Abstract
(1) Background: Cardiocrinum cordatum (Thunb.) Makino (Liliaceae) is a forest perennial herb distributed in East Asia. Although flower visitors for this plant species have been well reported, their contribution to pollination remains unknown. (2) Methods: We evaluated pollination contribution for visitors of C. cordatum flowers in a natural cool temperate forest. We investigated visiting frequency, the number of pollen grains per body surface, fruit set, and the mean number of seeds per fruit produced after a single visit of each visiting species. Combining the results of these experiments, we determined the most important pollinators of this species. (3) Results: For the population investigated in the study, the three most essential pollinators were the bumblebee (Bombus diversus tersatus) (Apidae), sweat bee (Halictidae sp.), and marmalade hoverfly (Episyrphus balteatus) (Syrphidae). Additionally, we found that the contribution of a flower-visiting ant species (Myrmica ruginodis Nylander (s.l.)) (Formicidae) is small. (4) Conclusions: Pollinator contributions differed among flower visitors. Our results underscore the insufficiency of current information about flower-visiting species to evaluate pollination contribution.
1. Introduction
The maintenance of genetic diversity is important for the conservation of wild plant species, and successful cross-pollination plays a major role in gene flow to maintain the genetic diversity of plant populations [1,2,3,4,5,6,7,8,9], especially when a population is fragmented [5,6,7,8,9,10]. Additionally, pollination is a central issue in studies on plant–animal interactions [11,12,13,14,15], plant reproductive biology [15,16,17,18,19,20], and on improvement of agricultural production [15,20,21,22,23,24,25,26,27,28]. For animal-pollinated flowers, pollination effectiveness differs among visitors of different species [3,5,12,13,14,15,19,29]. Some visitors obtain pollen or nectar from flowers without contributing as pollinators for some plant species, phenomena known as pollen or nectar theft [30], or nectar robbing for cases where a visitor damages a flower [31]. Therefore, the information on flower-visiting frequency may not be sufficient to evaluate the pollinator contribution of each animal species. Hence, it is necessary to identify which species significantly contribute as pollinators for specific plants.
Cardiocrinum cordatum (Thunb.) Makino (Liliaceae) is a temperate forest perennial herb distributed in East Asia. Although C. cordatum is still a common species in forest understories, most of the populations are highly fragmented due to the fragmentation of forests in lowland urban areas [32]. The situation is similar in the urban area of Obihiro city in Hokkaido, in which forests are fragmented into small pieces [33,34]. As the pollination process is critical for the maintenance of genetic diversity of fragmented populations [5,7,8,9,10], quantification of pollinator contribution is of utmost importance for future conservation of this species. Ohara et al. [32] suggested that the large nectar quantity and mild floral fragrance of C. cordatum attract pollinators; however, they did not investigate the contribution of flower visitors to pollination. In addition, they suggested that although this species is self-compatible, pollen limitation may also be present for this species. However, although flower visitors have been well documented for this species [32,35,36], their contribution to pollination has not been quantitatively investigated. Here, we examined the pollination contribution of each flower-visiting species using visiting frequency, the number of pollen grains on body surfaces, fruit set, and the mean number of seeds per fruit produced after a single visit in a wild plant population in a natural cool temperate forest in Hokkaido, Japan.
2. Materials and Methods
2.1. Study Species
Cardiocrinum cordatum (Thunb.) Makino (Liliaceae), including var. glehni, is a forest perennial herb of East Asia [32,37,38]. Hermaphroditic flowers (Figure 1a–e) have six stamens and one pistil and are self-compatible [7,32,39]. Although their basic structure is similar to lily flowers (e.g., Lilium auratum Lindl.), their flower tubes do not open as widely as those of L. auratum (Figure 1a–d). Seeds, contained in a capsule, have wings (Figure 1f,g) and are suggested to be dispersed by winds [32]. This species is classified as a monocarpic perennial; the entire aboveground part of a bolting rosette dies after producing seeds, often leaving (usually, one or two) small bulblets at the base of the parent bulbs [6,7,32,40,41,42,43]. Therefore, offspring from bulblets compensate for the loss of the parent plant after (apparently) monocarpic sexual reproduction, and occupy the same place as the parental plant [6,43]. Traditionally, the bulb of C. cordatum has been used as food [44,45].

