthones | Xanth. | 0.642 | 0.502 | 0.793 |
| Morphological and anatomical traits | ||||
| Photobiont type: | ||||
| Algae absent | Algae absent | 0.350 | 0.684 | 0.809 |
| Green | Green | 0.572 | 0.497 | 0.649 |
| Cyanobacteria | Cyanob. | 0.775 | 0.781 | 0.762 |
| Trentepohlia | Trentep. | 0.784 | 0.683 | 0.906 |
| Ascospore dark pigmentation: | ||||
| Spores not pigmented | Spores not pigm. | 0.660 | 0.648 | 0.714 |
| Spores pigmented | Spores pigm. | 0.642 | 0.451 | 0.789 |
| Ascospore septation: | ||||
| Spores one-celled | Spores 1-c. | 0.582 | 0.531 | 0.672 |
| Spores two-celled | Spores 2-c. | 0.643 | 0.494 | NA |
| Spores multi-celled | Spores multi-c. | 0.753 | 0.675 | 0.855 |
| Spores muriform | Spores murif. | 0.660 | 0.773 | 0.587 |
| Thallus type: | ||||
| Crustose | Crustose | 0.663 | 0.636 | 0.768 |
| Foliose | Foliose | 0.510 | 0.459 | 0.649 |
| Fruticose | Fruticose | 0.398 | 0.559 | 0.494 |
| Leprose | Leprose | 0.623 | 0.364 | 0.867 |
| Ascomata texture and pigmentation: | ||||
| Ascomata with carbonized structures | Ascom. carbo. | 0.708 | 0.709 | 0.816 |
| Ascomata with carbonized structures + Ascomata with pruina | Ascom. carbo. and pruina | 0.625 | 0.497 | 0.818 |
| Ascomata without carbonized structures | Ascom. no carbo. | 0.641 | 0.654 | 0.628 |
