The Effects of Native Shrub, Fencing, and Acorn Size on the Emergence of Contrasting Co-Occurring Oak in Mediterranean Grazed Areas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Species Description
2.2. Experimental Design
2.3. Statistical Methods
3. Results
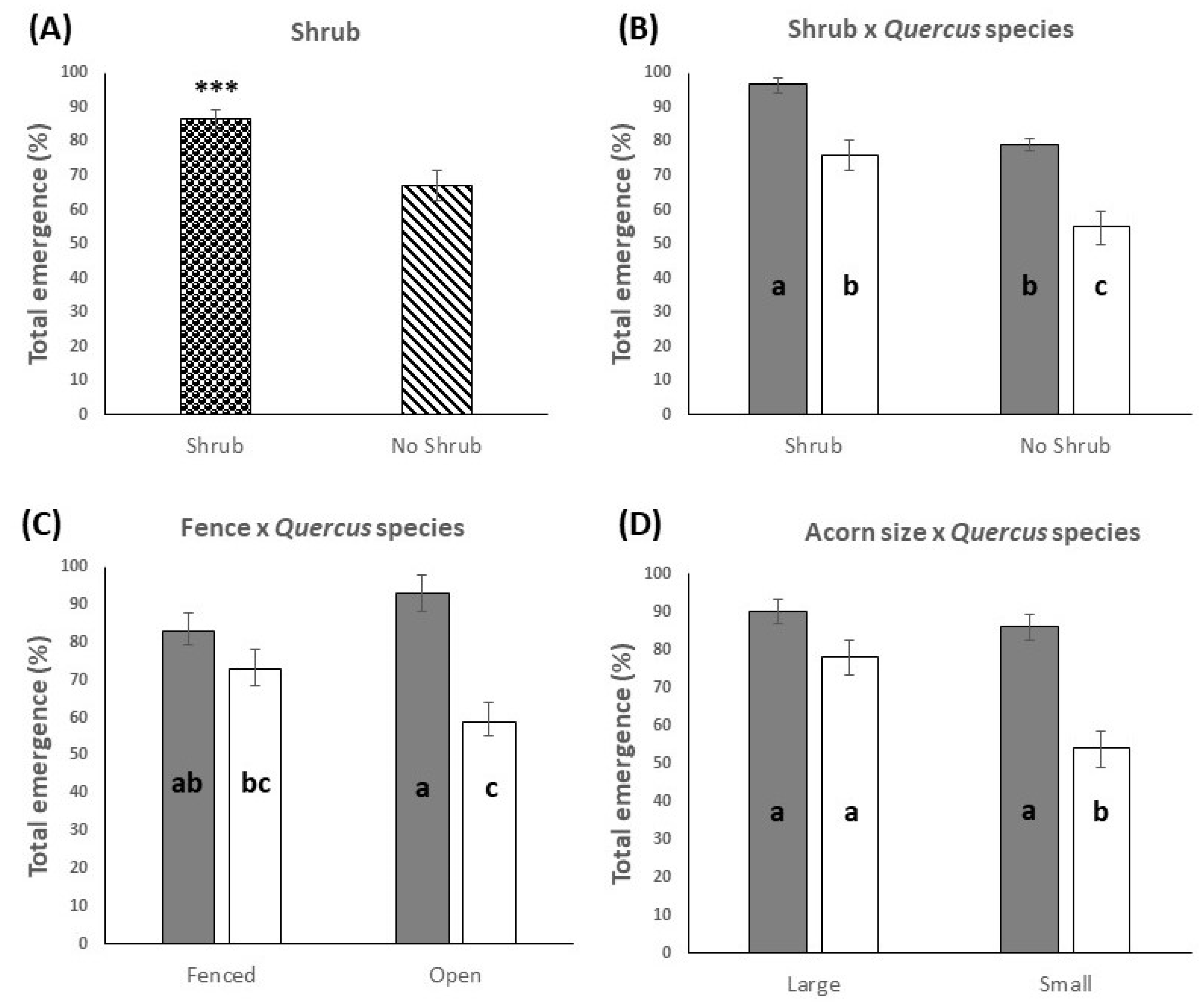
3.1. Emergence at the End of the Study Period
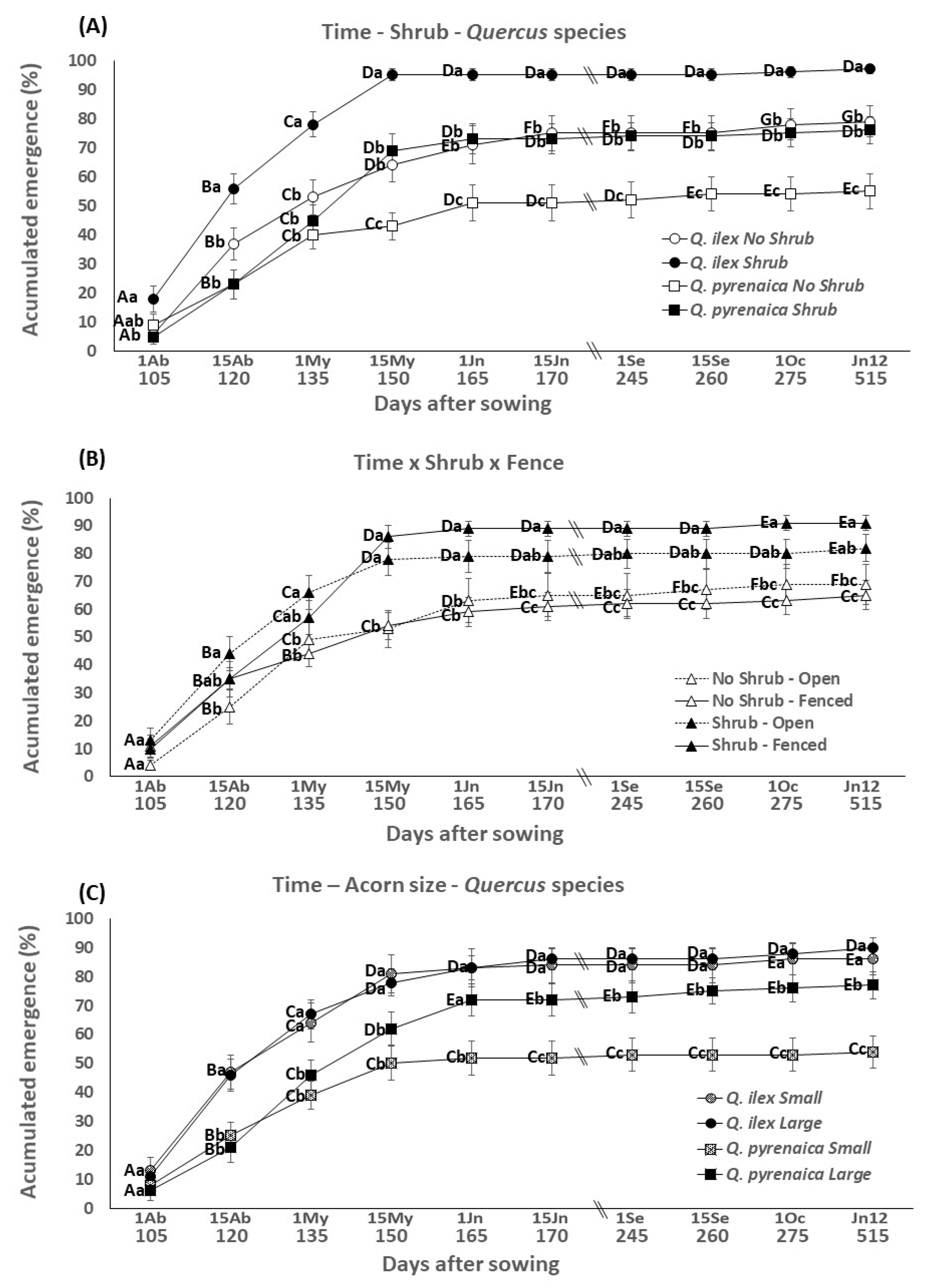
3.2. Emergence through the Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- McCreary, D. Managing and restoring California’s oak woodlands. Nat. Areas J. 2004, 24, 269–275. [Google Scholar]
- Bergmeier, E.; Petermann, J.; Schroder, E. Geobotanical survey of wood-pasture habitats in Europe: Diversity, threats and conservation. Biodivers. Conserv. 2010, 19, 2995–3014. [Google Scholar] [CrossRef]
- Beniston, M.; Diaz, H.F.; Bradley, R.S. Climatic change at high elevation sites: An overview. In Climatic Change at High Elevation Sites; Diaz, H.F., Beniston, M., Bradley, R.S., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 1–19. [Google Scholar]
- Rolo, V.; Plieninger, T.; Moreno, G. Facilitation of holm oak recruitment through two contrasted shrubs species in Mediterranean grazed woodlands. J. Veg. Sci. 2013, 24, 344–355. [Google Scholar] [CrossRef]
- Marañón, T.; Pugnaire, F.I.; Callaway, R.M. Mediterranean-climate oak savannas: The interplay between abiotic environment and species interactions. Web. Ecol. 2009, 9, 30–43. [Google Scholar] [CrossRef]
- Plieninger, T.; Bieling, C. Resilience-based perspectives to guiding high-nature-value farmland through socioeconomic change. Ecol. Soc. 2013, 18, 20. [Google Scholar] [CrossRef]
- Pulido, F.J.; Díaz, M.; Hidalgo de Trucios, S.J. Size structure and regeneration of Spanish holm oak Quercus ilex forests and dehesas: Effects of agroforestry use on their long-term sustainability. For. Ecol. Manag. 2001, 146, 1–13. [Google Scholar] [CrossRef]
- Pulido, F.J.; Díaz, M. Regeneration of a Mediterranean oak: A whole-cycle approach. Écoscience 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Navarro Cerrillo, R.M.; Fragueiro, B.; Ceaceros, C.; del Campo, A.; de Prado, R. Establishment of Quercus ilex L. subsp. ballota [Desf.] Samp. using different weed control strategies in southern Spain. Ecol. Eng. 2005, 25, 332–342. [Google Scholar] [CrossRef]
- Gómez, J.M.; Puerta-Piñero, C.; Schupp, E.W. Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 2008, 155, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pulido, F.; García, E.; Obrador, J.J.; Moreno, G. Multiple pathways for tree regeneration in anthropogenic savannas: Incorporating biotic and abiotic drivers into management schemes: Pathways for oak recruitment in savannas. J. Appl. Ecol. 2010, 47, 1272–1281. [Google Scholar] [CrossRef]
