Hybridisation of Malus sylvestris (L.) Mill. with Malus × domestica Borkh. and Implications for the Production of Forest Reproductive Material
Abstract
:1. Introduction
2. Materials and Methods
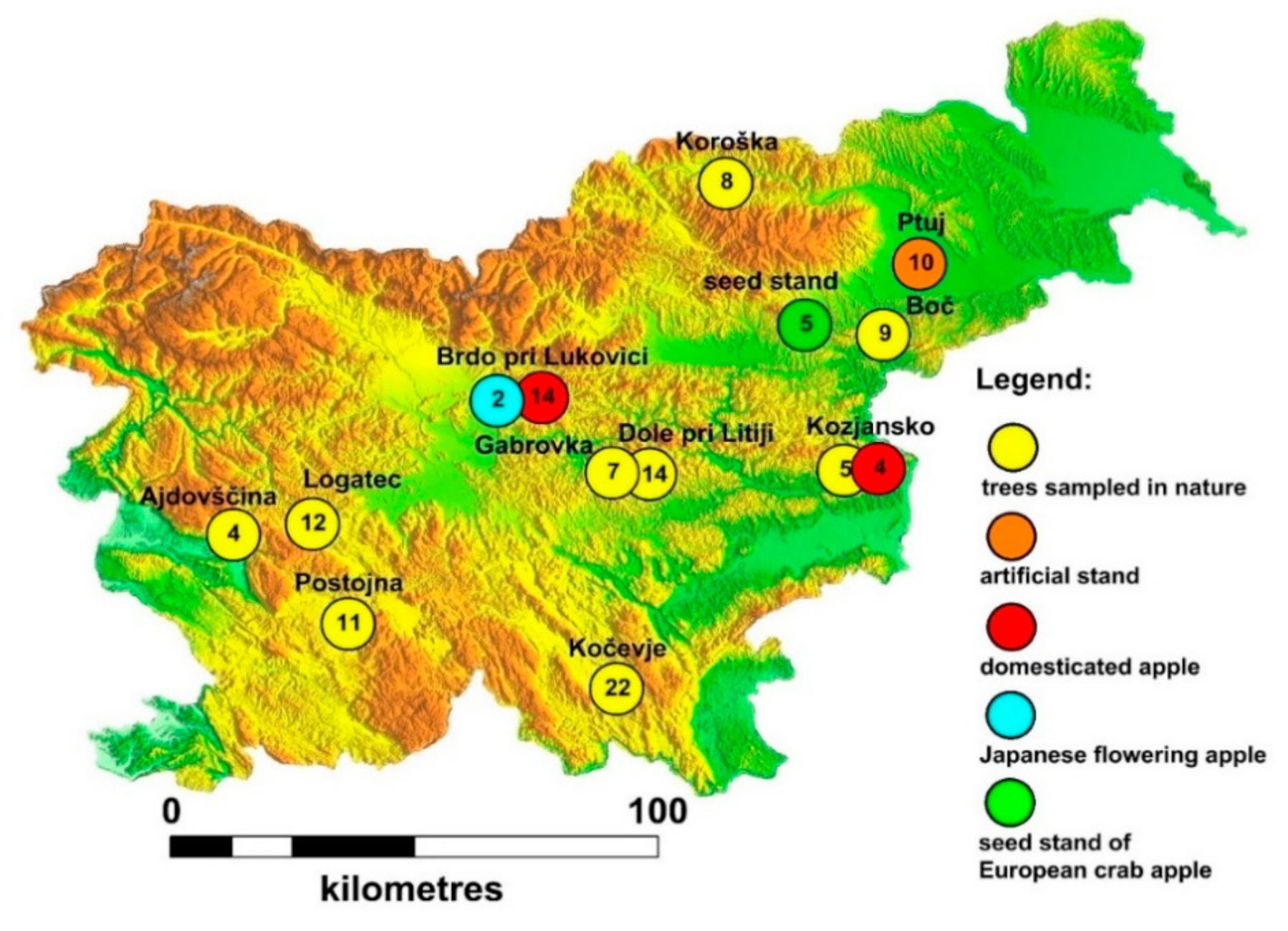
2.1. Area of Research and Sampling
2.2. Morphological Analysis
2.3. Genetic Analysis
3. Results
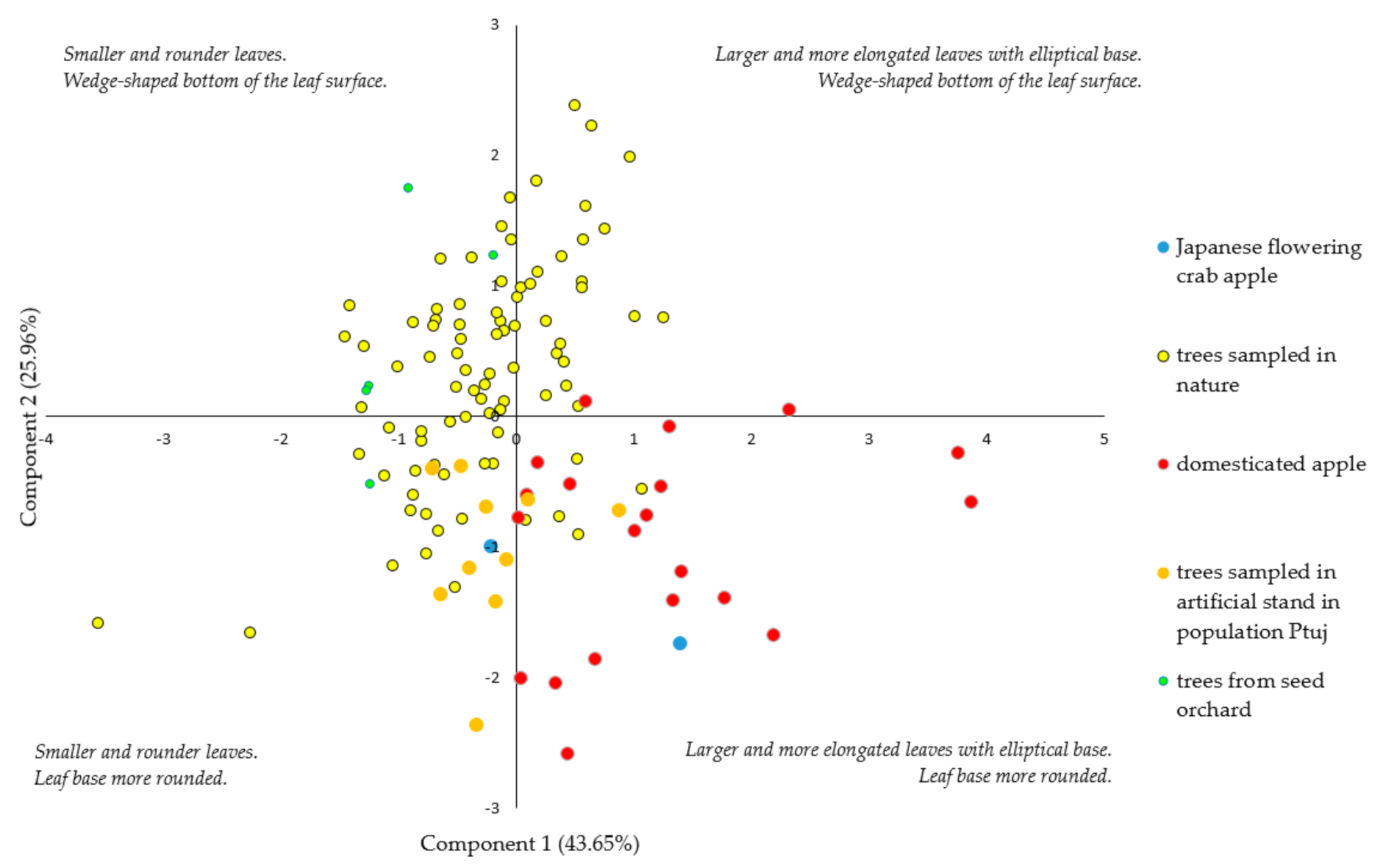
3.1. Morphological Identification of Trees
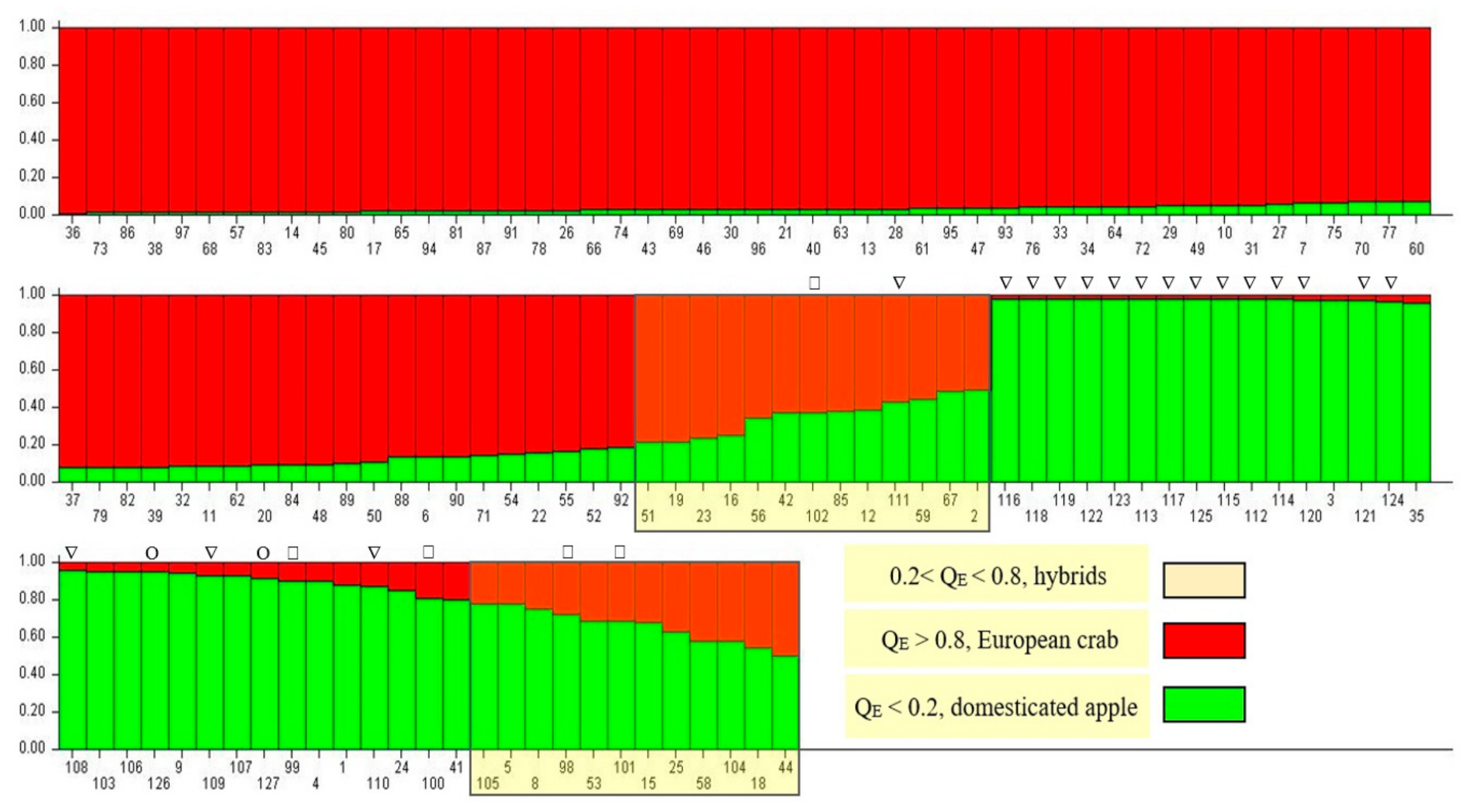
3.2. Genetic Identification of Trees
3.3. Comparison of Morphological and Genetic Identification of Trees
4. Discussion
4.1. Admixture Analysis and Genetic Identification of Trees
4.2. Comparison of Morphological and Genetic Traits
4.3. Implications for the Production of Forest Reproductive Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephan, B.R.; Wagner, I.; Kleinschmidt, J. Wild apple and pear (Malus sylvestris and Pyrus pyraster). In EUFORGEN Technical Guidelines for Genetic Conservation and Use; Bioversity International: Rome, Italy, 2003; p. 6. [Google Scholar]
- Spiecker, H. Manjšinske drevesne vrste—Izziv za večnamensko gozdarstvo [Minority tree species—A challenge for multipurpose forestry]. Gozdarski Vestnik 2006, 64, 123–133. [Google Scholar]
- Hamrick, J.L. Response of forest trees to global environmental changes. For. Ecol. Manag. 2004, 197, 322–335. [Google Scholar] [CrossRef]
- Cojzer, M.; Diaci, J.; Brus, R. Značilnosti zaraščanja na opuščenih kmetijskih zemljiščih v Halozah [Patterns of succession on abandoned agricultural land in Haloze]. Acta Silvae et Ligni. 2019, 119, 27–42. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.F.; Hellmann, J.J.; Boggs, C.L.; Ehrlich, P.R. Climate change hastens population extinctions. Proc. Natl. Acad. Sci. USA 2002, 99, 6070–6074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viršček Marn, M.; Stopar, M. Sorte Jabolk [Apple Varieties]; Kmečki Glas: Ljubljana, Slovenia, 1998. [Google Scholar]
- Coart, E. Molecular Contributions to the Conservation of Forest Genetic Resources in Flanders: Genetic Diversity of Malus sylvestris, Quercus spp. and Carpinus betulus. Ph.D. Thesis, University of Gent, Gent, Belgium, 2003. [Google Scholar]
- Jurick, W.M.; Janisewicz, W.J.; Saftner, R.A.; Vicos, I.; Gaskins, V.L.; Park, E.; Forsline, P.L.; Fazio, G.; Conway, W.S. Identification of wild apple germplasm (Malus spp.) accessions with resistance to the postharvest decay pathogens Penicillium expansum and Colletotrichum acutatum. Plant Breed. 2011, 130, 481–486. [Google Scholar] [CrossRef]
- Kik, C.; Korpelainen, H.; Vögel, R.; Asdal, Å.; Eliáš, P.; Draper, D. Malus sylvestris. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/172170/6841688 (accessed on 5 February 2021).