| Reproduction traits | ||||
| Ascomata type: | ||||
| No ascomata | No ascomata | 0.702 | 0.439 | 0.675 |
| Arthonia | Arthonia | 0.551 | 0.476 | 0.925 |
| Lecanora | Lecanora | 0.726 | 0.804 | 0.671 |
| Lecidea | Lecidea | 0.566 | 0.540 | 0.576 |
| Lirella | Lirella | 0.793 | 0.727 | 0.888 |
| Stalked | Stalked | 0.503 | 0.687 | 0.849 |
| Perithecia | Perithecia | 0.880 | 0.919 | 0.877 |
| Asexual reproduction of mycobiont: | ||||
| No asexual reproduction of mycobiont | No asex. repr. mycobiont | 0.800 | 0.506 | 0.720 |
| Hyphophores | Hyphophores | 0.604 | 0.850 | 0.771 |
| Pycnidia | Pycnidia | 0.637 | 0.602 | 0.689 |
| Sporodochia | Sporodochia | 0.696 | 0.766 | 0.998 |
| Asexual reproduction of both bionts: | ||||
| No asexual reproduction of both bionts | No asex. repr. both bionts | 0.695 | 0.642 | 0.809 |
| Isidia | Isidia | 0.548 | 0.647 | 0.576 |
| Soredia | Soredia | 0.695 | 0.408 | 0.644 |
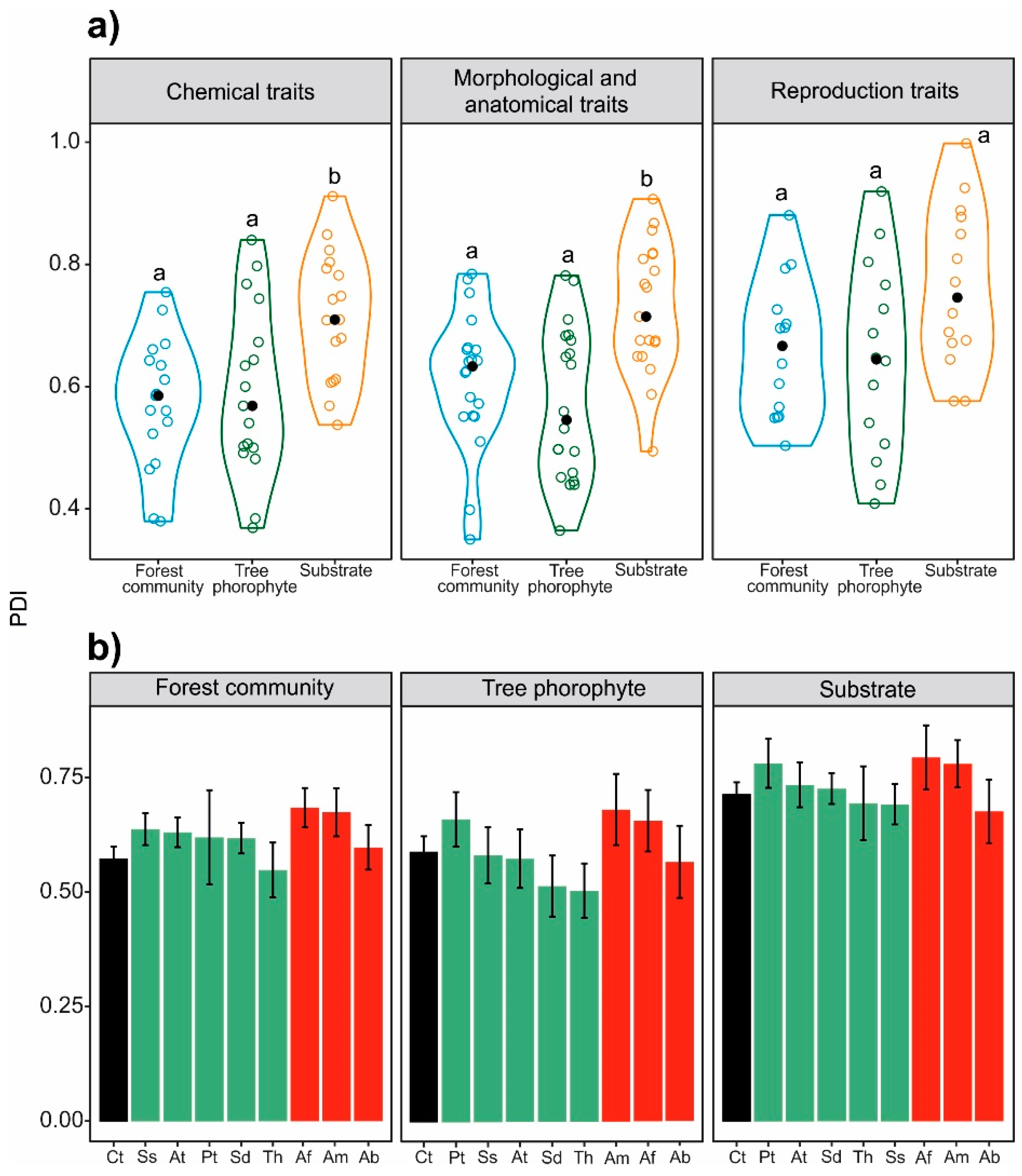
| Parameter | Forest Community | Tree Phorophyte | Substrate | ANOVA | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | F | p | |
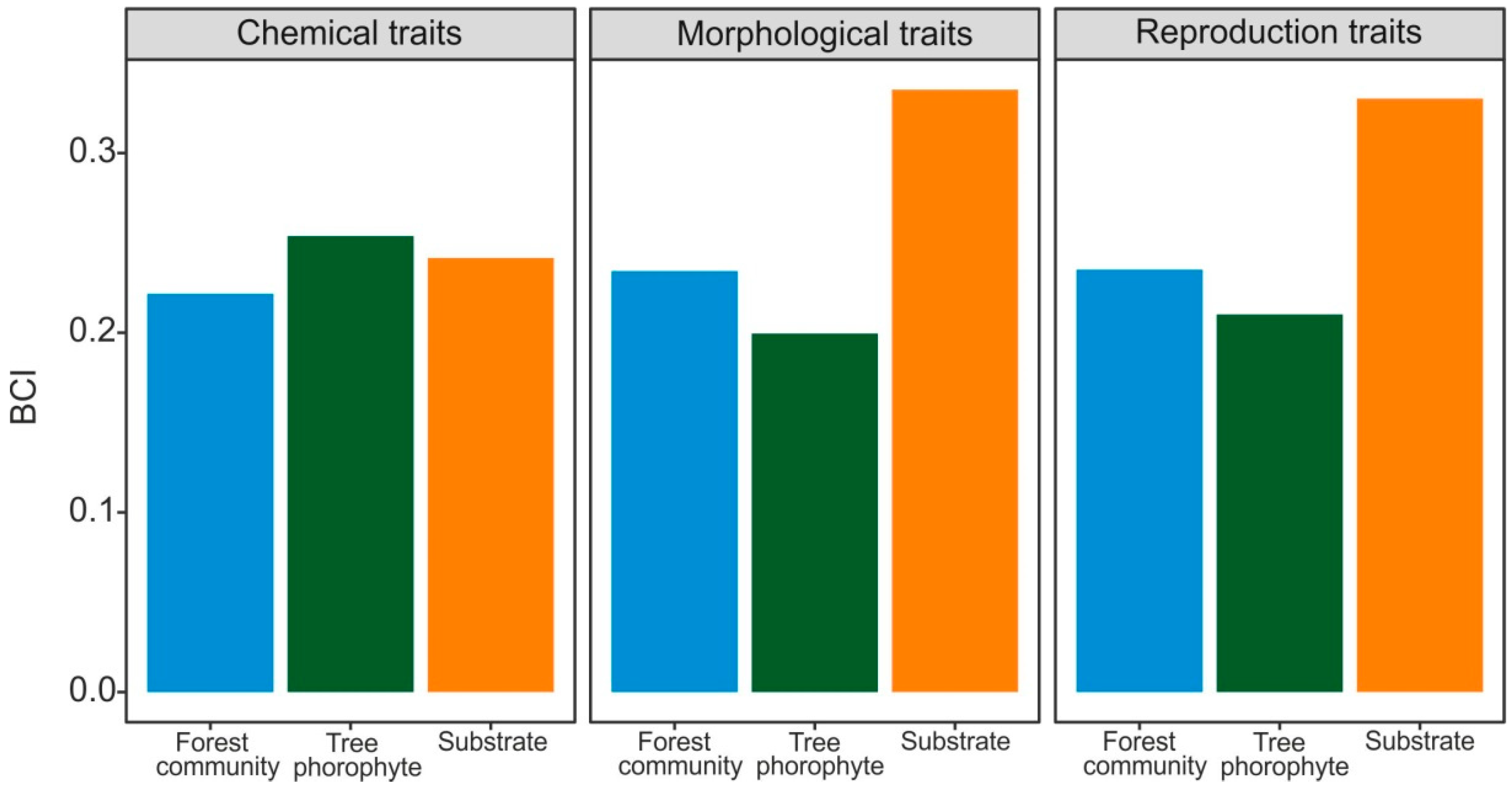
| Chemical traits | 0.574a | 0.106 | 0.590a | 0.139 | 0.715b | 0.105 | 7.212 | <0.01 |