- Bertness, M.D.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Porta, J.; López-Acevedo, M.; Roquero de Laburu, C. Edafología Para la Agricultura y el Medio Ambiente, 3rd ed.; Mundi-Prensa: Madrid, España, 2003. [Google Scholar]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer: Dortrecht, The Netherlands, 2007. [Google Scholar]
- Rousset, O.; Lepart, J. Shrub facilitation of Quercus humilis regeneration in succession on calcareous grasslands. J. Veg. Sci. 1999, 10, 493–502. [Google Scholar] [CrossRef]
- García, D.; Obeso, J.R. Facilitation by herbivore mediated nurse plants in a threatened tree, Taxus baccata: Local effects and landscape level consistency. Ecography 2003, 26, 739–750. [Google Scholar] [CrossRef]
- Baraza, E.; Zamora, R.; Hodar, J.A. Conditional outcomes in plant–herbivore interactions: Neighbours matter. Oikos 2006, 113, 148–156. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Pérez-Ramos, I.M.; Mendoza, I.; Matías, L.; Quero, J.L.; Castro, J.; Zamora, R.; Marañón, T. Oak seedling survival and growth along resource gradients in Mediterranean forests: Implications for regeneration in current and future environmental scenarios. Oikos 2008, 117, 1683–1699. [Google Scholar] [CrossRef]
- Torroba-Balmori, P.; Zaldívar, P.; Alday, J.G.; Fernández-Santos, B.; Martínez-Ruiz, C. Recovering Quercus species on reclaimed coal wastes using native shrubs as restoration nurse plants. Ecol. Eng. 2015, 77, 146–153. [Google Scholar] [CrossRef]
- Costa, A.; Villa, S.; Alonso, P.; García-Rodríguez, J.A.; Martín, F.J.; Martínez-Ruiz, C.; Fernández-Santos, B. Can native shrubs facilitate the early establishment of contrasted co-occurring oaks in Mediterranean grazed areas? J. Veg. Sci. 2017, 28, 1047–1056. [Google Scholar] [CrossRef]
- Rolo, V.; Moreno, G. Shrub encroachment and climate change increase the exposure to drought of Mediterranean wood-pastures. Sci. Total Environ. 2019, 660, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Callaway, R.M.; Valladares, F.; Lortie, C.J. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 2009, 97, 199–205. [Google Scholar] [CrossRef]
- Madrigal-González, J.; García-Rodríguez, J.A.; Zavala, M.A. Shrub encroachment shifts the bioclimatic limit between marcescent and sclerophyllous oaks along an elevation gradient in west-central Spain. J. Veg. Sci. 2014, 25, 514–524. [Google Scholar] [CrossRef]
- Michalet, R.; Brooker, R.W.; Cavieres, L.A.; Kikvidze, Z.; Lortie, C.J.; Pugnaire, F.I.; Valiente-Banuet, A.; Callaway, R.M. Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 2006, 9, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments: Facilitation and stress in arid environments. J. Ecol. 2005, 93, 748–757. [Google Scholar] [CrossRef]
