The Effects of Tree and Stand Traits on the Specific Leaf Area in Managed Scots Pine Forests of Different Ages
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Selection of Sample Trees
| Sample Plot | Age (Years) | Stand Density (Trees·ha−1) | Reineke’s Density Index | Basal Area (m2·ha−1) | Standing Stock (m3·ha−1) | Mean ± SE DBH (cm) | Mean ± SE Height (m) | Mean ± SE Stem Volume (m3) | Mean ± SE Aboveground Biomass of Tree (kg) | Mean ± SE Needle Biomass of Tree (kg) | Canopy Depth (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mid-aged stands | |||||||||||
| 1 | 59 | 703 | 633 | 31.2 | 312 | 24.1 ± 1.3 | 22.1 ± 0.4 | 0.50 ± 0.06 | 257 ± 32 | 9.0 ± 1.5 | 6.7 |
| 2 | 57 | 890 | 659 | 31.0 | 310 | 20.9 ± 1.5 | 20.3 ± 0.6 | 0.37 ± 0.06 | 188 ± 33 | 6.1 ± 1.1 | 6.2 |
| 3 | 55 | 1120 | 739 | 33.8 | 325 | 20.1 ± 1.3 | 20.1 ± 0.2 | 0.31 ± 0.04 | 160 ± 21 | 5.8 ± 1.0 | 6.3 |
| 4 | 53 | 1323 | 705 | 30.6 | 265 | 16.7 ± 1.6 | 16.5 ± 1.9 | 0.24 ± 0.05 | 120 ± 25 | 3.7 ± 0.8 | 5.8 |
| 5 | 55 | 1373 | 855 | 38.5 | 364 | 18.1 ± 1.2 | 19.6 ± 0.6 | 0.24 ± 0.03 | 112 ± 14 | 3.5 ± 0.5 | 6.2 |
| Mature stands | |||||||||||
| 6 | 82 | 476 | 575 | 30.5 | 319 | 28.5 ± 1.6 | 22.9 ± 0.5 | 0.71 ± 0.08 | 357 ± 42 | 9.9 ± 1.2 | 7.5 |
| 7 | 82 | 590 | 615 | 31.5 | 301 | 25.2 ± 1.8 | 21.4 ± 0.4 | 0.51 ± 0.08 | 244 ± 34 | 6.4 ± 1.0 | 6.4 |
| 8 | 82 | 672 | 608 | 30.1 | 275 | 24.3 ± 1.5 | 20.3 ± 0.6 | 0.48 ± 0.06 | 235 ± 33 | 6.6 ± 1.1 | 6.4 |
| 9 | 82 | 756 | 715 | 35.7 | 334 | 24.3 ± 1.7 | 21.2 ± 0.5 | 0.48 ± 0.08 | 244 ± 39 | 8.3 ± 1.5 | 7.0 |
| 10 | 82 | 824 | 658 | 31.5 | 286 | 21.4 ± 1.3 | 19.6 ± 0.3 | 0.34 ± 0.05 | 167 ± 26 | 5.1 ± 1.0 | 6.2 |
2.3. Needle Measurement
2.4. Biomass Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- The achieved SLA values of Scots pine needles growing in Poland are within the range of previously noted values for trees of this species of similar age classes.
- The smallest SLA values are found in the upper part of the crown, which is in line with SLA’s known relation to the light exposure of needles.
- Mid-aged stands of Scots pine have higher SLA values than mature ones.
- Dominant trees in mid-aged stands have a lower SLA than more shaded intermediate ones, which is probably due to the different lighting conditions within the canopy.
- Contrary to expectations, no clear relationship was observed between the stand density and the SLA.