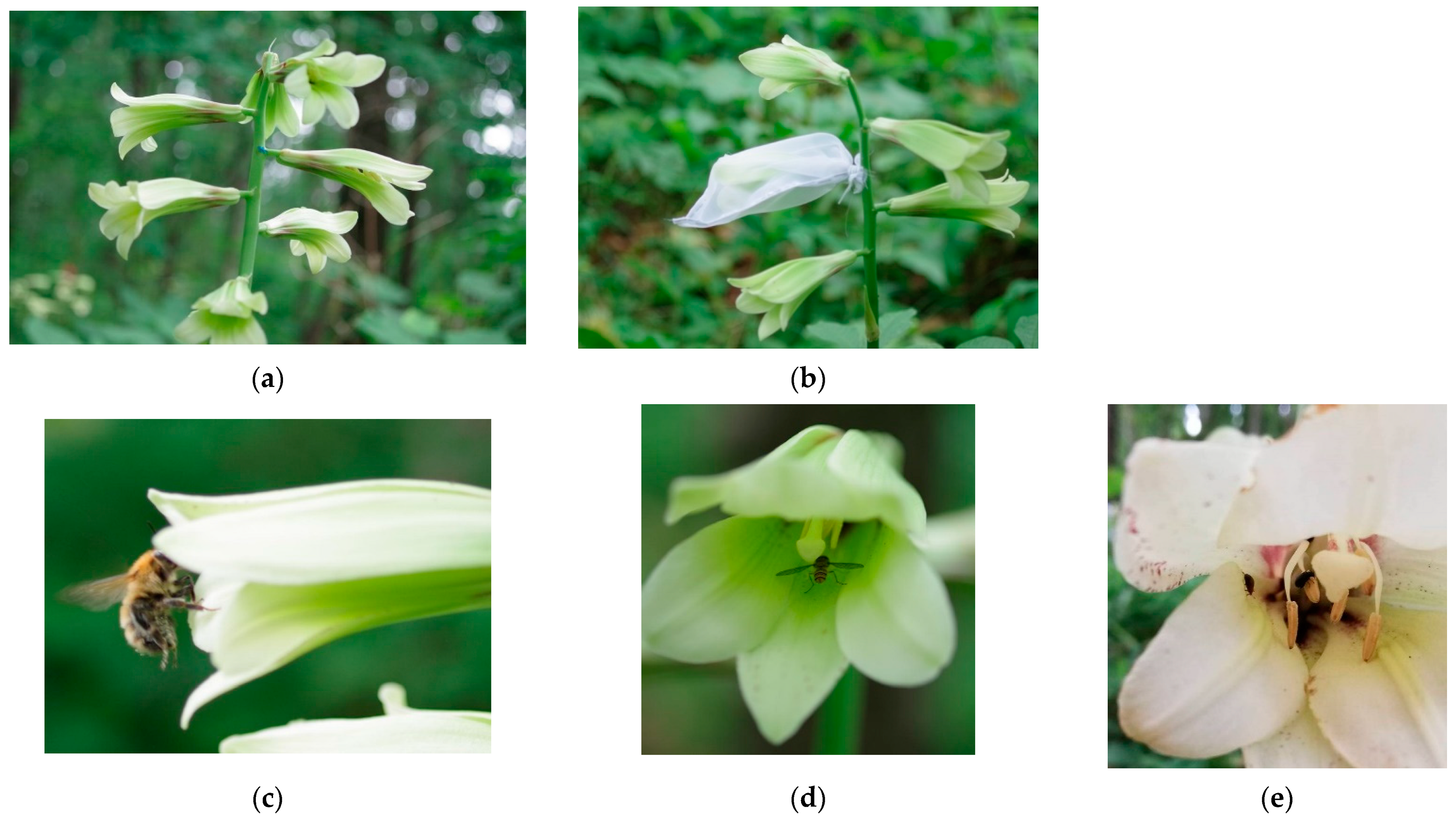
Figure 1.
Flowers of Cardiocrinum cordatum (Thunb.) Makino. (a) An inflorescence; (b) pollinator exclusion experiment using polyester bags; (c) a bumblebee (Bombus diversus tersatus); (d) a marmalade hoverfly (Episyrphus balteatus); (e) leaf beetles (Chrysomelidae sp.); (f,g) fruits and seeds. Photographs were taken at Hokkaido Obihiro Agricultural High School (a–e) in July and (f,g) in October, in 2020, by (a–d) Kohei Koyama and (e–g) Riko Komamura. The original high-resolution images are available as Supplementary Materials.
2.2. Study Site
The study was performed using a wild population in a natural cool temperate deciduous forest (45°52′ N 143°11′ E, altitude: 69 m a.s.l.) located in the campus area of the Hokkaido Obihiro Agricultural High School. The mean annual temperature was 7.2 °C and the precipitation was 937 mm at the Japan Meteorological Agency Obihiro Weather Station (6 km from the study site) during 1998–2017 [46]. The understory vegetation comprises a mixture of native species, which includes C. cordatum, Sasa chartacea (Makino) Makino & Shibata, Pachysandra terminalis Siebold & Zucc. [47], and Phryma esquirolii H.Lév. The population of C. cordatum at the site was comprised of approximately 150–200 individuals and separated from other populations in Obihiro city. The canopy openness before canopy closure was 27.1% [47] and after canopy closure was 4.9% (estimated with hemispherical photographs and the software CanopOn2 [48]).
2.3. Monitoring of Flower Visitors
In lowland forests in Obihiro, flowering of C. cordatum occurs during a relatively short period during mid to late July. Each flower lasts approximately one week at the study site. According to our field observation, the pollen was mature and the pistil was receptive simultaneously at the beginning of the first day of the flowering. We marked flowers and determined its age (0 day = the first day of flowering) by monitoring them every day. We investigated the flower visit frequency during midday hours in July in 2020 (11:00 AM–12:40 PM, 9:50 AM–11:00 AM, and 10:50 AM–12:10 PM on 17–19 July, respectively). On each day, an observation was made by one or two observers. Each observer sat in front of one or two individual plants, monitored the flowers on them for 10 min, and moved to next one or two plants. During each 10-min observation, the tentative name of every species that visited the flowers was recorded. Species visits were classified either as (1) those that entered the flower (i.e., those that arrived at the adaxial surface of the corolla) or (2) those that did not enter the flower (i.e., those that arrived only at the external (abaxial) surface of the corolla). Whenever possible, each individual flower-visiting species was captured into either a vial (5, 15, or 20 mL) or a plastic bag (Ziploc, Asahi Kasei, Tokyo, Japan), depending on the body size. Some visitors were recorded as described but were not successfully captured. Separate containers were used for each individual visitor. Immediately after capturing, each container was stored in a Styrofoam box with ice packs. These containers were moved to a household freezer within the same day and kept until the pollen count assays, as described below. After the pollen counts, one of the authors (T.Y.) identified captured species morphologically.