- Pigott, C.D.; Pigott, S. Water as a determinant of the distribution of trees at the boundary of the Mediterranean zone. J. Ecol. 1993, 81, 557. [Google Scholar] [CrossRef]
- Niinemets, U.; Valladares, F. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar]
- Rivas-Martínez, S.; Gandullo Gutiérrez, J.M.; Allué Andrade, J.L.; Montero de Burgos, J.L.; González Rebollar, J.L. Mapa de Series de Vegetación de España 1: 400 000 y Memoria; Instituto Nacional para la Conservación de la Naturaleza: Madrid, España, 1987. [Google Scholar]
- Quero, J.L.; Villar, R.; Maranon, T.; Zamora, R.; Poorter, L. Seed-mass effects in four Mediterranean Quercus species (Fagaceae) growing in contrasting light environments. Am. J. Bot. 2007, 94, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-González, J.; Ruiz-Benito, P.; Ratcliffe, S.; Rigling, A.; Wirth, C.; Zimmermann, N.E.; Zweifel, R.; Zavala, M.A. Competition drives oak species distribution and functioning in Europe: Implications under global change. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Gil-Pelegrin, E., Peguero-Pina, J., Sancho-Knapik, D., Eds.; Tree Physiology; Springer: Cham, Switzerland, 2017; Volume 7, pp. 513–538. [Google Scholar] [CrossRef]
- Lloret, F.; Casanovas, C.; Peñuelas, J. Seedling survival of Mediterranean shrubland species in relation to root: Shoot ratio, seed size and water and nitrogen use. Funct. Ecol. 1999, 13, 210–216. [Google Scholar] [CrossRef]
- Luis Calabuig, E.; Montserrat, P. Mapa fitoclimático de la provincia de Salamanca. In Estudio Integrado y Multidisciplinario de la Dehesa Salmantina; Centro Pirenaico de Biología Experimental: Salamanca, España, 1979; Volume 3, pp. 157–181. [Google Scholar]
- Fernández-Santos, B.; Gómez-Gutiérrez, J.M.; Moreno-Marcos, G. Effects of disturbance caused by traditional Spanish rural land use on the regeneration of Cytisus multiflorus. Appl. Veg. Sci. 1999, 2, 239–250. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef]
- Bonfil, C. The effect of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am. J. Bot. 1998, 85, 79–87. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, V.; Barrio, I.C.; Villar, R. Within-population variability influences early seedling establishment in four Mediterranean oaks. Acta Oecol. 2012, 41, 82–89. [Google Scholar] [CrossRef]
- Smit, C.; Ouden, J.; Vandenberghe, C.; Müller-Schärer, H. Nurse plants, tree saplings and grazing pressure: Changes in facilitation along a biotic environmental gradient. Oecologia 2007, 152, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; García, D.; Zamora, R. Impact of vertebrate acorn- and seedling-predators on a Mediterranean Quercus pyrenaica forest. For. Ecol. Manag. 2003, 180, 125–134. [Google Scholar] [CrossRef]