- Some parameters of mid-aged stands, like DBH, height, steam volume, aboveground, and needle biomass were negatively correlated with SLA. This has not been observed in mature stands.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierce, L.L.; Running, S.W.; Walker, J. Regional-scale relationships of leaf area index to specific leaf area and leaf nitrogen content. Ecol. Appl. 1994, 4, 313–321. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Kelliher, F.M.; Körner, C.; Lloyd, J.; Leuning, R. Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: A global ecology scaling exercise. Annu. Rev. Ecol. Syst. 1994, 25, 629–662. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar] [CrossRef]
- Falster, D.S.; Duursma, R.A.; FitzJohn, R.G. How functional traits influence plant growth and shade tolerance across the life cycle. Proc. Natl. Acad. Sci. USA 2018, 115, E6789. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- White, M.A.; Thornton, P.E.; Running, S.W.; Nemani, R.R. Parameterization and Sensitivity Analysis of the BIOME?BGC Terrestrial Ecosystem Model: Net Primary Production Controls. Earth Interact. 2000, 4, 1–85. [Google Scholar] [CrossRef]
- Nippert, J.B.; Marshall, J.D. Sources of variation in ecophysiological parameters in Douglas-fir and grand fir canopies. Tree Physiol. 2003, 23, 591–601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kellomäki, S.; Oker-Blom, P. Specific needle area of Scots pine and its dependence on light conditions inside the canopy. Silva Fenn. 1981, 15, 190–198. [Google Scholar] [CrossRef]
- Chen, H.Y.H.; Klinka, K.; Kayahara, G.J. Effects of light on growth, crown architecture, and specific leaf area for naturally established Pinus contorta var. latifolia and Pseudotsuga menziesii var. glauca saplings. Can. J. For. Res. 1996, 26, 1149–1157. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Nagel, L.M.; O’Hara, K.L. The influence of stand structure on ecophysiological leaf characteristics of Pinus ponderosa in western Montana. Can. J. For. Res. 2001, 31, 2173–2182. [Google Scholar] [CrossRef]
- Temesgen, H.; Weiskittel, A.R. Leaf mass per area relationships across light gradients in hybrid spruce crowns. Trees 2006, 20, 522–530. [Google Scholar] [CrossRef]
- Goudie, J.W.; Parish, R.; Antos, J.A. Foliage biomass and specific leaf area equations at the branch, annual shoot and whole-tree levels for lodgepole pine and white spruce in British Columbia. For. Ecol. Manag. 2016, 361, 286–297. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, X.; Wang, X.; Zhang, J. The effects of stand structure on specific needle area in closed-canopy Chinese pine plantations. J. For. Res. 2013, 18, 445–453. [Google Scholar] [CrossRef]
- England, J.R.; Attiwill, P.M. Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees 2006, 20, 79. [Google Scholar] [CrossRef]
