Is the Seasonal Variation in Frost Resistance and Plant Performance in Four Oak Species Affected by Changing Temperatures?
Abstract
1. Introduction
- (1)
- Is there a difference in seasonal variation in FR, photosynthetic parameters, and leaf functional traits between the four species and between different temperature treatments?
- (2)
- Is there a trade-off between FR and photosynthetic performance, and does it change under different temperature treatments?
- (3)
- Are functional traits involved in this trade-off and can they be used to describe it?
2. Materials and Methods
2.1. Measuring of FR and Photosynthesic Parameters
2.2. Measuring the Leaf Functional Traits
2.3. Statistical Analysis
3. Results
3.1. Seasonal Variation and Species-Specific Responses
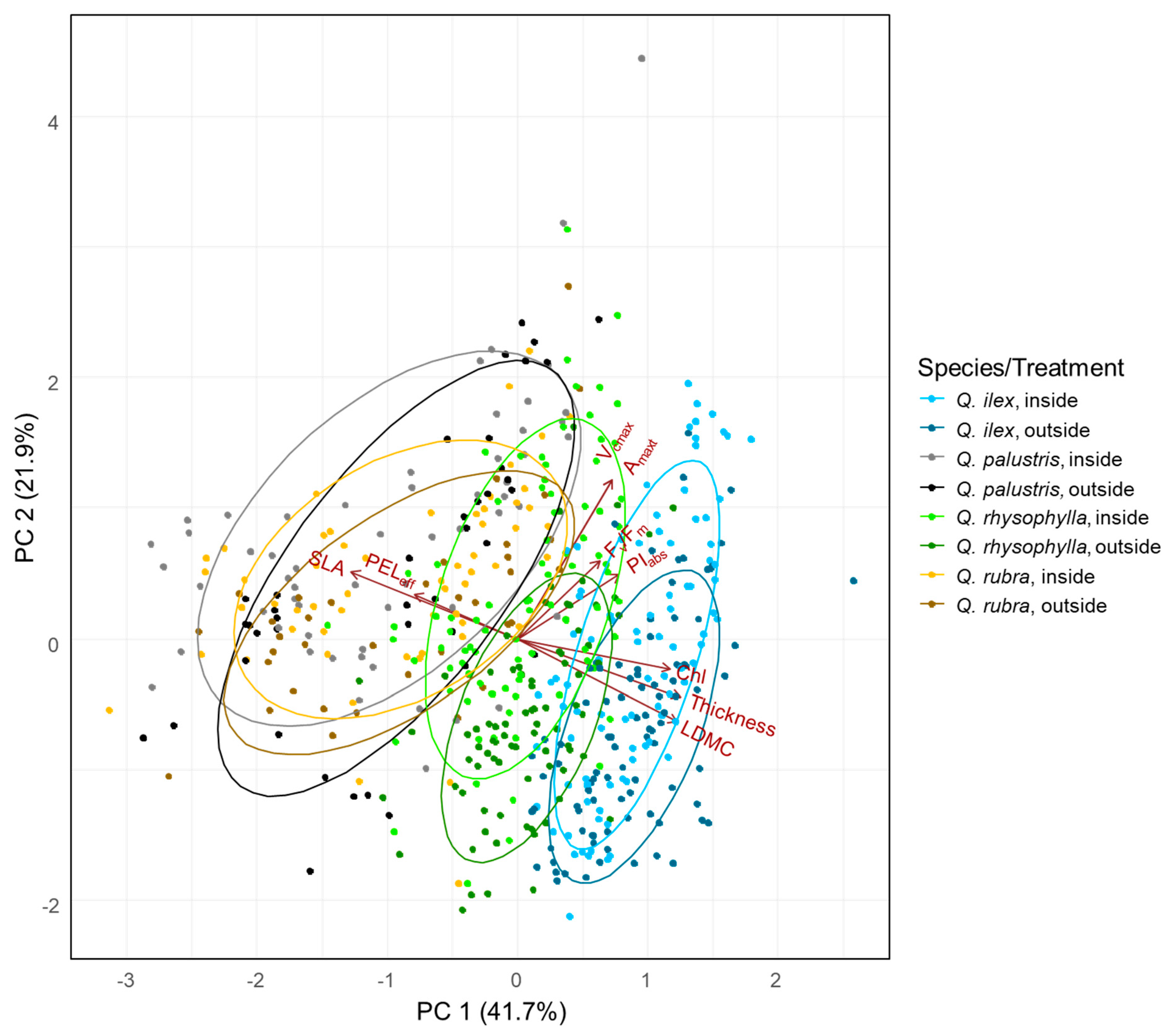
3.2. Trade-Off between Frost Resistance and Photosynthetic Performance
3.3. Effects of Traits on the Trade-Off between Resistance and Performance
4. Discussion
4.1. Influence of Temperature on Plant Traits
4.2. Seasonal Variation and Species-Specific Responses
4.3. Effects of Traits on the Trade-Off between Resistance and Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gast, A.; Römermann, C.; Bucher, S.F. Special issue in honour of Prof. Reto J. Strasser—Seasonal variation and trade-off between frost resistance and photosynthetic performance in woody species. Photosynthetica 2020, 58, 331–340. [Google Scholar] [CrossRef]
- Larcher, W.; Bauer, H. Ecological significance of resistance to low temperature. In Physiological Plant Ecology I; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 403–437. [Google Scholar]
- Sakai, A.; Larcher, W. Frost Survival of Plants: Responses and Adaptation to Freezing Stress; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Öquist, G.; Huner, N.P. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Bucher, S.F.; Feiler, R.; Buchner, O.; Neuner, G.; Rosbakh, S.; Leiterer, M.; Römermann, C. Temporal and spatial trade-offs between resistance and performance traits in herbaceous plant species. Environ. Exp. Bot. 2018, 157, 187–196. [Google Scholar] [CrossRef]
- Römermann, C.; Bucher, S.F.; Hahn, M.; Bernhardt-Römermann, M. Plant functional traits—Fixed facts or variable depending on the season? Folia Geobot. 2016, 51, 143–159. [Google Scholar] [CrossRef]
- Bucher, S.F.; Rosbakh, S. Foliar summer frost resistance measured via electrolyte leakage approach as related to plant distribution, community composition and plant traits. Funct. Ecol. 2021. [Google Scholar] [CrossRef]