- Smit, C.; Ouden, J.; Díaz, M. Facilitation of Quercus ilex recruitment by shrubs in Mediterranean open woodlands. J. Veg. Sci. 2008, 19, 193–200. [Google Scholar] [CrossRef]
- Joët, T.; Ourcival, J.-M.; Dussert, S. Ecological significance of seed desiccation sensitivity in Quercus ilex. Ann. Bot. 2013, 111, 693–701. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Rodríguez-Calcerrada, J.; Ourcival, J.M.; Rambal, S. Quercus ilex recruitment in a drier world: A multi-stage demographic approach. Perspect. Plant. Ecol. Evol. Syst. 2013, 15, 106–117. [Google Scholar] [CrossRef]
- Farnsworth, E. The Ecology and Physiology of Viviparous and Recalcitrant Seeds. Annu. Rev. Ecol. Syst. 2000, 31, 107–138. [Google Scholar] [CrossRef]
- Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks physiological ecology. Exploring the functional diversity of genus Quercus L. Tree Physiol. 2017, 7, 547. [Google Scholar]
- Pugnaire, F.I.; Armas, C.; Maestre, F.T. Positive plant interactions in the Iberian Southeast: Mechanisms, environmental gradients, and ecosystem function. J. Arid Environ. 2011, 75, 1310–1320. [Google Scholar] [CrossRef]
- Smit, C.; Ouden, J.D.; Müller-Schärer, H. Unpalatable plants facilitate tree sapling survival in wooded pastures. J. Appl. Ecol. 2006, 43, 305–312. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Gómez, J.M.; Zamora, R.; Boettinger, J.L. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. J. Veg. Sci. 2005, 16, 191–198. [Google Scholar] [CrossRef]
- Muhamed, H.; Touzard, B.; Le Bagousse-Pinguet, Y.; Michalet, R. The role of biotic interactions for the early establishment of oak seedlings in coastal dune forest communities. For. Ecol. Manag. 2013, 297, 67–74. [Google Scholar] [CrossRef]
- Perea, R.; San Miguel, A.; Gil, L. Leftovers in seed dispersal: Ecological implications of partial seed consumption for oak regeneration. J. Ecol. 2011, 99, 194–201. [Google Scholar] [CrossRef]
- Perea, R.; San Miguel, A.; Martínez-Jáuregui, M.; Valbuena-Carabaña, M.; Gil, L. Effects of seed quality and seed location on the removal of acorns and beechnuts. Eur. J. For. Res. 2012, 131, 623–631. [Google Scholar] [CrossRef]
- Del Arco, J.M.; Beltrán, D.; Martínez-Ruiz, C. Risk for the natural regeneration of Quercus species due to the expansion of rodent species (Microtus arvalis). Behav. Ecol. Sociobiol. 2018, 72. [Google Scholar] [CrossRef]
- Leiva, M.J.; Fernández-Alés, R. Holm-oak (Quercus ilex subsp. balIota) acorns infestation by insects in Mediterranean dehesas and shrublands: Its effect on acorn germination and seedling emergence. For. Ecol. Manag. 2005, 212, 221–229. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.; Marañón, T.; Lobo, J.M.; Verdú, J.R. Acorn removal and dispersal by the dung beetle Thorectes lusitanicus Jeckel: Ecological and evolutionary implications. Ecol. Entomol. 2007, 32, 349–356. [Google Scholar] [CrossRef]