- Larsen, A.S.; Asmussen, C.B.; Coart, E.; Olrik, D.C.; Kjær, E.D. Hybridization and genetic variation in Danish populations of European crab apple (Malus sylvestris). Tree Genet. Genomes 2006, 2, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Jacques, D.; Vandermijnsbrugge, K.; Lemaire, S.; Antofie, A.; Lateur, M. Natural distribution and variability of wild apple (Malus sylvestris) in Belgium. Belg. J. Bot. 2009, 142, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Reim, S.; Proft, A.; Heinz, S.; Höfer, M. Diversity of the European indigenous wild apple Malus sylvestris (L.) Mill. in the East Ore Mountains (Osterzgebirge), Germany: I. Morphological characterization. Genet. Resour. Crop Evol. 2012, 59, 1101–1114. [Google Scholar] [CrossRef]
- Ignatov, A.; Bodishevskaya, A. Malus. In Wild Crop Relatives, Genomic and Breeding Resources: Temperate Fruits; Kole, C., Ed.; Springer: Clemson, SC, USA, 2011; pp. 45–64. [Google Scholar]
- Forte, A.V.; Ignatov, A.N.; Ponomarenko, V.V.; Dorokhov, D.B.; Savelev, N. Phylogeny of the Malus (Apple Tree) Species, Inferred from the Morphological Traits and Molecular DNA Analysis. Russ. J. Genet. 2002, 38, 1150–1160. [Google Scholar] [CrossRef]
- Duan, N.; Bai, Y.; Sun, H.; Wang, N.; Li, M.; Wang, X.; Jiao, W.; Legall, N.; Mao, L.; Wan, S.; et al. Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Gladieux, P.; Smulders, M.J.M.; Roldan-Ruiz, I.; Laurens, F.; Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New insight into the history of domesticated apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties. PLoS Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef] [Green Version]
- Lenormand, T. Gene flow and the limits to natural selection. Trends Ecol. Evol. 2002, 17, 183–189. [Google Scholar] [CrossRef]
- Lynch, M. The genetic interpretation of inbreeding depression and outbreeding depression. Evolution 1991, 45, 622–692. [Google Scholar] [CrossRef] [PubMed]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridization and introgression. Ann. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Feurtey, A.; Cornille, A.; Shykoff, J.; Snirc, A.; Giraud, T. Crop-to wild gene flow and its consequences for a wild fruit tree: Towards a comprehensive conservation strategy of the wild apple in Europe. Evol. Appl. 2017, 10, 180–188. [Google Scholar] [CrossRef]
- Bleeker, W.; Schmitz, U.; Ristow, M. Interspecific hybridization between alien and native plant species in Germany and its consequences for native biodiversity. Biol. Conserv. 2007, 137, 248–253. [Google Scholar] [CrossRef]
- Czarna, A.; Nowińska, R.; Gawrońska, B. Malus × oxysepala (M. × domestica Borkh. × M. sylvestris Mill.)—New spontaneous apple hybrid. Acta Soc. Bot. Pol. 2013, 82, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Cornille, A.; Gladieux, P.; Giraud, T. Crop-to-wild gene flow and spatial genetic structure in the closest wild relatives of the cultivated apple. Evol. Appl. 2013, 6, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldán–Ruiz, I.; Gladieux, P. The domestication and evolutionary ecology of apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.L.; Henk, A.D.; Forsline, P.L.; Richards, C.M.; Volk, G.M. Identification of interspecific hybrids among domesticated apple and its wild relatives. Tree Genet. Genomes 2012, 8, 1223–1235. [Google Scholar] [CrossRef]