- Gurvich, D.E.; Diaz, S.; Falczuk, V.; Harguindeguy, N.P.; Cabido, M.; Thorpe, P.C. Foliar resistance to simulated extreme temperature events in contrasting plant functional and chorological types. Glob. Chang. Biol. 2002, 8, 1139–1145. [Google Scholar] [CrossRef]
- Bucher, S.F.; Bernhardt-Römermann, M.; Römermann, C. Chlorophyll fluorescence and gas exchange measurements in field research: An ecological case study. Photosynthetica 2018, 56, 1161–1170. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: Bristol, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Srivastava, A. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Lin, Y.S.; Wright, I.J.; Medlyn, B.E.; Crous, K.Y.; Ellsworth, D.S. A test of the ‘one-point method’ for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytol. 2016, 210, 1130–1144. [Google Scholar] [CrossRef]
- Grassi, G.; Vicinelli, E.; Ponti, F.; Cantoni, L.; Magnani, F. Seasonal and interannual variability of photosynthetic capacity in relation to leaf nitrogen in a deciduous forest plantation in northern Italy. Tree Physiol. 2005, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.V.; Farquhar, G. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Perezharguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- de Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.; Bardgett, R.D. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Poorter, H.; Garnier, E. Ecological significance of inherent variation in relative growth rate and its components. In Handbook of Functional Plant Ecology; Pugnaire, F., Valladares, F., Eds.; Marcel Dekker Inc.: Yew York, NY, USA, 1999; pp. 81–120. [Google Scholar]
- Grime, J.P.; Cornelissen, J.H.C.; Thompson, K.; Hodgson, J.G. Evidence of a causal connection between anti-herbivore defence and the decomposition rate of leaves. Oikos 1996, 77, 489–494. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Curran, P.J.; Windham, W.R.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Filella, I.; Serrano, L.; Serra, J.; Peñuelas, J. Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci. 1995, 35, 1400–1405. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A. Why and what for the leaves are yellow in autumn? On the interpretation of optical spectra of senescing leaves (Acerplatanoides L.). J. Plant Physiol. 1995, 145, 315–320. [Google Scholar]
- Bucher, S.F.; Auerswald, K.; Tautenhahn, S.; Geiger, A.; Otto, J.; Müller, A.; Römermann, C. Inter-and intraspecific variation in stomatal pore area index along elevational gradients and its relation to leaf functional traits. Plant Ecol. 2016, 217, 229–240. [Google Scholar] [CrossRef]