- Chaichi, M.R.; Saravi, M.M.; Malekian, A. Effects of livestock trampling on soil physical properties and vegetation cover (Case Study: Lar Rangeland, Iran). Int. J. Agric. Biol. 2005, 7, 1560–8530. [Google Scholar]
- Sigcha, F.; Pallavicini, Y.; Camino, M.J.; Martínez-Ruiz, C. Effects of short-term grazing exclusion on vegetation and soil in early succession of a Subhumid Mediterranean reclaimed coal mine. Plant. Soil 2018, 426, 197–209. [Google Scholar] [CrossRef]
- Komatsu, H.; Katayama, A.; Hirose, S.; Kume, A.; Higashi, N.; Ogawa, S.; Otsuki, K. Reduction in soil water availability and tree transpiration in a forest with pedestrian trampling. Agric. For. Meteorol. 2007, 146, 107–114. [Google Scholar] [CrossRef]
- Ciapała, S.; Adamski, P.; Zielonka, T. Tree ring analysis as an indicator of environmental changes caused by tourist trampling—A potential method for the assessment of the impact of tourists. Geochronometria 2014, 41, 392–399. [Google Scholar] [CrossRef]
- Salomón, R.; Lorenzo, Z.; Valbuena-Carabaña, M.; Nicolás, J.L.; Gil, L. Seed recalcitrant behavior of Iberian Quercus: A multispecies comparison. Austrian J. For. Sci. 2012, 129, 182–201. [Google Scholar]
- Urbieta, I.R.; Pérez-Ramos, I.M.; Zavala, M.A.; Marañón, T.; Kobe, R.K. Soil water content and emergence time control seedling establishment in three co-occurring Mediterranean oak species. Can. J. For. Res. 2008, 38, 2382–2393. [Google Scholar] [CrossRef]
- Quero, J.L.; Villar, R.; Perez-Ramos, I.M.; González-Rodríguez, V.; Urbieta, I.R.; Gómez-Aparicio, L.; Zavala, M.A.; Marañon, T.; Navarro-Cerrillo, R.M.; Zamora, R.; et al. Implicaciones ecológicas del peso de semilla en especies del género Quercus. Evidencias en condiciones controladas y experimentos de campo. In Proceedings of the 5° Congreso Forestal Español, Ávila, España; S.E.C.F.-Junta de Castilla y León: Ávila, España, 2009. [Google Scholar]
- Gómez, J.M. Bigger is not always better: Conflicting selective pressures on seed size in Quercus ilex. Evolution 2004, 58, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Castro-Díez, P.; Puyravaud, J.P.; Cornelissen, J.H.C. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 2000, 124, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.M.; Davis, F.W.; Mahall, B.E. The relative importance of factors affecting age-specific seedling survival of two co-occurring oak species in southern California. For. Ecol. Manag. 2008, 255, 3063–3074. [Google Scholar] [CrossRef]