- Larsen, A.S.; Jensen, M.; Kjær, E.D. Crossability between wild (Malus sylvestris) and cultivated (M. × domestica) apples. Silvae Genet. 2008, 57, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Larsen, A.D.; Kjær, E.D. Pollen mediated gene flow in a native population of Malus sylvestris and its implications for contemporary gene conservation management. Conserv. Genet. 2009, 10, 1637–1646. [Google Scholar] [CrossRef]
- Reim, S.; Höltken, A.; Höfer, M. Diversity of the European indigenous wild apple (Malus sylvestris (L.) Mill.) in the East Ore Mountains (Osterzgebirge), Germany: II. Genetic characterization. Genet. Resour. Crop. Evol. 2013, 60, 879–892. [Google Scholar] [CrossRef]
- Wagner, I.; Schmitt, H.P.; Maurer, W.; Tabel, U. Isozyme Polymorphism and Genetic Structure of Malus sylvestris (L.) Mill. Native in Western Areas of Germany with respect to Malus × domestica Borkh. Acta Hortic. 2004, 663, 545–550. [Google Scholar] [CrossRef]
- Wagner, I.; Maurer, W.D.; Lemmen, P.; Schmitt, H.P.; Wagner, M.; Binder, M.; Patzak, P. Hybridization and Genetic diversity in Wild Apple (Malus sylvestris (L.) Mill.) from Various Regions in Germany and from Luxembourg. Silvae Genet. 2014, 63, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Omasheva, M.Y.; Flachowsky, H.; Ryabushkina, N.A.; Pozharskiy, A.S.; Galiakparov, N.N.; Hanke, M.V. To what extent do wild apples in Kazahstan retain their genetic integrity? Tree Genet. Genomes 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Meirmans, P.; Rong, J.; Bartsch, D.; Ghosh, A.; De Jong, T.J. Introgression of crop alleles into wild or weedy populations. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 325–345. [Google Scholar] [CrossRef] [Green Version]
- Sagnard, F.; Deu, M.; Dembélé, D. Genetic diversity, structure, gene flow and evolutionary relationships within the Sorghum bicolor wild–weedy–crop complex in a western African region. Theor. Appl. Genet. 2011, 123, 1231–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenczewski, E.; Ronfort, J.; Chèvre, A.M. Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environ. Biosafety Res. 2003, 2, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornille, A.; Feurtey, A.; Gelin, U.; Ropars, J.; Misvanderbrugge, K.; Gladieux, P.; Giraud, T. Anthropogenic and natural drivers of gene flow in a temperate wild fruit tree: A basis for conservation and breeding programs in apples. Evol. Appl. 2015, 8, 373–384. [Google Scholar] [CrossRef]
- Delplancke, M.; Alvarez, N.; Anahí, E.; Joly, H.; Benoit, L.; Brouck, E.; Arrigo, N. Gene flow among wild and domesticated almond species: Insights from chloroplast and nuclear markers. Evol. Appl 2012, 5, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Reim, S.; Lochshmidt, F.; Proft, A.; Wolf, H.; Wolf, H. Species delimitation, genetic diversity and structure of the European indigenous wild pear (Pyrus pyraster) in Saxony, Germany. Genet. Resour. Crop. Evol. 2016, 63, 6. [Google Scholar] [CrossRef]