- Chaerle, L.; Saibo, N.; Van Der Straeten, D. Tuning the pores: Towards engineering plants for improved water use efficiency. Trends Biotechnol. 2005, 23, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, M.R.G.; Hedrich, R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Garnier, E.; Salager, J.-L.; Laurent, G.; Sonie, L. Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol. 1999, 143, 119–129. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A.; Heilmeier, H. Internal leaf anatomy and photosynthetic resource-use efficiency: Interspecific and intraspecific comparisons. Tree Physiol. 2001, 21, 251–259. [Google Scholar] [CrossRef]
- Agustí, S.; Enríquez, S.; Frost-Christensen, H.; Sand-Jensen, K.; Duarte, C.M. Light harvesting among photosynthetic organisms. Fun Ecol. 1994, 8, 273–279. [Google Scholar] [CrossRef]
- Enríquez, S.; Duarte, C.M.; Sand-Jensen, K.; Nielsen, S.L. Broad-scale comparison of photosynthetic rates across phototrophic organisms. Oecologia 1996, 108, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.L.; Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Scaling maximum growth rates across photosynthetic organisms. Fun Ecol. 1996, 10, 167–175. [Google Scholar] [CrossRef]
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Neuner, G.; Pramsohler, M. Freezing and high temperature thresholds of photosystem 2 compared to ice nucleation, frost and heat damage in evergreen subalpine plants. Physiol. Plant. 2006, 126, 196–204. [Google Scholar] [CrossRef]
- Adams, W.W.; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. Bioscience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Holland, V.; Koller, S.; Brüggemann, W. Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis. Plant Biol. 2014, 16, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Kadereit, J.W.; Körner, C.; Kost, B.; Sonnewald, U. Strasburger-Lehrbuch der Pflanzenwissenschaften; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Clark, A.; Landolt, W.; Bucher, J.; Strasser, R. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Hilu, K.W.; Randall, J.L. Convenient method for studying grass leaf epidermis. Taxon 1984, 33, 413–415. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The Vegan Package. Community Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Wickham, H.; Chang, W. Devtools: Tools to Make Developing R Code Easier, R package version 1.13.3; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Vu, V.Q. Ggbiplot: A ggplot2 Based Biplot. 0.55 edn. Available online: http://github.com/vqv/ggbiplot (accessed on 16 March 2021).