- Mendoza, E.; Dirzo, R. Seed tolerance to predation: Evidence from the toxic seeds of the buckeye tree (Aesculus californica; Sapindaceae). Am. J. Bot. 2009, 96, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Piñero, C.; Gómez, J.M.; Zamora, R. Species-specific effects on topsoil development affect Quercus ilex seedling performance. Acta Oecol. 2006, 29, 65–71. [Google Scholar] [CrossRef]
- Metcalfe, D.J.; Grubb, P.J. The responses to shade of seedlings of very small-seeded tree and shrub species from tropical rain forest in Singapore. Funct. Ecol. 1997, 11, 215–221. [Google Scholar] [CrossRef]
- Fernández-Santos, B.; Moro, D.; Martínez-Ruiz, C.; Fernández, M.J.; Martín, F.J. Efectos del peso de la bellota y de la edad del árbol productor en las características regenerativas de Quercus ilex subsp. ballota. In Avances en la Restauración de Sistemas Forestales. Técnicas de Implantación; Martínez-Ruiz, C., Lario Leza, F.J., Fernández-Santos, B., Eds.; SECF-AEET: Madrid, Spain, 2013; pp. 198–202. [Google Scholar]
- Alonso-Crespo, I.M.; Silla, F.; Jiménez del Nogal, P.; Fernández, M.J.; Martínez-Ruiz, C.; Fernández-Santos, B. Effect of the mother tree age and acorn weight in the regenerative characteristics of Quercus faginea. Eur. J. For. Res. 2020, 139, 513–523. [Google Scholar] [CrossRef]
- Green, P.T.; Juniper, P.A. Seed mass, seedling herbivory and the reserve effect in tropical rainforest seedlings. Funct. Ecol. 2004, 18, 539–547. [Google Scholar] [CrossRef]
- Bossema, I. Jays and oaks: An eco-ethological study of a symbiosis. Behaviour 1979, 70, 1–117. [Google Scholar] [CrossRef]
- Choler, P.; Michalet, R.; Callaway, R.M. Facilitation and competition on gradientes in alpine plant communities. Ecology 2001, 82, 3295–3308. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A. Restoring Quercus pyrenaica forests using pioneer shrubs as nurse plants. Appl. Veg. Sci. 2006, 9, 137–142. [Google Scholar] [CrossRef]
- Wang, D.; Ba, L. Ecology of meadow steppe in northeast China. Rangel. J. 2008, 30, 247. [Google Scholar] [CrossRef]
- Madrigal-González, J.; García-Rodríguez, J.A.; Alarcos-Izquierdo, G. Testing general predictions of the stress gradient hypothesis under high inter- and intra-specific nurse shrub variability along a climatic gradient. J. Veg. Sci. 2012, 23, 52–61. [Google Scholar] [CrossRef]
- Laliberté, E.; Bouchard, A.; Cogliastro, A. Optimizing hardwood reforestation in old fields: The effects of treeshelters and environmental factors on tree seedling growth and physiology. Restor. Ecol. 2008, 16, 270–280. [Google Scholar] [CrossRef]
- Moreno-Rodríguez, J.M. Principales Conclusiones de la Evaluación Preliminar de los Impactos en España Por Efecto del Cambio Climático; Departamento de Ciencias Ambientales, Universidad de Castilla-La Mancha (UCLM): Toledo, España, 2005; pp. 1–35. [Google Scholar]