- Macková, L.; Vit, P.; Urfus, T. Crop-to-wild hybridization in cherries—Empirical evidence from Prunus fruticose. Evol. Appl. 2018, 11, 1748–1759. [Google Scholar] [CrossRef] [Green Version]
- Bourguiba, H.; Scotti, I.; Sauvage, C.; Zhebentyayeva, T.; Ledbetter, C.; Krška, B.; Remay, A.; D’Onofrio, C.; Iketani, H.; Christen, D.; et al. Genetic Structure of a Worldwide Germplasm Collection of Prunus armeniaca L. Reveals Three Major Diffusion Routes for Varieties Coming From the Species Center of Origin. Front. Plant Sci. 2020, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, P.; Woeste, K.; Zhang, S. Gene flow among wild and cultivated common walnut (Juglans regia) trees in the Qinling Mountains revealed by microsatellite markers. J. For. Res. 2020, 11–13. [Google Scholar] [CrossRef]
- Brunet, J.E.; Zalpa, F.P.; Santini, A. Hybridization and introgression between the exotic Siberian elm Ulmus pumila and the native field elm Ulmus minor. Biol. Invasions 2013, 15, 2717–2730. [Google Scholar] [CrossRef]
- Broeck, A.V.; Cox, K.; Villar, M. Natural hybridization and potential seed set of sympatric Populus nigra and Populus ×canadensis along the river IJzer in Flanders (Belgium). Plant Ecol. Evol. 2012, 3, 341–349. [Google Scholar] [CrossRef]
- Brus, R. Dendrologija za Gozdarje [Dendrology or Foresters]; Biotechnical Faculty: Ljubljana, Slovenia, 2008. [Google Scholar]
- Coart, E.; Vekemans, X.; Smulders, M.J.; Wagner, I.; Huylenbroeck, J.; Van Bockstaele, E.; Roldan—Ruiz, I. Genetic variation in the endangered wild apple (Malus sylvestris (L.) Mill.) in Belgium as revealed by amplified fragment length polymorphism and microsatellite markers. Mol. Ecol. 2003, 12, 845–857. [Google Scholar] [CrossRef]
- Godec, B. Jablanove Sorte Travniških Sadovnjakov [Apple Varieties of Meadow Orchards]; Agricultural Institute of Slovenia: Ljubljana, Slovenia, 2006. [Google Scholar]
- National List of Forest Seed Objects in Slovenia. Available online: https://www.uradni-list.si/ (accessed on 25 February 2018).
- IBM SPSS Statistics for Windows, version 21.0; IBM Corp: Armonk, NY, USA, 2012.
- Perrier, X.; Jacquemound-Collet, J.P. DARwin Software. 2006. Available online: http://darwin.cirad.fr/ (accessed on 11 March 2021).
- Qiagen, DNeasy® Plant Handbook. Available online: www.qiagen.com (accessed on 31 March 2015).
- Patocchi, A.; Fernandez-Fernandez, F.; Evans, K.; Gobbin, D.; Rezzonico, F.; Budichevskaia, A.; Dunemann, F.; Stankiewicz-Kosyl, M.; Mathis-Jeanneteau, F.; Durel, C.E.; et al. Development and test of 21 multiplex SSRs spanning most of the apple genome. Tree Genet. Genomes 2008, 5, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multi-locus genotype. Genetics 2000, 155, 945–959. [Google Scholar]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Cornille, A.; Giraud, T.; Bellard, C.; Tellier, A.; Cam, B.; Smulders, M.J.M.; Kleinschmit, J.; Roldan-Ruiz, I.; Gladieux, P. Postglacial recolonization history of the European crab apple (Malus sylvestris Mill.), a wild contributor to the domesticated apple. Mol. Ecol. 2013, 22, 2249–2263. [Google Scholar] [CrossRef]