- Weiskittel, A.R.; Temesgen, H.; Wilson, D.S.; Maguire, D.A. Sources of within- and between-stand variability in specific leaf area of three ecologically distinct conifer species. Ann. For. Sci. 2008, 65, 103. [Google Scholar] [CrossRef][Green Version]
- Fellner, H.; Dirnberger, G.F.; Sterba, H. Specific leaf area of European Larch (Larix decidua Mill.). Trees 2016, 30, 1237–1244. [Google Scholar] [CrossRef]
- Giuggiola, A.; Bugmann, H.; Zingg, A.; Dobbertin, M.; Rigling, A. Reduction of stand density increases drought resistance in xeric Scots pine forests. For. Ecol. Manag. 2013, 310, 827–835. [Google Scholar] [CrossRef]
- Tahvonen, O.; Pihlainen, S.; Niinimäki, S. On the economics of optimal timber production in boreal Scots pine stands. Can. J. For. Res. 2013, 43, 719–730. [Google Scholar] [CrossRef]
- Curtis, R.O.; Marshall, D.D.; Bell, J.F. LOGS: A pioneering example of silvicultural research in coast Douglas-fir. J. For. 1997, 95, 19–25. [Google Scholar]
- Kuliešis, A.; Saladis, J.; Kuliešis, A.A. Development and productivity of young Scots pine stands by regulating density. Balt. For. 2010, 16, 235–246. [Google Scholar]
- Nilsson, U.; Agestam, E.; Ekö, P.-M.; Elfving, B.; Fahlvik, N.; Johansson, U.; Karlsson, K.; Lundmark, T.; Wallentin, C. Thinning of Scots pine and Norway spruce monocultures in Sweden. Studia For. Suec. 2010, 219, 1–46. [Google Scholar]
- Mäkinen, H.; Hynynen, J.; Isomäki, A. Intensive management of Scots pine stands in southern Finland: First empirical results and simulated further development. For. Ecol. Manag. 2005, 215, 37–50. [Google Scholar] [CrossRef]
- del Río, M.; Calama, R.; Cañellas, I.; Roig, S.; Montero, G. Thinning intensity and growth response in SW-European Scots pine stands. Ann. For. Sci. 2008, 65, 308. [Google Scholar] [CrossRef]
- Valinger, E.; Elfving, B.; Mörling, T. Twelve-year growth response of Scots pine to thinning and nitrogen fertilisation. For. Ecol. Manag. 2000, 134, 45–53. [Google Scholar] [CrossRef]
- Węgiel, A.; Bembenek, M.; Łacka, A.; Mederski, P.S. Relationship between stand density and value of timber assortments: A case study for Scots pine stands in north-western Poland. N. Z. J. For. Sci. 2018, 48, 12. [Google Scholar] [CrossRef]
- Janssens, I.A.; Sampson, D.A.; Cermak, J.; Meiresonne, L.; Riguzzi, F.; Overloop, S.; Ceulemans, R. Above- and belowground phytomass and carbon storage in a Belgian Scots pine stand. Ann. For. Sci. 1999, 56, 81–90. [Google Scholar] [CrossRef]