- Plieninger, T.; Rolo, V.; Moreno, G. Large-scale patterns of Quercus ilex, Quercus suber, and Quercus pyrenaica regeneration in Central-Western Spain. Ecosystems 2010, 13, 644–660. [Google Scholar] [CrossRef]
| Source | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Shrub | 1.681 | 1 | 1.681 | 20.016 | <0.001 |
| Fence | 0.001 | 1 | 0.001 | 0.012 | 0.915 |
| Quercus species | 2.585 | 1 | 2.585 | 30.770 | <0.001 |
| Size | 0.195 | 1 | 0.195 | 2.316 | 0.133 |
| Site | 0.691 | 4 | 0.173 | 2.056 | 0.097 |
| Shrub × Fence | 0.211 | 1 | 0.211 | 2.509 | 0.118 |
| Shrub × Quercus | 0 | 1 | 0 | 0.004 | 0.950 |
| Shrub × Size | 0.183 | 1 | 0.183 | 2.175 | 0.145 |
| Fence × Quercus | 0.724 | 1 | 0.724 | 8.621 | 0.005 |
| Fence × Size | 0.008 | 1 | 0.008 | 0.096 | 0.758 |
| Quercus × Size | 0.064 | 1 | 0.064 | 0.757 | 0.387 |
| Error | 5.460 | 65 | 0.084 |
| Source | Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Time | 85.208 | 13 | 6.554 | 236.622 | <0.001 |
| Time × Shrub | 2.007 | 13 | 0.154 | 5.574 | <0.001 |
| Time × Fence | 0.342 | 13 | 0.026 | 0.95 | 0.5 |
| Time × Quercus | 1.214 | 13 | 0.093 | 3.37 | <0.001 |
| Time × Size | 0.672 | 13 | 0.052 | 1.867 | 0.03 |
| Time × Shrub × Fence | 1.824 | 13 | 0.14 | 5.066 | <0.001 |
| Time × Shrub × Quercus | 0.791 | 13 | 0.061 | 2.196 | 0.008 |
| Time × Shrub × Size | 0.188 | 13 | 0.014 | 0.523 | 0.912 |
| Time × Fence × Quercus | 0.529 | 13 | 0.041 | 1.469 | 0.123 |
| Time × Fence × Size | 0.054 | 13 | 0.004 | 0.149 | 1 |
| Time × Quercus × Size | 0.51 | 13 | 0.039 | 1.415 | 0.146 |
| Error (Time) | 24.847 | 897 | 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Hernández, R.; Vicente Villardón, J.L.; Martínez-Ruiz, C.; Fernández-Santos, B. The Effects of Native Shrub, Fencing, and Acorn Size on the Emergence of Contrasting Co-Occurring Oak in Mediterranean Grazed Areas. Forests 2021, 12, 307. https://doi.org/10.3390/f12030307
Díaz-Hernández R, Vicente Villardón JL, Martínez-Ruiz C, Fernández-Santos B. The Effects of Native Shrub, Fencing, and Acorn Size on the Emergence of Contrasting Co-Occurring Oak in Mediterranean Grazed Areas. Forests. 2021; 12(3):307. https://doi.org/10.3390/f12030307
Chicago/Turabian StyleDíaz-Hernández, Roberto, José Luis Vicente Villardón, Carolina Martínez-Ruiz, and Belén Fernández-Santos. 2021. "The Effects of Native Shrub, Fencing, and Acorn Size on the Emergence of Contrasting Co-Occurring Oak in Mediterranean Grazed Areas" Forests 12, no. 3: 307. https://doi.org/10.3390/f12030307
APA StyleDíaz-Hernández, R., Vicente Villardón, J. L., Martínez-Ruiz, C., & Fernández-Santos, B. (2021). The Effects of Native Shrub, Fencing, and Acorn Size on the Emergence of Contrasting Co-Occurring Oak in Mediterranean Grazed Areas. Forests, 12(3), 307. https://doi.org/10.3390/f12030307