- Koopman, W.J.M.; Yinghui, L.; Coart, E.; Van de Weg, E.; Vosman, B.; Roldan Ruiz, I.; Marinus, J.M.; Smulders, M. Linked vs. Unlinked markers: Multi-locus microsatellite haplotype-sharing as a tool to estimate gene flow and introgression. Mol. Ecol. 2007, 16, 243–256. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlex 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Ruhsam, M.; Jessop, W.; Cornille, A.; Renny, J.; Worrell, R. Crop—To–Wild introgression in the European wild apple Malus sylvestris in Northern Britain. Int. J. For. Res. 2018, 92, 85–96. [Google Scholar] [CrossRef]
- Ganopoulos, I.V.; Aravanopoulos, F.A.; Tsaftaris, A. Genetic differentiation and gene flow between wild and cultivated Prunus avium: An analysis of molecular genetic evidence at a regional scale. Plant Biosyst. 2013, 147, 1–8. [Google Scholar] [CrossRef]
- Reim, S.; Proft, A.; Heinz, S.; Lochschmidt, F.; Hofer, M.; Trober, U.; Wolf, H. Pollen movement in a Malus sylvestris population and conclusions for conservation measures. Plant Genet. Resour. 2015, 15, 12–20. [Google Scholar] [CrossRef]
- Worrel, R.; Ruhsam, M.; Renny, J.; Jessop, W.; Findlay, G. The Ecology and Genetics of Scotland’s Native Wild apple: Malus sylvestris. Reforesting Scotl. 2018, 56, 32–34. [Google Scholar]
- Reiseberg, L.H.; Ellstrand, N.C.; Arnold, M. What can molecular and morphological markers tell us about plant hybridization? Crit. Rev. Plant Sci. 2011, 12, 213–241. [Google Scholar] [CrossRef]
- Watano, Y.; Kanai, A.; Tani, N. Genetic structure of hybrid zones between Pinus pumila and Pinus parviflora var. Pentaphylla (Pinaceae) reveals by molecular hybrid index analysis. Am. J. Bot. 2004, 91, 65–72. [Google Scholar] [CrossRef]
- Kišek, M.; Jarni, K.; Brus, R. Morfološka variabilnost lesnike (Malus sylvestris (L.) Mill.) v Sloveniji in smernice za njeno dolgoročno ohranitev [Morphological Variability of European Crab Apple (Malus sylvestris (L.) Mill.) in Slovenia and Guidelines for its Long-term Preservation]. Gozdarski Vestnik. 2015, 9, 355–368. [Google Scholar]
- Ballian, D.; Kajba, D. Oplemenjivanje Šumskog Drveća i Očuvanje Njegove Genetske Raznolikosti [Breeding of Forest Trees and Preservation of Its Genetic Diversity]; Faculty of Forestry: Zagreb, Croatia, 2011. [Google Scholar]
- Graudal, L.; Kjær, E.D.; Canger, S. A systematic approach to the conservation of genetic resources of trees and shrubs in Denmark. For. Ecol. Manag. 1995, 73, 117–134. [Google Scholar] [CrossRef]
- Kleinschmit, J.R.G.; Hosius, B.; Leinemann, L. Gefahrdung von Wildapfelsamenplantagen durch Genfluss [Risk of gene flow in wild apple seed orchards]. Forstarchiv 2012, 83, 19–25. [Google Scholar] [CrossRef]