- Wright, I.J.; Leishman, M.R.; Read, C.; Westoby, M. Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct. Plant Biol. 2006, 33, 407–419. [Google Scholar] [CrossRef]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between saplings and adult trees: An integration of field results by meta-analysis. Tree Physiol. 2002, 22, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Kawasaki, T.; Nakano, T.; Chiba, Y. Leaf-age effects on seasonal variability in photosynthetic parameters and its relationships with leaf mass per area and leaf nitrogen concentration within a Pinus densiflora crown. Tree Physiol. 2008, 28, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.S.; Day, M.E.; Berlyn, G.P. Regulation of foliar plasticity in conifers: Developmental and environmental factors. J. Sustain. For. 2009, 28, 48–62. [Google Scholar] [CrossRef]
- Niinemets, Ü. Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 2002, 22, 515–535. [Google Scholar] [CrossRef]
- Mencuccini, M.; Martínez-Vilalta, J.; Vanderklein, D.; Hamid, H.A.; Korakaki, E.; Lee, S.; Michiels, B. Size-mediated ageing reduces vigour in trees. Ecol. Lett. 2005, 8, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Vanderklein, D.; Martínez-Vilalta, J.; Lee, S.; Mencuccini, M. Plant size, not age, regulates growth and gas exchange in grafted Scots pine trees. Tree Physiol. 2007, 27, 71–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Socha, J.; Tymińska-Czabańska, L.; Grabska, E.; Orzeł, S. Site index models for main forest-forming tree species in Poland. Forests 2020, 11, 301. [Google Scholar] [CrossRef]
- Węgiel, A.; Bielinis, E.; Polowy, K. Macronutrient stocks in Scots pine stands of different densities. Forests 2018, 9, 593. [Google Scholar] [CrossRef]
- Wertz, B.; Bembenek, M.; Karaszewski, Z.; Ochał, W.; Skorupski, M.; Strzeliński, P.; Węgiel, A.; Mederski, P.S. Impact of stand density and tree social status on aboveground biomass allocation of Scots pine Pinus sylvestris L. Forests 2020, 11, 765. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P. A Re-Evaluation of Reineke’s Rule and Stand Density Index. For. Sci. 2005, 51, 304–320. [Google Scholar] [CrossRef]
- Węgiel, A.; Polowy, K. Aboveground carbon content and storage in mature Scots pine stands of different densities. Forests 2020, 11, 240. [Google Scholar] [CrossRef]
- Mencuccini, M.; Bonosi, L. Leaf/sapwood area ratios in Scots pine show acclimation across Europe. Can. J. For. Res. 2001, 31, 442–456. [Google Scholar] [CrossRef]
- Goude, M.; Nilsson, U.; Holmström, E. Comparing direct and indirect leaf area measurements for Scots pine and Norway spruce plantations in Sweden. Eur. J. For. Res. 2019, 138, 1033–1047. [Google Scholar] [CrossRef]
- Han, Q.; Kawasaki, T.; Katahata, S.; Mukai, Y.; Chiba, Y. Horizontal and vertical variations in photosynthetic capacity in a Pinus densiflora crown in relation to leaf nitrogen allocation and acclimation to irradiance. Tree Physiol. 2003, 23, 851–857. [Google Scholar] [CrossRef]
- Marshall, J.D.; Monserud, R.A. Foliage height influences specific leaf area of three conifer species. Can. J. For. Res. 2003, 33, 164–170. [Google Scholar] [CrossRef]