- Larcher, W. Ökophysiologie der Pflanzen; Eugen Ulmer: Stuttgart, Germany, 1994. [Google Scholar]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; with 47 Tables; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- de Rigo, D.; Caudullo, G. Quercus ilex in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office EU: Luxembourg, 2016; pp. 152–153. [Google Scholar]
- Rosbakh, S.; Römermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alpine Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.; Baker, N.; Woolhouse, H. Changes in chlorophyll content and organization during senescence of the primary leaves of Phaseolus vulgaris L. in relation to photosynthetic electron transport. J. Exp. Bot. 1981, 32, 1009–1020. [Google Scholar] [CrossRef]
- Hänninen, H. Does climatic warming increase the risk of frost damage in northern trees? Plant Cell Environ. 1991, 14, 449–454. [Google Scholar] [CrossRef]
- Murray, M.; Cannell, M.; Smith, R. Date of budburst of fifteen tree species in Britain following climatic warming. J. Appl. Ecol. 1989, 26, 693–700. [Google Scholar] [CrossRef]
- Hänninen, H. Effects of climatic warming on northern trees: Testing the frost damage hypothesis with meteorological data from provenance transfer experiments. Scand J. For. Res. 1996, 11, 17–25. [Google Scholar] [CrossRef]
- Katz, R.W.; Brown, B.G. Extreme events in a changing climate: Variability is more important than averages. Clim. Chang. 1992, 21, 289–302. [Google Scholar] [CrossRef]
- Troeng, E.; Linder, S. Gas exchange in a 20-year-old stand of Scots pine: I. Net photosynthesis of current and one-year-old shoots within and between seasons. Physiol. Plant. 1982, 54, 7–14. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Steer, B. The dynamics of leaf growth and photosynthetic capacity in Capsicum frutescens L. Ann. Bot. 1971, 35, 1003–1015. [Google Scholar] [CrossRef]
- Jurik, T.W. Temporal and spatial patterns of specific leaf weight in successional northern hardwood tree species. Am. J. Bot. 1986, 73, 1083–1092. [Google Scholar] [CrossRef]
- Palacio, S.; Milla, R.; Albuixech, J.; Pérez-Rontomé, C.; Camarero, J.J.; Maestro, M.; Montserrat-Martí, G. Seasonal variability of dry matter content and its relationship with shoot growth and nonstructural carbohydrates. New Phytol. 2008, 180, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Heide, O.M. Growth and dormancy in Norway spruce ecotypes (Picea abies) I. Interaction of photoperiod and temperature. Physiol. Plant. 1974, 30, 1–12. [Google Scholar] [CrossRef]