| Morphological Trait | Abbreviation |
|---|---|
| Leaf area | A (cm2) |
| Leaf circumference 1 | LC (cm) |
| Leaf width/Leaf length ratio 1 | W/LL |
| Shape coefficient 1 | SC |
| Leaf length | LL (cm) |
| Leaf width | W (cm) |
| Position of max. leaf width (%) | PW% |
| Width at 70% | W70% (cm) |
| Width at 80% | W80% (cm) |
| Angle at 5% length | A5% (°) |
| Angle at 15% length | A15% (°) |
| Petiole length | PL (cm) |
| Locus Name | Allele Size Range | Nucleotide Sequence of the forward and Reverse Primer (5′→3′) | Repeated Motif | Number of Alleles per Locus | PIC Value | Studies in which the Locus Was Previously Studied |
|---|---|---|---|---|---|---|
| CH01h10 * | 94–114 | tgc aaa gat agg tag ata tat gcc a agg agg gat tgt ttg tgc ac | (ag)21 | 5 | 0.91 | [10,28,44,53,54] |
| CH04c07 * | 98–135 | ggc ctt cca tgt ctc aga ag cct cat gcc ctc cac taa ca | (ga) | 8 | 0.87 | [10,28,53] |
| CH01h01 * | 114–134 | gaa aga ctt gca gtg gga gc gga gtg ggt ttg aga agg tt | (ag)25.5 | 6 | 0.85 | [10,28,44,53] |
| CH02b03b ** | 77–109 | ata agg ata caa aaa ccc tac aca g gac atg ttt ggt tga aaa ctt g | (ga)22 | 8 | 0.90 | [23,54] |
| CH02b12 ** | 101–143 | ggc agg ctt tac gat tat gc ccc act aaa agt tca cag gc | (ga)26 | 13 | 0.92 | [44,53] |
| MS06g03 ** | 154–190 | cgg agg gtg tgc tgc cga ag gcc cag ccc ata tct gct | (ga) | 9 | 0.93 | [23,53] |
| CH02b07 *** | 180–202 | cca gac aag tca tca caa cac tc atg tcg atg tcg ctc tgt tg | (ga) | 7 | 0.90 | [10,54] |
| CH02c11 *** | 219–239 | tga agg caa tca ctc tgt gc ttc cga gaa tcc tct tcg ac | (ga) | 7 | 0.70 | [10,28,54] |
| CH03d11 *** | 115–181 | acc cca cag aaa cct tct cc caa ctg caa gaa tcg cag ag | (ga) | 6 | 0.91 | [54] |
| CH02a10 *** | 143–177 | atg cca atg cat gag aca aa aca cgc agc tga aac act tg | (ga) | 6 | 0.94 | [54] |
| Morphological Characteristics | Component 1 | Component 2 | Component 3 |
|---|---|---|---|
| Leaf area (A) | 0.944 | −0.189 | 0.093 |
| Leaf length (LL) | 0.739 | −0.597 | 0.001 |
| Leaf width (W) | 0.305 | 0.017 | 0.916 |
| Max. leaf width (PW%) | −0.101 | −0.025 | 0.928 |
| Width at 70% (W70%) | 0.933 | 0.183 | 0.221 |
| Width at 80% (W80%) | 0.900 | 0.189 | 0.204 |
| Angle at 5% length (A5%) | −0.028 | 0.949 | −0.048 |
| Angle at 15% length (A15%) | 0.008 | 0.986 | 0.050 |
| Petiole length (PL) | 0.696 | −0.184 | −0.228 |
| Eigenvalues | 9.92 | 2.33 | 1.66 |
| % of variability (%) | 43.65 | 25.96 | 18.48 |
| Cumulative variability (%) | 43.65 | 69.62 | 88.10 |
| Group 1 | Group 2 | Group 3 | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average | CV (%) | MSE | Average | CV (%) | MSE | Average | CV (%) | MSE | ||
| A (cm2) | 16.04 | 20.03 | 3.21 | 17.01 | 13.42 | 2.28 | 23.51 | 26.53 | 6.24 | <0.001 *** |
| LC (cm2) | 17.25 | 10.45 | 1.80 | 16.99 | 6.33 | 1.08 | 25.24 | 18.77 | 4.74 | <0.001 *** |
| W/LL | 0.60 | 10.37 | 0.60 | 0.74 | 7.76 | 0.60 | 0.44 | 13.53 | 0.6 | <0.001 *** |