2.4. Pollen Counts
We followed the pollen count procedure as described in [29], with some modifications. The hind legs of the bees and bumblebees were removed before counting, because pollen grains on these parts do not contribute to pollination [29,49]. Distilled water (1.0–6.0 mL, depending on the body size) was pipetted into each sample-containing vial. Samples from plastic bags were moved into 15 or 20 mL vials. These vials were shaken manually for 1 min. Distilled water was used instead of an isotonic sucrose solution [29] because the pollen grains of this species do not explode in distilled water. Because the number of C. cordatum pollen grains attached to the insect body differed greatly, we adopted different methods depending on insect species. For the bumblebee (Bombus diversus tersatus), sweat bee (Halictidae sp.), and marmalade hoverfly (Episyrphus balteatus), which contained many pollen grains attached to them, we took subsamples (60 μL per body) from the shaken water inside each vial with a micropipette (M200; MonotaRO, Amagasaki, Japan) and counted the number of pollen grains under an optical microscope (ECLIPSE E600; Nikon, Tokyo, Japan) (Figure 2). This process was repeated five times, and the total number of pollen grains in the vial was estimated by the volumetric ratio. If Ziploc plastic bags were used to capture the flower visitor, we also washed the inner surface of the bags with 6 mL distilled water. The bags were shaken, and the number of pollen grains inside each bag was calculated by sampling five 60 μL subsamples, as described above, and the values obtained were added to those on the body surfaces. For remaining species with small pollen counts, we counted all pollen found in the distilled water. After shaking, the distilled water in the vial was put on a small open-top transparent plastic container. A piece of graph paper was attached to the bottom surface of the container to facilitate counting, and the pollen grains were counted under the optical microscope.
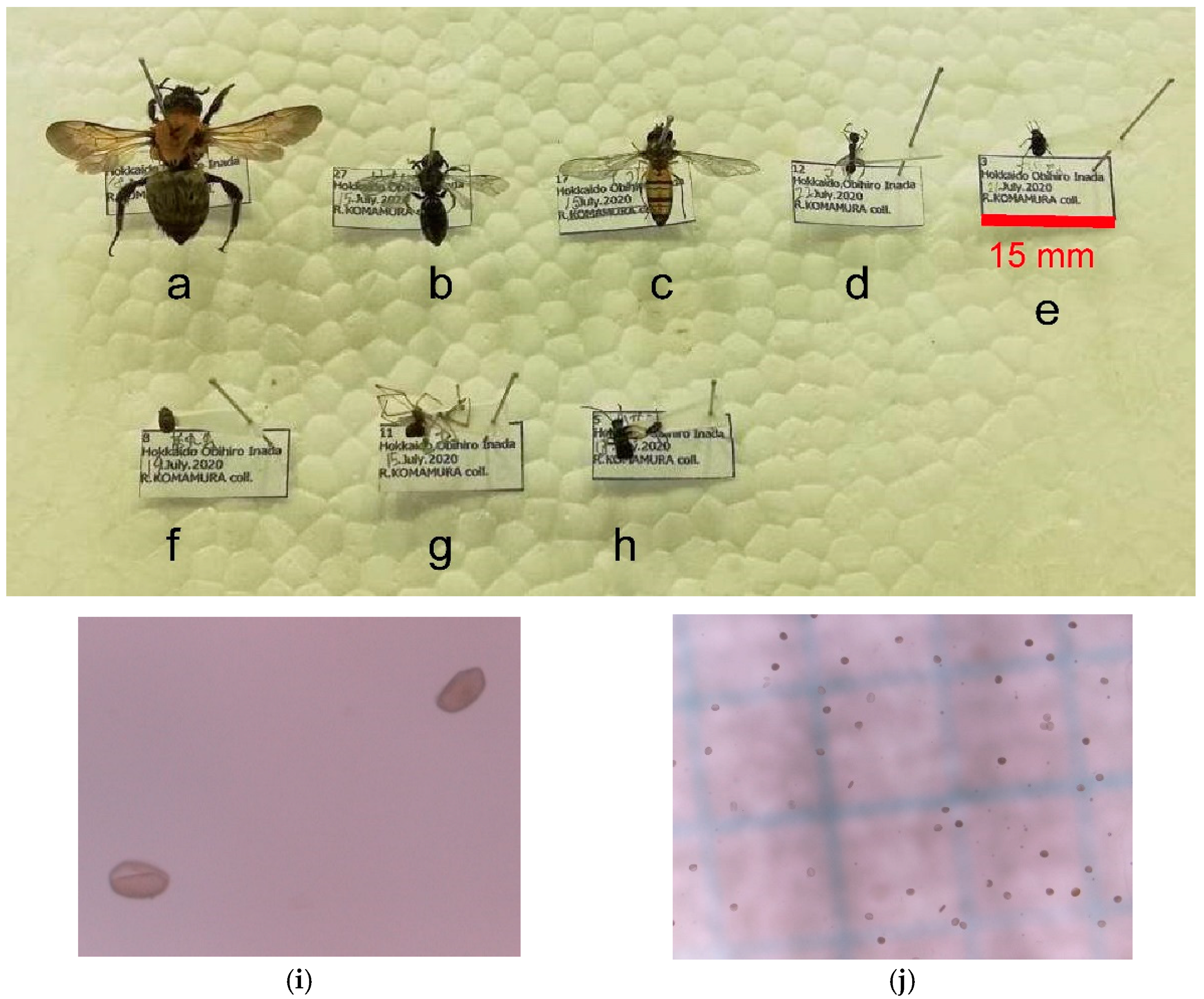
Figure 2.