- Xiao, C.-W.; Ceulemans, R. Allometric relationships for needle area of different needle age classes in young Scots pines: Needles, branches and trees. Forestry 2004, 77, 369–382. [Google Scholar] [CrossRef]
- Day, M.E.; Greenwood, M.S.; White, A.S. Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiol. 2001, 21, 1195–1204. [Google Scholar] [CrossRef]
- van Hees, A.F.M.; Bartelink, H.H. Needle area relationships of Scots pine in the Netherlands. For. Ecol. Manag. 1993, 58, 19–31. [Google Scholar] [CrossRef]
- Barna, M. Adaptation of European beech (Fagus sylvatica L.) to different ecological conditions: Leaf size variation. Pol. J. Ecol. 2004, 52, 35–45. [Google Scholar]
- Konôpka, B.; Pajtík, J.; Marušák, R.; Bošeľa, M.; Lukac, M. Specific leaf area and leaf area index in developing stands of Fagus sylvatica L. and Picea abies Karst. For. Ecol. Manag. 2016, 364, 52–59. [Google Scholar] [CrossRef]
- Mäkinen, H.; Isomäki, A. Thinning intensity and long-term changes in increment and stem form of Scots pine trees. For. Ecol. Manag. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Del Rio, M.; Montero, G.; Bravo, F. Analysis of diameter-density relationships and self-thinning in non-thinned even-aged Scots pine stands. For. Ecol. Manag. 2001, 142, 79–87. [Google Scholar] [CrossRef]
- Karolewski, P.; Giertych, M.J.; Żmuda, M.; Jagodziński, A.M.; Oleksyn, J. Season and light affect constitutive defenses of understory shrub species against folivorous insects. Acta Oecologica 2013, 53, 19–32. [Google Scholar] [CrossRef]
- Peeters, P.J.; Sanson, G.; Read, J. Leaf biomechanical properties and the densities of herbivorous insect guilds. Funct. Ecol. 2007, 21, 246–255. [Google Scholar] [CrossRef]
- Dominy, N.J.; Grubb, P.J.; Jackson, R.V.; Lucas, P.W.; Metcalfe, D.J.; Svenning, J.-C.; Turner, I.M. In tropical lowland rain forests monocots have tougher leaves than dicots, and include a new kind of tough leaf. Ann. Bot. 2008, 101, 1363–1377. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N.; et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Giertych, M.J.; Żmuda, M.; Mąderek, E.; Adamczyk, D.; Karolewski, P. Decomposition of herbivore-damaged leaves of understory species growing in oak and pine stands. Forests 2021, 12, 304. [Google Scholar] [CrossRef]
- Hager, H.; Sterba, H. Specific leaf area and needle weight of Norway spruce (Picea abies) in stands of different densities. Can. J. For. Res. 1985, 15, 389–392. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kull, O. Effects of light availability and tree size on the architecture of assimilative surface in the canopy of Picea abies: Variation in shoot structure. Tree Physiol. 1995, 15, 791–798. [Google Scholar] [CrossRef][Green Version]