| FC | 0.67 | 8.46 | 0.06 | 0.74 | 5.47 | 0.04 | 0.50 | 22.24 | 0.11 | <0.001 *** |
| LL (cm) | 6.08 | 10.98 | 0.67 | 5.66 | 7.48 | 0.42 | 7.62 | 11.95 | 0.91 | <0.001 *** |
| W (cm) | 4.57 | 9.74 | 0.45 | 4.54 | 5.15 | 0.23 | 4.41 | 14.17 | 0.62 | <0.001 *** |
| PW% | 44.67 | 15.95 | 7.13 | 45.41 | 5.15 | 2.34 | 38.17 | 15.71 | 5.99 | 0.005 * |
| W70% (cm) | 3.10 | 12.67 | 0.39 | 3.45 | 8.55 | 0.30 | 3.61 | 16.97 | 0.61 | <0.001 *** |
| W80% (cm) | 2.37 | 15.67 | 0.37 | 2.72 | 11.33 | 0.31 | 2.86 | 18.47 | 0.53 | <0.001 *** |
| A5% (°) | 57.91 | 12.06 | 6.99 | 67.95 | 6.28 | 4.27 | 53.55 | 13.50 | 7.23 | <0.001 *** |
| A15% (°) | 52.22 | 7.00 | 3.65 | 59.09 | 4.35 | 2.57 | 48.72 | 9.54 | 4.65 | <0.001 *** |
| SL (cm) | 2.95 | 19.88 | 0.59 | 3.20 | 10.13 | 0.32 | 3.82 | 16.51 | 0.63 | <0.001 *** |
| Trees Sampled in Nature | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | SR | A | AE | HT | FIS | FST | HO | HE | |
| CH01h10 | 95 | 88–166 | 23 | 6.63 | 0.92 | 0.13 | 0.09 | 0.72 | 0.83 |
| CH04c07 | 96 | 88–190 | 21 | 5.79 | 0.89 | 0.16 | 0.10 | 0.68 | 0.81 |
| CH01h01 | 92 | 100–135 | 15 | 4.93 | 0.85 | 0.34 | 0.07 | 0.53 | 0.79 |
| CH02b03b | 97 | 71–110 | 21 | 6.59 | 0.91 | 0.18 | 0.08 | 0.68 | 0.83 |
| CH02b12 | 95 | 110–174 | 29 | 6.84 | 0.93 | 0.35 | 0.10 | 0.54 | 0.84 |
| MS06g03 | 96 | 148–198 | 25 | 6.74 | 0.93 | 0.34 | 0.11 | 0.55 | 0.83 |
| CH02b07 | 97 | 92–127 | 15 | 6.33 | 0.90 | −0.03 | 0.07 | 0.86 | 0.83 |
| CH02c11 | 97 | 195–246 | 22 | 3.08 | 0.70 | −0.39 | 0.05 | 0.93 | 0.67 |
| CH03d11 | 97 | 95–179 | 25 | 7.70 | 0.92 | 0.02 | 0.07 | 0.84 | 0.86 |
| CH02a10 | 96 | 133–176 | 25 | 7.27 | 0.93 | 0.26 | 0.09 | 0.63 | 0.85 |
| average | 22.1 | 6.19 | 0.89 | 0.14 | 0.08 | 0.70 | 0.81 | ||
| Group 1 (European Crab Apple *) | |||||||||
| CH01h10 | 70 | 88–166 | 21 | 7.20 | 0.89 | 0.00 | 0.19 | 0.72 | 0.72 |
| CH04c07 | 71 | 94–190 | 17 | 6.40 | 0.86 | −0.02 | 0.19 | 0.71 | 0.70 |
| CH01h01 | 70 | 100–135 | 15 | 5.70 | 0.83 | 0.11 | 0.17 | 0.62 | 0.69 |
| CH02b03b | 71 | 73–109 | 19 | 6.90 | 0.89 | 0.01 | 0.20 | 0.70 | 0.71 |
| CH02b12 | 69 | 110–174 | 24 | 7.00 | 0.91 | 0.18 | 0.15 | 0.63 | 0.77 |
| MS06g03 | 70 | 150–198 | 22 | 7.10 | 0.92 | 0.21 | 0.20 | 0.59 | 0.74 |
| CH02b07 | 71 | 92–127 | 14 | 6.50 | 0.86 | −0.05 | 0.13 | 0.79 | 0.76 |
| CH02c11 | 71 | 195–246 | 17 | 5.80 | 0.71 | −0.50 | 0.08 | 0.98 | 0.66 |
| CH03d11 | 71 | 95–179 | 25 | 7.50 | 0.92 | −0.03 | 0.14 | 0.82 | 0.79 |
| CH02a10 | 71 | 133–176 | 23 | 7.60 | 0.93 | 0.28 | 0.14 | 0.57 | 0.80 |
| average | 19.70 | 6.77 | 0.87 | 0.02 | 0.16 | 0.71 | 0.73 | ||
| Group 2 (Hybrids *) | |||||||||
| CH01h10 | 23 | 88–117 | 15 | 3.64 | 0.91 | −0.12 | 0.23 | 0.78 | 0.69 |
| CH04c07 | 23 | 88–125 | 14 | 3.28 | 0.86 | −0.25 | 0.24 | 0.83 | 0.66 |
| CH01h01 | 22 | 104–133 | 9 | 2.58 | 0.80 | 0.35 | 0.45 | 0.29 | 0.44 |
| CH02b03b | 24 | 71–110 | 15 | 3.79 | 0.90 | 0.06 | 0.34 | 0.56 | 0.59 |
| CH02b12 | 24 | 110–151 | 19 | 4.20 | 0.92 | 0.08 | 0.22 | 0.65 | 0.71 |