Different species visiting the flowers of Cardiocrinum cordatum (Thunb.) Makino. (a) A bumblebee (Bombus diversus tersatus), (b) a sweat bee (Halictidae sp.), (c) a marmalade hoverfly (Episyrphus balteatus), (d) an ant (Myrmica ruginodis (s.l.)), (e) a leaf beetle (Chrysomelidae sp.), (f) a sap beetle (Nitidulidae sp.), (g) a spider (Araneae sp.), and (h) an earwig (Dermaptera sp.). (i,j) Pollen grains of C. cordatum were observed during the counting. The photographs were taken by Riko Komamura in 2020. The original high-resolution images are available as Supplementary Materials.
2.5. Pollinator Exclusion Experiment
Following the procedures as described in [2,7,12,17,18,25,50], we performed a pollinator exclusion experiment by covering the flower with polyester bags (0.6 mm × 0.9 mm mesh) (Figure 1b). One selected flower was covered for each individual. Before blooming, 85 flower buds were covered with bags. Among them, 15 flowers were assigned as complete pollinator exclusion treatment [18,25], and were covered with the bags during the entire flowering period. The remaining 70 flowers were used to estimate the pollination contribution per visit as described below.
Following the procedures as described in [50], the pollination contribution per visit was investigated during the daytimes of 13−22 July 2020. On each observation day, for each target flower for observation, the bag on one flower was temporarily removed, and an observer sat in front of it until the first visitor entered that flower. In this particular experiment, only those that entered the flower (as described above) were counted as visitors. After the visitor left, the flower was covered again with the same bag, and the observer moved to the next target flower. These visited flowers were kept bagged until the end of the flowering period. Whenever possible, flower-visiting species were captured just after they left the flowers. These captured species were also used for pollen counts as described above.
2.6. Estimating Fruit Set and Seed Number per Fruit
We calculated fruit set (i.e., fruit-to-flower ratio [51,52]) and the seed number per fruit for the 85 bagged fruits used for the pollinator exclusion experiment and, additionally, for different 84 uncovered flowers (i.e., open pollination treatment [15,18,29]) from 15 individuals in the same population. The bags were removed when the flowering period was complete. Then, the fruits were covered again with the same bags before fruit maturation to prevent a loss of seeds by wind dispersal. After maturation, all fruits from the bagged flowers and 22 selected fruits from uncovered flowers were sampled and their seeds were oven-dried at 70 °C for 70 h. For each fruit, the dry mass of 20 selected seeds and the total seed mass were measured with a precision balance and, the total number of seed in each fruit was estimated by the mass ratio. All statistical analyses were performed with statistical software R Version 4.0.4 [53]. All the datasets and the R codes used in this article are available in the Supplementary Materials.
3. Results
3.1. Flower-Visiting Species
The species composition differed among flowers of different ages (Table 1 and Table 2). For young flowers, just after the beginning of flowering (flower age, 0–1 day), the most frequent visitors were bumblebees and marmalade hoverflies, followed by ants, sweat bees, and leaf beetles. By contrast, old flowers (age, >2 days) were seldom visited by bumblebees, whereas the same ant species continued to visit the old flowers. Other species (sap beetles, earwigs, and spiders) occasionally arrived at the outer surface of the corolla but they did not enter the flower tube.

Table 1.
The observed frequency of flower-visiting species per unit observational time (visits hour−1 flower−1). Flowering age = 0 is defined as the first day of the flowering for each individual flower. In each cell, the fraction X/Y indicates that a total of Y individuals arrived at the flower per hour (including those that only arrived at the outer (abaxial) surface of the corolla), and among them, X entered inside a flower tube (i.e., the adaxial surface of a corolla).

Table 2.
The total number of visits of each species in relation to flower age.
3.2. Number of Pollen Grains on the Body Surface
The number of pollen grains on body surfaces differed greatly among the flower visitors (Table 3). Bumblebees and sweat bees carried much more pollen than did the rest of the species, and marmalade hoverflies carried a moderate amount of pollen. For the rest of the species, including the ants, the number of pollen grains on the body surfaces was very small.

Table 3.
The total number of pollen grains attached on the surface of the flower-visiting animals.
3.3. Pollination Contribution per Single Visit
Although this species is known to be self-compatible [7,32,39], in our experiment, only one undeveloped fruit that contained no seed was produced from 15 flowers used for complete pollinator exclusion, and all the other ovules were aborted after flowering. Pollination contribution was confirmed by the observation of fruits produced after single visits for bumblebees, sweat bees, and marmalade hoverflies (Table 4). The fruit set of flowers visited by bumblebees (31.8%) was comparable to those of uncovered flowers (32.1%). Among single-visited flowers, the mean number of seeds per fruit was the highest for the bumblebee-visited flowers. However, the mean number of seeds per fruit produced after a single visit of bumblebee was lower than those of uncovered flowers. This may be due to pollen limitation, as previously suggested (but not directly tested) [32]. One flower visited by an ant produced a fruit with a small number of seeds, suggesting a possible small contribution of ants as pollinators, but the present limited data do not allow us to confirm the contribution of ants.