- Monserud, R.A.; Marshall, J.D. Allometric crown relations in three northern Idaho conifer species. Can. J. For. Res. 1999, 29, 521–535. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]

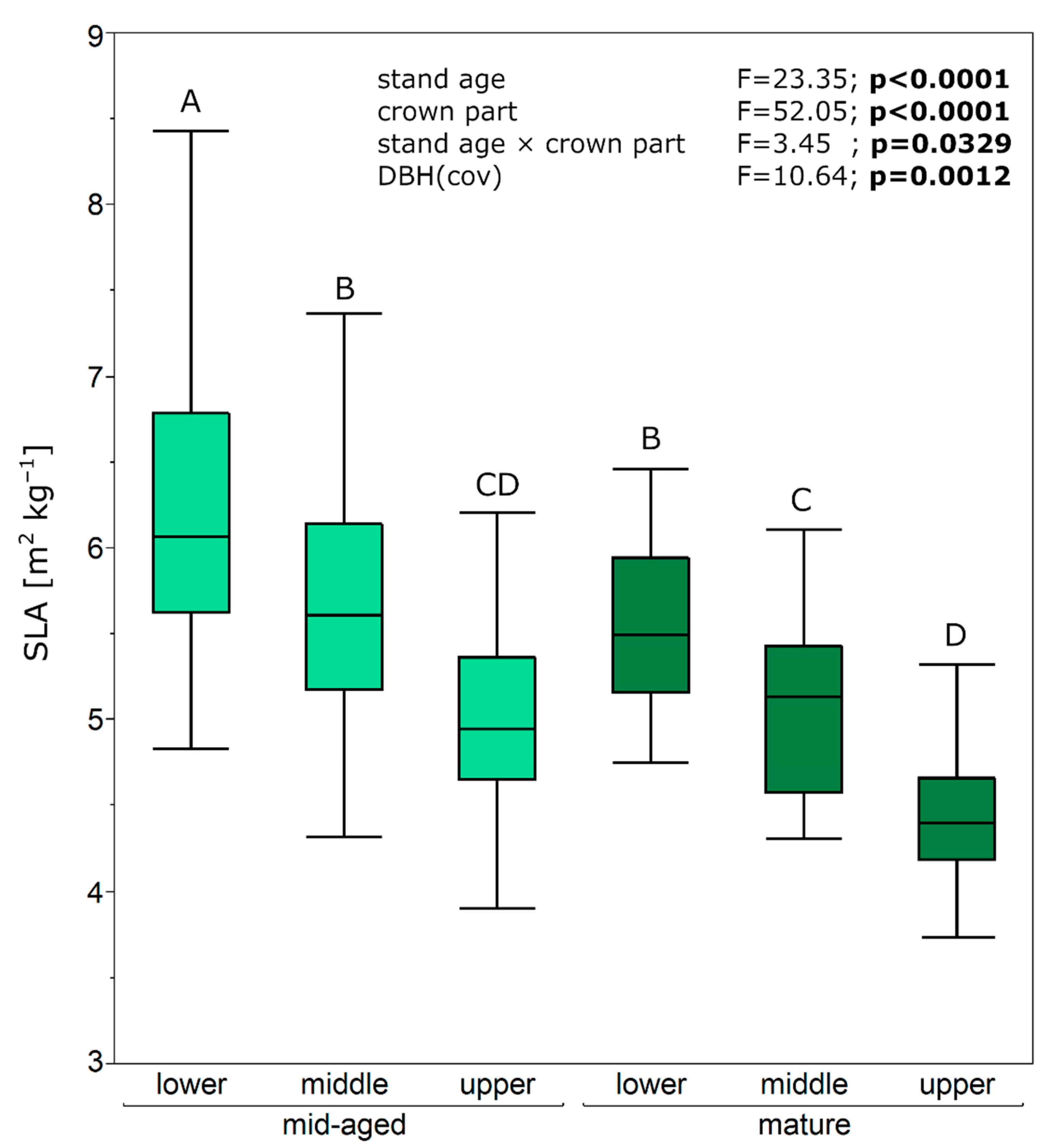
| Stand Age (Development Stage) | Crown Part | N | Needle Length (mm) | Needle Width (mm) | Needle Area (mm2) | Needle Weight (mg) |
|---|---|---|---|---|---|---|
| Mid-aged | Upper | 48 | 63.8 ± 0.3 | 1.35 ± 0.004 | 87.6 ± 0.6 | 17.4 ± 0.64 |
| Middle | 48 | 57.3 ± 0.2 | 1.23 ± 0.004 | 71.1 ± 0.4 | 12.4 ± 0.44 | |
| Lower | 48 | 52.7 ± 0.2 | 1.14 ± 0.004 | 61.1 ± 0.4 | 9.5 ± 0.33 | |
| Mature | Upper | 49 | 58.7 ± 0.2 | 1.35 ± 0.005 | 80.4 ± 0.5 | 17.6 ± 0.57 |
| Middle | 50 | 53.0 ± 0.2 | 1.23 ± 0.004 | 66.3 ± 0.4 | 13.1 ± 0.42 | |
| Lower | 50 | 48.0 ± 0.2 | 1.14 ± 0.004 | 55.6 ± 0.4 | 10.1 ± 0.33 | |
| ANCOVA | Df | |||||
| Stand age | 1 | F | 52.80 | 2.05 | 32.92 | 1.59 |
| p | <0.0001 | 0.1540 | <0.0001 | 0.2080 | ||
| Crown part | 2 | F | 69.80 | 85.61 | 113.08 | 147.24 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Stand age × crown part | 2 | F | 0.13 | 0.08 | 0.20 | 0.1111 |
| p | 0.8755 | 0.9193 | 0.8164 | 0.8949 | ||
| DBH(cov) | 1 | F | 13.12 | 12.51 | 21.00 | 31.27 |
| p | 0.0003 | 0.0005 | <0.0001 | <0.0001 | ||
| Df error | 286 |
| Plot Number | Stand Density (Trees·ha−1) | Mean (±SE) SLA of Different Part of Crown (m2 kg−1) | Mean (±SE) SLA of Whole Crown (m2 kg−1) | |||
|---|---|---|---|---|---|---|
| Upper | Middle | Lower | ||||
| Mid-aged stands | ||||||
| 1 | 703 | 4.89 ± 0.11 b | 5.50 ± 0.15 b | 6.31 ± 0.20 b | 5.57 ± 0.14 b | |
| 2 | 890 | 5.25 ± 0.29 b | 5.54 ± 0.37 b | 6.44 ± 0.47 b | 5.72 ± 0.23 b | |
| 3 | 1120 | 4.98 ± 0.15 b | 5.96 ± 0.18 b | 6.47 ± 0.18 b | 5.80 ± 0.15 b | |
| 4 | 1323 | 5.01 ± 0.14 b | 5.59 ± 0.16 b | 6.04 ± 0.16 b | 5.53 ± 0.12 b | |
| 5 | 1373 | 6.22 ± 0.50 a | 7.17 ± 0.29 a | 7.77 ± 0.36 a | 7.11 ± 0.25 a | |
| ANCOVA | Df | |||||
| Stand density | 4 | F | 5.55 | 5.92 | 2.89 | 9.78 |
| p | 0.0010 | 0.0007 | 0.0331 | <0.0001 | ||
| DBH(cov) | 1 | F | 3.84 | 7.49 | 4.17 | 12.28 |
| p | 0.0563 | 0.1461 | 0.0472 | 0.0197 | ||
| Df error | 44 | |||||
| Mature stands | ||||||
| 1 | 476 | 4.83 ± 0.14 a | 5.06 ± 0.16 ab | 5.44 ± 0.15 | 5.11 ± 0.10 ab | |
| 2 | 590 | 4.90 ± 0.11 a | 5.45 ± 0.16 a | 5.70 ± 0.18 | 5.34 ± 0.10 ab | |
| 3 | 672 | 4.35 ± 0.08 b | 4.71 ± 0.10 b | 5.52 ± 0.11 | 4.86 ± 0.11 b | |
| 4 | 756 | 4.84 ± 0.18 a | 5.55 ± 0.10 a | 5.81 ± 0.17 | 5.40 ± 0.12 a | |
| 5 | 824 | 4.32 ± 0.12 b | 4.93 ± 0.15 c | 5.39 ± 0.16 | 4.88 ± 0.12 b | |
| ANCOVA | Df | |||||
| Stand density | 4 | F | 8.33 | 8.25 | 2.24 | 6.47 |
| p | <0.0001 | <0.0001 | 0.0794 | 0.0003 | ||
| DBH(cov) | 1 | F | 12.80 | 7.38 | 11.43 | 13.26 |
| p | 0.0009 | 0.0094 | 0.0015 | 0.0007 | ||
| Df error | 44 | |||||
| Social Position of a Tree | Mean (±SE) SLA of Different Part of Crown (m2 kg−1) | Mean (±SE) SLA of Whole Crown (m2 kg−1) | ||||