| MS06g03 | 24 | 148–189 | 12 | 3.09 | 0.89 | 0.01 | 0.28 | 0.64 | 0.65 |
| CH02b07 | 24 | 98–127 | 12 | 3.88 | 0.87 | −0.22 | 0.23 | 0.82 | 0.67 |
| CH02c11 | 24 | 195–236 | 12 | 2.41 | 0.64 | −0.52 | 0.14 | 0.84 | 0.55 |
| CH03d11 | 24 | 95–129 | 16 | 3.58 | 0.84 | −0.26 | 0.20 | 0.85 | 0.68 |
| CH02a10 | 24 | 139–169 | 16 | 3.46 | 0.92 | −0.04 | 0.26 | 0.71 | 0.68 |
| average | 14.00 | 3.39 | 0.86 | −0.09 | 0.26 | 0.70 | 0.63 | ||
| Group 3 (Domesticated Apple *) | |||||||||
| CH01h10 | 30 | 88–111 | 10 | 2.46 | 0.83 | −0.38 | 0.49 | 0.59 | 0.42 |
| CH04c07 | 30 | 92–121 | 12 | 2.57 | 0.81 | −0.03 | 0.55 | 0.38 | 0.36 |
| CH01h01 | 28 | 104–131 | 8 | 2.17 | 0.83 | −0.07 | 0.48 | 0.46 | 0.43 |
| CH02b03b | 30 | 71–103 | 9 | 2.54 | 0.84 | −0.22 | 0.42 | 0.59 | 0.49 |
| CH02b12 | 29 | 110–155 | 17 | 3.17 | 0.87 | −0.02 | 0.45 | 0.49 | 0.48 |
| MS06g03 | 30 | 153–189 | 12 | 2.73 | 0.82 | −0.25 | 0.37 | 0.65 | 0.52 |
| CH02b07 | 30 | 92–127 | 10 | 2.73 | 0.78 | −0.39 | 0.32 | 0.74 | 0.53 |
| CH02c11 | 30 | 195–238 | 13 | 2.36 | 0.68 | −0.68 | 0.18 | 0.94 | 0.56 |
| CH03d11 | 30 | 108–179 | 14 | 2.93 | 0.84 | −0.38 | 0.26 | 0.85 | 0.62 |
| CH02a10 | 29 | 139–176 | 14 | 2.99 | 0.85 | −0.38 | 0.27 | 0.86 | 0.62 |
| average | 11.90 | 2.67 | 0.81 | −0.28 | 0.38 | 0.65 | 0.50 | ||
| Tree Identification Based on Genetic Data (Bayesian Method) | Tree Identification Based on Morphological Data (Ward Method) | |||||
|---|---|---|---|---|---|---|
| Trees | (%) | Group 1 (European Crab Apple Trees) (%) | Group 2 (Hybrids) (%) | Group 3 (Domesticated Apple Trees) (%) | Total (%) | |
| Group 1 (European crab apple) | 68 | 70.10% | 39 (40.20%) | 29 (29.90%) | 0 (0.00%) | 70.10% |
| Group 2 (hybrids) | 21 | 21.64% | 12 (12.37%) | 7 (7.21%) | 2 (2.06%) | 21.64% |
| Group 3 (domesticated apple) | 8 | 8.26% | 6 (6.18%) | 0 (0.00%) | 2 (2.06%) | 8.26% |
| Total | 97 | 100 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kišek, M.; Jarni, K.; Brus, R. Hybridisation of Malus sylvestris (L.) Mill. with Malus × domestica Borkh. and Implications for the Production of Forest Reproductive Material. Forests 2021, 12, 367. https://doi.org/10.3390/f12030367
Kišek M, Jarni K, Brus R. Hybridisation of Malus sylvestris (L.) Mill. with Malus × domestica Borkh. and Implications for the Production of Forest Reproductive Material. Forests. 2021; 12(3):367. https://doi.org/10.3390/f12030367
Chicago/Turabian StyleKišek, Mateja, Kristjan Jarni, and Robert Brus. 2021. "Hybridisation of Malus sylvestris (L.) Mill. with Malus × domestica Borkh. and Implications for the Production of Forest Reproductive Material" Forests 12, no. 3: 367. https://doi.org/10.3390/f12030367
APA StyleKišek, M., Jarni, K., & Brus, R. (2021). Hybridisation of Malus sylvestris (L.) Mill. with Malus × domestica Borkh. and Implications for the Production of Forest Reproductive Material. Forests, 12(3), 367. https://doi.org/10.3390/f12030367