- Demarez, V. Seasonal variation of leaf chlorophyll content of a temperate forest. Inversion of the PROSPECT model. Int. J. Remote. Sens. 1999, 20, 879–894. [Google Scholar] [CrossRef]
- Gindel, I. Stomatal number and size as related to soil moisture in tree xerophytes in Israel. Ecology 1969, 50, 263–267. [Google Scholar] [CrossRef]
- Bucher, S.F.; Auerswald, K.; Grün-Wenzel, C.; Higgins, S.I.; Jorge, J.G.; Römermann, C. Stomatal traits relate to habitat preferences of herbaceous species in a temperate climate. Flora 2017, 229, 107–115. [Google Scholar] [CrossRef]
- Sierra-Almeida, A.; Cavieres, L.A. Summer freezing resistance decreased in high-elevation plants exposed to experimental warming in the central Chilean Andes. Oecologia 2010, 163, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.; Jalili, A. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Friend, A.D.; Stevens, A.K.; Knox, R.G.; Cannell, M.G.R. A process-based, terrestrial biosphere model of ecosystem dynamics (Hybrid v3. 0). Ecol. Model. 1997, 95, 249–287. [Google Scholar] [CrossRef]
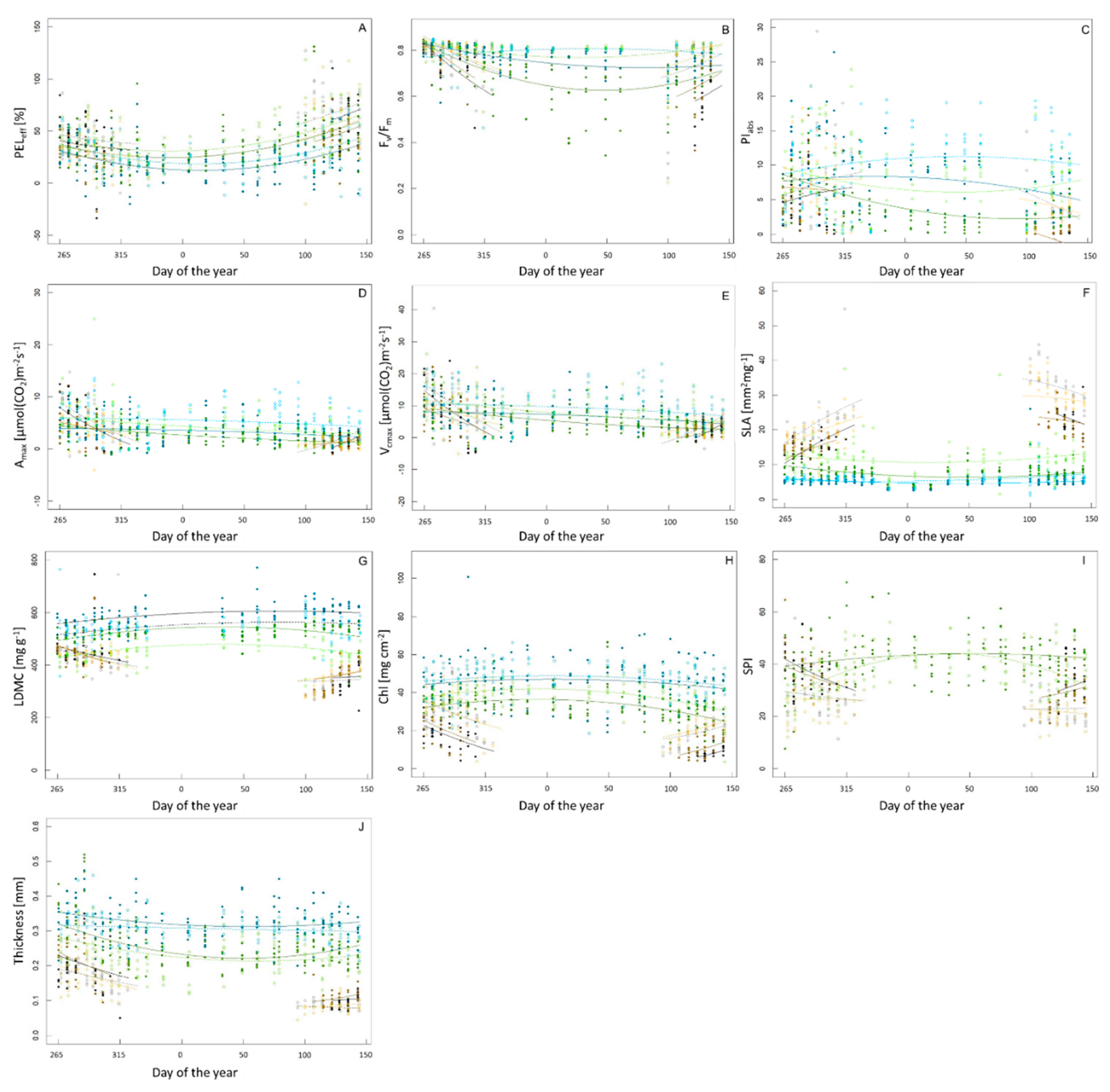

| Species | Lifeform | Canopy Duration [d] | PELeff [%] | Fv/Fm | PIabs | Amax [µmol(CO2)m−2 s−1] | Vcmax [µmol(CO2)m−2 s−1] | SLA [mm2 mg−1] | LDMC [mg g−1] | Chl [mg cm−2] | SPI | Thickness [mm] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA | F4,794 = 38.7 *** R2 = 0.16 | F7,647 = 15.9 *** R2 = 0.15 | F4,650 = 30.2 *** R2 = 0.16 | F7,764 = 10.7 *** R2 = 0.09 | F7,752 = 8.0 *** R2 = 0.07 | F7,748 = 289 *** R2 = 0.73 | F7,688 = 170 *** R2 = 0.63 | F7,781 = 155.2 *** R2 = 0.58 | F5,516 = 69.6 *** R2 = 0.40 | F4,794 = 299.2 *** R2 = 0.60 | ||
| Q. ilex inside | evergreen | 27.4 [−13.0; 77.4] | 0.79 [0.46; 0.85] | 9.7 [0.03; 21.5] | 5.3 [−0.38; 13.6] | 9.4 [−0.34; 23.0] | 5.8 [1.1; 16.1] | 545.9 [358.1; 767.6] | 46.9 [18.5; 66.4] | - | 0.31 [0.21; 0.46] | |
| Q. ilex outside | evergreen | 23.2 [−19.8; 76.2] | 0.76 [0.57; 0.83] | 8.2 [1.3; 26.4] | 3.3 [−1.1; 9.9] | 6.3 [−3.5; 20.6] | 5.1 [2.6; 26.0] | 588.2 [491.5; 770.6] | 45.3 [19.2; 100.8] | - | 0.32 [0.20; 0.48] | |