Table 4.
Pollination contribution of each species, estimated as the total number of seeds in each fruit produced after a single visit of each individual insect, with the prior exclusion and subsequent exclusion of the other visitors by covering the flowers with bags.
4. Discussion
Our results suggest that bumblebees (Bombus diversus tersatus [syn. Bombus tersatus]), which feed on both nectar [54] and pollen [55], are the most important pollinators for this species for the population investigated in the present study. Generally, insects that visit young flowers with abundant pollen are important pollinators [50]. Bumblebees visited young flowers (flower age, 0–1 day) (Table 1). Accordingly, bumblebees carried more pollen than other pollinators did (Table 3). Pollination efficiency is also determined by match and mismatch between flower morphology (including the size of flower parts) and animal morphology [30,31]. All of the observed bumblebees entered into the inner (adaxial) surface of the corolla (Table 1), and their large body length (ca. 1–2 cm), together with the tube-shaped morphology of the C. cordatum flower (Figure 1), forced them into contact with the stigma when they entered the flower to extract the nectar. These observations are consistent with the results that they are important pollinators, as confirmed with the pollinator exclusion experiment (Table 4). Several species of bumblebees (genus Bombus) are generally well known to be effective pollinators [29,56], and are recognized as flower visitors of C. cordatum [32,35]. However, in previous studies on C. cordatum, the contribution of bumblebees to pollination remains elusive. The results of the present study are consistent with the aforementioned previous findings and provide further quantitative evidence confirming that B. diversus tersatus is an important pollinator for C. cordatum. Additionally, B. diversus has wide foraging range [33]. This indicates that the fragmented populations of C. cordatum in the lowland urban area might be genetically connected to the nearby populations with the contribution of bumblebees as pollinators.
Ohara et al. [32] and Nishizawa and Ohara [6] discussed that the large nectar quantity and mild flower fragrance of C. cordatum attract pollinators. Nevertheless, not all flower visitors may have access to nectar because the length of the proboscis is known to limit the ability of flower-visiting insects to access nectar [5,57,58]. Among the flower visitors observed in the present study, bumblebees (B. diversus) have the longest proboscis (approximate size 10.5–13.5 mm [59]), which is longer than that of sweat bees (Halictidae sp., typically, <2 mm [60]) or marmalade hoverflies (Episyrphus balteatus, 2.1–2.6 mm [57]). The flower tube of C. cordatum (Liliaceae) does not open as widely as that of Lilium auratum (Figure 1a,c,d). Therefore, a flower visitor with a short proboscis may not have access to the nectar of C. cordatum. The long, tube-shaped flower morphology of C. cordatum with narrow opening corolla may serve as a mechanism that limits visitor access to its nectar, thus restricting the nectar access to bumblebees that are the most efficient pollinators.
van Rijn et al. [61] reported that marmalade hoverflies feed on both pollen and nectar. Consistent with their results, we observed that they gathered or fed on pollen dropped from the anthers to the surface of the petals (Figure 1d). These behaviors may explain the observed high pollen amount on their body surface (Table 3). We also observed that sweat bees and marmalade hoverflies were occasionally attached to the stigma, consistent with their contribution as pollinators (Table 4). Ants are generally considered as nectar thieves [62], and their contribution to plant reproduction is thought to be low in many cases [62], although they indeed in some cases contribute as pollinators [63,64]. Although the ants observed in the present study (Myrmica ruginodis (s.l.)) were frequent visitors (Table 1), the number of pollen grains on the body surfaces of the ant species was small (Table 3). Additionally, we observed that the ants, because of their small body size (ca. 5 mm), rarely came into contact with the stigma. These results indicate that the ant species may not play a major role as pollinators. Other species, including sap beetles (Nitidulidae sp.), earwigs (Dermaptera sp.), and spiders (Araneae sp.) occasionally arrived at the outer surface of the corolla, but they did not enter inside the flower tube (Table 1). The number of pollen grains on their body surfaces was small (Table 3), indicating that they are not pollinators, at least in our present results.
Our study has several limitations. First, we investigated only a single population for a single flowering period. In general, reproductive patterns of plants differ among populations of the same species from different environments [6,7,36,39]. Second, the existence of nocturnal floral visitors was not investigated in the present study. Although we are unaware of any reports on nocturnal visitors for this plant species, further studies, including nighttime investigations, are needed in future studies. Third, we did not investigate the genetic composition or quality component of pollens. Self-pollination causes inbreeding depression, and hence outcross pollen is generally considered as of higher quality than self-pollen [1,3,8,25,62,63,65]. Matsuki et al. [3] reported that certain beetle species carry genetically more diverse outcross pollen than bumblebees. Rostás et al. [62,63] further argued that fruit or seed set may not give a sufficient estimate of contribution as pollinators and emphasized the importance of investigation of seedling viability and offspring vigor, which are determined by the genetic composition of pollen. Given these important limitations, further studies are needed to reconfirm our findings before generalization.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/f12040452/s1.
Author Contributions
Conceptualization, R.K. and K.K.; methodology, R.K., K.K. and Y.K.; investigation, R.K. and K.K.; identification of insect species, T.Y.; writing—original draft preparation, R.K. and K.K.; writing—review and editing, R.K., K.K., Y.K. and L.G.; Supervision, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Japan Society for the Promotion of Science (KAKENHI Grant Number 18K06406).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the datasets and the R codes used in this article are available in the Supplementary Materials.