| Morphological traits | 0.612a | 0.111 | 0.571a | 0.124 | 0.727b | 0.106 | 9.618 | <0.001 |
| Reproduction traits | 0.661a | 0.113 | 0.644a | 0.157 | 0.762a | 0.132 | 3.099 | 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łubek, A.; Kukwa, M.; Jaroszewicz, B.; Czortek, P. Composition and Specialization of the Lichen Functional Traits in a Primeval Forest—Does Ecosystem Organization Level Matter? Forests 2021, 12, 485. https://doi.org/10.3390/f12040485
Łubek A, Kukwa M, Jaroszewicz B, Czortek P. Composition and Specialization of the Lichen Functional Traits in a Primeval Forest—Does Ecosystem Organization Level Matter? Forests. 2021; 12(4):485. https://doi.org/10.3390/f12040485
Chicago/Turabian StyleŁubek, Anna, Martin Kukwa, Bogdan Jaroszewicz, and Patryk Czortek. 2021. "Composition and Specialization of the Lichen Functional Traits in a Primeval Forest—Does Ecosystem Organization Level Matter?" Forests 12, no. 4: 485. https://doi.org/10.3390/f12040485
APA StyleŁubek, A., Kukwa, M., Jaroszewicz, B., & Czortek, P. (2021). Composition and Specialization of the Lichen Functional Traits in a Primeval Forest—Does Ecosystem Organization Level Matter? Forests, 12(4), 485. https://doi.org/10.3390/f12040485