|---|---|---|---|---|---|---|
| Upper | Middle | Lower | ||||
| Mid-aged stands | ||||||
| Dominant | 5.10 ± 0.09 b | 5.89 ± 0.16 | 6.63 ± 0.16 a | 5.92 ± 0.14 ab | ||
| Codominant | 5.03 ± 0.28 b | 5.63 ± 0.13 | 6.17 ± 0.27 b | 5.67 ± 0.26 b | ||
| Intermediate | 6.90 ± 0.74 a | 6.88 ± 0.58 | 7.67 ± 0.93 a | 7.15 ± 0.48 a | ||
| ANCOVA | Df | |||||
| Social position | 2 | F | 8.99 | 3.15 | 4.10 | 4.67 |
| p | 0.0005 | 0.0522 | 0.0231 | 0.0142 | ||
| DBH(cov) | 1 | F | 2.21 | 4.34 | 5.26 | 5.85 |
| p | 0.1438 | 0.0428 | 0.0264 | 0.0196 | ||
| Df error | 46 | |||||
| Mature stands | ||||||
| Dominant | 4.51 ± 0.07 | 5.00 ± 0.08 | 5.47 ± 0.08 | 4.99 ± 0.06 | ||
| Codominant | 4.86 ± 0.13 | 5.40 ± 0.15 | 5.91 ± 0.12 | 5.37 ± 0.12 | ||
| Intermediate | 4.86 ± 0.23 | 5.23 ± 0.21 | 5.45 ± 0.19 | 5.18 ± 0.19 | ||
| ANCOVA | Df | |||||
| Social position | 2 | F | 3.07 | 1.95 | 2.51 | 2.06 |
| p | 0.0563 | 0.1539 | 0.0929 | 0.1388 | ||
| DBH(cov) | 1 | F | 0.08 | 0.03 | 3.05 | 0.43 |
| p | 0.7777 | 0.8584 | 0.0678 | 0.5139 | ||
| Df error | 46 | |||||
| SLA | DBH | Tree Height | Stem Volume | Needle Biomass of Tree | Aboveground Biomass of Tree | |
|---|---|---|---|---|---|---|
| Mid-aged stands | ||||||
| Crown part | Upper | −0.342 | −0.425 | −0.329 | −0.325 | −0.347 |
| Middle | −0.294 | −0.243 | −0.297 | −0.284 | −0.296 | |
| Lower | −0.270 | −0.260 | −0.323 | −0.289 | −0.313 | |
| Whole crown | −0.348 | −0.340 | −0.370 | −0.358 | −0.369 | |
| Mature stands | ||||||
| Crown part | Upper | −0.193 | 0.029 | −0.136 | −0.144 | −0.107 |
| Middle | −0.191 | −0.029 | −0.164 | −0.139 | −0.161 | |
| Lower | −0.348 | −0.241 | −0.309 | −0.292 | −0.2937 | |
| Whole crown | −0.269 | −0.093 | −0.223 | −0.212 | −0.207 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błasiak, A.; Węgiel, A.; Łukowski, A.; Sułkowski, S.; Turski, M. The Effects of Tree and Stand Traits on the Specific Leaf Area in Managed Scots Pine Forests of Different Ages. Forests 2021, 12, 396. https://doi.org/10.3390/f12040396
Błasiak A, Węgiel A, Łukowski A, Sułkowski S, Turski M. The Effects of Tree and Stand Traits on the Specific Leaf Area in Managed Scots Pine Forests of Different Ages. Forests. 2021; 12(4):396. https://doi.org/10.3390/f12040396
Chicago/Turabian StyleBłasiak, Agnieszka, Andrzej Węgiel, Adrian Łukowski, Sławomir Sułkowski, and Mieczysław Turski. 2021. "The Effects of Tree and Stand Traits on the Specific Leaf Area in Managed Scots Pine Forests of Different Ages" Forests 12, no. 4: 396. https://doi.org/10.3390/f12040396
APA StyleBłasiak, A., Węgiel, A., Łukowski, A., Sułkowski, S., & Turski, M. (2021). The Effects of Tree and Stand Traits on the Specific Leaf Area in Managed Scots Pine Forests of Different Ages. Forests, 12(4), 396. https://doi.org/10.3390/f12040396