Acknowledgments
We thank Norikuni Kumano for useful advices on pollen counts. We also thank staff members of Hokkaido Obihiro Agricultural High School for permitting us to perform the fieldwork at the study site.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Collevatti, R.G.; Estolano, R.; Garcia, S.F.; Hay, J.D. Seed abortion in the bat pollinated Neotropical tree species, Caryocar brasiliense (Caryocaraceae). Botany 2009, 87, 1110–1115. [Google Scholar] [CrossRef]
- Kunitake, Y.K.; Hasegawa, M.; Miyashita, T.; Higuchi, H. Role of a seasonally specialist bird Zosterops japonica on pollen transfer and reproductive success of Camellia japonica in a temperate area. Plant Spec. Biol. 2004, 19, 197–201. [Google Scholar] [CrossRef]
- Matsuki, Y.; Tateno, R.; Shibata, M.; Isagi, Y. Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. Am. J. Bot. 2008, 95, 925–930. [Google Scholar] [CrossRef]
- Nakamura, M.; Nanami, S.; Okuno, S.; Hirota, S.K.; Matsuo, A.; Suyama, Y.; Tokumoto, H.; Yoshihara, S.; Itoh, A. Genetic diversity and structure of apomictic and sexually reproducing Lindera species (Lauraceae) in Japan. Forests 2021, 12, 227. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nikkeshi, A.; Chishiki, A. Identification of effective pollinators of Primula sieboldii E. Morren in a wild habitat in Hiroshima, Japan. Plant Spec. Biol. 2020. [CrossRef]
- Nishizawa, M.; Ohara, M. The role of sexual and vegetative reproduction in the population maintenance of a monocarpic perennial herb, Cardiocrinum cordatum var. glehnii. Plant Spec. Biol. 2018, 33, 289–304. [Google Scholar] [CrossRef]
- Narumi, T.; Ohara, M. Variation in reproductive modes and population genetic structures of a monocarpic perennial herb, Cardiocrinum cordatum, in relation to habitat fragmentation. Plant Spec. Biol. 2018, 33, 248–258. [Google Scholar] [CrossRef]
- Al-Qthanin, R.N.; Alharbi, S.A. Spatial structure and genetic variation of a mangrove species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests 2020, 11, 1287. [Google Scholar] [CrossRef]
- Lu, J.-T.; Qiu, Y.-H.; Lu, J.-B. Effects of landscape fragmentation on genetic diversity of male-biased dioecious plant Pistacia chinensis bunge populations. Forests 2019, 10, 792. [Google Scholar] [CrossRef]
- Collevatti, R.G.; Grattapaglia, D.; Hay, J.D. High resolution microsatellite based analysis of the mating system allows the detection of significant biparental inbreeding in Caryocar brasiliense, an endangered tropical tree species. Heredity 2001, 86, 60–67. [Google Scholar] [CrossRef]
- Geerts, S.; Coetzee, A.; Rebelo, A.G.; Pauw, A. Pollination structures plant and nectar-feeding bird communities in Cape fynbos, South Africa: Implications for the conservation of plant–bird mutualisms. Ecol. Res. 2020, 35, 838–856. [Google Scholar] [CrossRef]
- Herrera, C.M. Components of pollinator “quality”: Comparative analysis of a diverse insect assemblage. Oikos 1987, 50, 79–90. [Google Scholar] [CrossRef]
- Herrera, C.M. Pollinator abundance, morphology, and flower visitation rate: Analysis of the “quantity” component in a plant-pollinator system. Oecologia 1989, 80, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. A general framework for effectiveness concepts in mutualisms. Ecol. Lett. 2017, 20, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Conceição, S.I.R.; Fernandes, J.; Borges da Silva, E.; Caperta, A.D. Reproductive output and insect behavior in hybrids and apomicts from Limonium ovalifolium and L. binervosum complexes (Plumbaginaceae) in an open cross-pollination experiment. Plants 2021, 10, 169. [Google Scholar] [CrossRef]
- Tokumoto, Y.; Matsushita, M.; Kishimoto-Yamada, K.; Nikkeshi, A.; Isogimi, T.; Nakagawa, M. Floral visitors and reproductive success in two sequentially flowering Lindera shrubs (Lauraceae) of central Japan. J. For. Res. 2019, 24, 42–51. [Google Scholar] [CrossRef]
- Yamasaki, E.; Sakai, S. Wind and insect pollination (ambophily) of Mallotus spp. (Euphorbiaceae) in tropical and temperate forests. Aust. J. Bot. 2013, 61, 60–66. [Google Scholar] [CrossRef]
- Giblin, D.E. Variation in floral longevity between populations of Campanula rotundifolia (Campanulaceae) in response to fitness accrual rate manipulation. Am. J. Bot. 2005, 92, 1714–1722. [Google Scholar] [CrossRef]
- Soley, N.M.; Sipes, S.D. Reproductive biology and pollinators of the invasive shrub Autumn olive (Elaeagnus umbellata Thunberg). Plant Spec. Biol. 2020. [CrossRef]
- Hou, S.; Zhao, T.; Yang, D.; Li, Q.; Liang, L.; Wang, G.; Ma, Q. Selection and validation of reference genes for quantitative RT-PCR analysis in Corylus heterophylla Fisch. × Corylus avellana L. Plants 2021, 10, 159. [Google Scholar] [CrossRef]