| Q. rhysophylla inside | evergreen | 43.3 [−4.2; 118.7] | 0.80 [0.51; 0.84] | 8.0 [0.04; 23.9] | 4.2 [−0.26; 25.0] | 7.6 [−0.22; 26.2] | 11.7 [1.4; 37.5] | 456.2 [320.2; 579.3] | 38.0 [3.6; 52.1] | 37.6 [18.6; 56.1] | 0.23 [0.11; 0.37] | |
| Q. rhysophylla outside | evergreen | 34.6 [−1.6; 131.2] | 0.70 [0.34; 0.85] | 4.2 [0.12; 18.2] | 2.5 [−1.3; 9.6] | 5.1 [−3.9; 19.0] | 7.5 [3.3; 13.2] | 522.4 [332.2; 575.2] | 32.8 [13.7; 66.5] | 42.1 [7.6; 71.2] | 0.26 [0.17; 0.52] | |
| Q. palustris inside | deciduous | 266–331; 105–155 | 53.3 [−9.5; 127.3] | 0.77 [0.25; 0.84] | 6.3 [0.31; 29.4] | 3.5 [−1.4; 14.8] | 6.5 [−3.5; 40.5] | 26.4 [14.0; 54.8] | 391.9 [269.0; 744.8] | 24.5 [6.9; 52.9] | 25.5 [11.4; 57.6] | 0.14 [0.06; 0.33] |
| Q. palustris outside | deciduous | 266–323; 133–155 | 47.8 [−33.7; 110.5] | 0.72 [0.39; 0.84] | 3.2 [0.02; 13.6] | 3.9 [−0.87; 12.4] | 6.6 [−4.9; 24.0] | 17.4 [11.6; 32.4] | 417.4 [226.4; 745.9] | 14.4 [4.1; 36.2] | 35.7 [21.0; 55.3] | 0.16 [0.05; 0.35] |
| Q. rubra inside | deciduous | 266–331; 105–155 | 41.1 [−19.9; 117.7] | 0.78 [0.23; 0.84] | 6.6 [0.16; 21.5] | 2.7 [−4.1; 12.1] | 5.0 [−5.1; 16.4] | 23.5 [6.8; 39.3] | 402.4 [274.0; 494.6] | 25.6 [3.9; 48.7] | 23.9 [12.0; 42.7] | 0.13 [0.05; 0.26] |
| Q. rubra outside | deciduous | 266–308; 118–155 | 36.0 [−27.4; 81.3] | 0.73 [0.37; 0.84] | 3.4 [0.02; 17.2] | 2.7 [−0.99; 12.2] | 5.0 [−1.6; 22.1] | 18.3 [11.4; 30.7] | 404.1 [277.6; 656.4] | 15.7 [4.3; 31.25] | 33.7 [17.4; 64.5] | 0.16 [0.08; 0.33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preißer, M.; Bucher, S.F. Is the Seasonal Variation in Frost Resistance and Plant Performance in Four Oak Species Affected by Changing Temperatures? Forests 2021, 12, 369. https://doi.org/10.3390/f12030369
Preißer M, Bucher SF. Is the Seasonal Variation in Frost Resistance and Plant Performance in Four Oak Species Affected by Changing Temperatures? Forests. 2021; 12(3):369. https://doi.org/10.3390/f12030369
Chicago/Turabian StylePreißer, Maggie, and Solveig Franziska Bucher. 2021. "Is the Seasonal Variation in Frost Resistance and Plant Performance in Four Oak Species Affected by Changing Temperatures?" Forests 12, no. 3: 369. https://doi.org/10.3390/f12030369
APA StylePreißer, M., & Bucher, S. F. (2021). Is the Seasonal Variation in Frost Resistance and Plant Performance in Four Oak Species Affected by Changing Temperatures? Forests, 12(3), 369. https://doi.org/10.3390/f12030369