- Bentrup, G.; Hopwood, J.; Adamson, N.L.; Vaughan, M. Temperate agroforestry systems and insect pollinators: A review. Forests 2019, 10, 981. [Google Scholar] [CrossRef]
- Tran, X.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Reduced pollination efficiency compromises some physicochemical qualities in gac (Momordica cochinchinensis Spreng.) fruit. Agronomy 2021, 11, 190. [Google Scholar] [CrossRef]
- Fernández, F.J.; Garay, J.; Móri, T.F.; Csiszár, V.; Varga, Z.; López, I.; Gámez, M.; Cabello, T. Theoretical foundation of the control of pollination by hoverflies in a greenhouse. Agronomy 2021, 11, 167. [Google Scholar] [CrossRef]
- Aizen, M.A.; Aguiar, S.; Biesmeijer, J.C.; Garibaldi, L.A.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Morales, C.L.; Ngo, H.; et al. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Chang. Biol. 2019, 25, 3516–3527. [Google Scholar] [CrossRef] [PubMed]
- Cusser, S.; Neff, J.L.; Jha, S. Natural land cover drives pollinator abundance and richness, leading to reductions in pollen limitation in cotton agroecosystems. Agr. Ecosyst. Environ. 2016, 226, 33–42. [Google Scholar] [CrossRef]
- McGrady, C.M.; Troyer, R.; Fleischer, S.J. Wild bee visitation rates exceed pollination thresholds in commercial Cucurbita agroecosystems. J. Econ. Entomol. 2020, 113, 562–574. [Google Scholar] [CrossRef]
- Pfister, S.C.; Eckerter, P.W.; Schirmel, J.; Cresswell, J.E.; Entling, M.H. Sensitivity of commercial pumpkin yield to potential decline among different groups of pollinating bees. R. Soc. Open Sci. 2017, 4, 170102. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, D.; Fründ, J.; Albrecht, M.; Garratt, M.P.D.; Kleijn, D.; Pickles, B.J.; Potts, S.G.; An, J.; Andersson, G.K.S.; Bänsch, S.; et al. Wild insect diversity increases inter-annual stability in global crop pollinator communities. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210212. [Google Scholar] [CrossRef]
- Nikkeshi, A.; Inoue, H.; Arai, T.; Kishi, S.; Kamo, T. The bumblebee Bombus ardens ardens (Hymenoptera: Apidae) is the most important pollinator of Oriental persimmon, Diospyros kaki (Ericales: Ebenaceae), in Hiroshima, Japan. Appl. Entomol. Zool. 2019, 54, 409–419. [Google Scholar] [CrossRef]
- Hargreaves, A.L.; Harder, L.D.; Johnson, S.D. Consumptive emasculation: The ecological and evolutionary consequences of pollen theft. Biol. Rev. 2009, 84, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.E.; Bronstein, J.L.; Manson, J.S.; Richardson, L. Nectar robbing: Ecological and evolutionary perspectives. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 271–292. [Google Scholar] [CrossRef]
- Ohara, M.; Narumi, T.; Yoshizane, T.; Okayasu, T.; Masuda, J.; Kawano, S. 7: Cardiocrinum cordatum (Thunb.) Makino (Liliaceae). Plant Spec. Biol. 2006, 21, 201–207. [Google Scholar] [CrossRef]
- Nagamitsu, T.; Tsukuba, S.-A.; Ushirokita, F.; Konno, Y. Foraging habitats and floral resource use by colonies of long- and short-tongued bumble bee species in an agricultural landscape with kabocha squash fields. Appl. Entomol. Zool. 2012, 47, 181–190. [Google Scholar] [CrossRef]
- Konno, Y. Present status of remnant forests in Obihro, eastern Hokkaido, Japan. In Global Perspective in Forest Conservation and Sustainable Agriculture; Obihiro Asia and the Pacific Seminar on Education for Rural Development (OASERD): Obihiro, Japan, 2002; pp. 39–46. [Google Scholar]
- Matsumura, C.; Yokoyama, J.; Washitani, I. Invasion status and potential ecological impacts of an invasive alien bumblebee, Bombus terrestris L. (Hymenoptera: Apidae) naturalized in Southern Hokkaido, Japan. Glob. Environ. Res. 2004, 8, 51–66. [Google Scholar]
- Cao, G.-X.; Kudo, G. Size-dependent sex allocation in a monocarpic perennial herb, Cardiocrinum cordatum (Liliaceae). Plant Ecol. 2008, 194, 99–107. [Google Scholar] [CrossRef]
- Lu, R.-S.; Chen, Y.; Tamaki, I.; Sakaguchi, S.; Ding, Y.-Q.; Takahashi, D.; Li, P.; Isagi, Y.; Chen, J.; Qiu, Y.-X. Pre-quaternary diversification and glacial demographic expansions of Cardiocrinum (Liliaceae) in temperate forest biomes of Sino-Japanese Floristic Region. Mol. Phylogenet. Evol. 2020, 143, 106693. [Google Scholar] [CrossRef]
- Lu, R.-S.; Li, P.; Qiu, Y.-X. The complete chloroplast genomes of three Cardiocrinum (Liliaceae) species: Comparative genomic and phylogenetic analyses. Front. Plant Sci. 2017, 7, 2054. [Google Scholar] [CrossRef]
- Cao, G.X.; Worley, A.C. Life history trade-offs and evidence for hierarchical resource allocation in two monocarpic perennials. Plant Biol. 2013, 15, 158–165. [Google Scholar] [CrossRef]
- Koyama, K.; Hidaka, Y.; Ushio, M. Dynamic scaling in the growth of a non-branching plant, Cardiocrinum cordatum. PLoS ONE 2012, 7, e45317. [Google Scholar] [CrossRef]
- Araki, K.; Shimatani, K.; Nishizawa, M.; Yoshizane, T.; Ohara, M. Growth and survival patterns of Cardiocrinum cordatum var. glehnii (Liliaceae) based on a 13-year monitoring study: Life history characteristics of a monocarpic perennial herb. Botany 2010, 88, 745–752. [Google Scholar] [CrossRef]
- Kondo, T.; Sato, C.; Baskin, J.M.; Baskin, C.C. Post-dispersal embryo development, germination phenology, and seed dormancy in Cardiocrinum cordatum var. glehnii (Liliaceae s. str.), a perennial herb of the broadleaved deciduous forest in Japan. Am. J. Bot. 2006, 93, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hayafune, T.; Utech, F.H.; Ohara, M. Inter-populational variation, but no-annual variation within populations, in terms of reproductive size and genetic structure in a monocarpic perennial herb, Cardiocrinum cordatum var. glehnii. Plant Spec. Biol. 2019, 34, 27–30. [Google Scholar] [CrossRef]
- Hori, K.; Watanabe, T.; Devkota, H.P. Phenolic acid derivatives, flavonoids and other bioactive compounds from the leaves of Cardiocrinum cordatum (Thunb.) Makino (Liliaceae). Plants 2021, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Hosokawa, K.; Anetai, M.; Shibata, T.; Mukai, R.; Yoshida, K.-i.; Ashida, H. Antagonistic effect of the ainu-selected traditional beneficial plants on the transformation of an aryl hydrocarbon receptor. J. Food Sci. 2012, 77, C420–C429. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: http://www.jma.go.jp (accessed on 14 September 2020).
- Iwabe, R.; Koyama, K.; Komamura, R. Shade avoidance and light foraging of a clonal woody species, Pachysandra terminalis. Plants. under review.
- Takenaka, A. CanopOn 2 ver. 2.03c. 2009. Available online: http://takenaka-akio.org/etc/canopon2/index.html (accessed on 26 September 2020).
- Parker, A.J.; Tran, J.L.; Ison, J.L.; Bai, J.D.K.; Weis, A.E.; Thomson, J.D. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod-Plant Int. 2015, 9, 197–203. [Google Scholar] [CrossRef]
- Sakai, S. Handbook of Methods in Ecological Research 2: Field Methods in Pollination Ecology; Kyritsu Publishing: Tokyo, Japan, 2015. (In Japanese) [Google Scholar]
- Sutherland, S.D. Why hermaphroditic plants produce many more flowers than fruits: Experimental tests with Agave mckelveyana. Evolution 1987, 41, 750–759. [Google Scholar] [CrossRef]
- Koyama, K.; Tashiro, M. No effect of selective maturation on fruit traits for a bird-dispersed species, Sambucus racemosa. Plants 2021, 10, 376. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kobayashi, S.; Inoue, K.; Kato, M. Evidence of pollen transfer efficiency as the natural selection factor favoring a large corolla of Campanula punctata pollinated by Bombus diversus. Oecologia 1997, 111, 535–542. [Google Scholar] [CrossRef]
- Katayama, E. Studies on the development of the broods of Bombus diversus Smith (Hymenoptera, Apidae): II. Brood development and feeding habits. Kontyu 1966, 34, 8–17. [Google Scholar]
- Darvill, B.; Knight, M.E.; Goulson, D. Use of genetic markers to quantify bumblebee foraging range and nest density. Oikos 2004, 107, 471–478. [Google Scholar] [CrossRef]
- van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Inouye, D.W. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia 1980, 45, 197–201. [Google Scholar] [CrossRef]
- Inoue, M.N.; Yokoyama, J. Competition for flower resources and nest sites between Bombus terrestris (L.) and Japanese native bumblebees. Appl. Entomol. Zool. 2010, 45, 29–35. [Google Scholar] [CrossRef][Green Version]
- Inoue, T.; Kato, M. Hana ni Hikiyose Rareru Dobutsu—Hana to Doubutsu no Kyoshinka; Heibonsya: Tokyo, Japan, 1993. (In Japanese) [Google Scholar]
- van Rijn, P.C.J.; Kooijman, J.; Wäckers, F.L. The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control 2013, 67, 32–38. [Google Scholar] [CrossRef]
- Rostás, M.; Bollmann, F.; Saville, D.; Riedel, M. Ants contribute to pollination but not to reproduction in a rare calcareous grassland forb. PeerJ 2018, 6, e4369. [Google Scholar] [CrossRef]
- Rostás, M.; Tautz, J. Ants as pollinators of plants and the role of floral scents. In All Flesh Is Grass: Plant-Animal Interrelationships; Dubinsky, Z., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 149–161. [Google Scholar] [CrossRef]
- de Vega, C.; Herrera, C.M.; Dötterl, S. Floral volatiles play a key role in specialized ant pollination. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 32–42. [Google Scholar] [CrossRef]
- Collevatti, R.G.; Amara, M.E.C.; Lopes, F.S. Role of pollinators in seed set and a test of pollen limitation hypothesis in the tropical weed Triumfetta semitriloba (Tiliaceae). Rev. Biol. Trop. 1997, 45, 1401–1407. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).