Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Conventional Storage and Cryostorage of Seeds
2.3. Stratification
2.4. Germination and Seedling Emergence Test and Seedling Growth
2.5. DNA Isolation and Assessment of Global DNA Methylation Levels
2.6. Statistical Analysis
3. Results
3.1. Germination, Seedling Emergence and DNA Methylation in P. communis Seeds after WC Adjustment and Cryostorage for 24 h
3.2. Germination, Seedling Emergence and DNA Methylation in P. communis Seeds Conventionally Stored for 2 Years Prior to Cryostorage
3.3. DNA Methylation and the Height of Seedlings Derived from Cryostored Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D TLC | 2-dimensional thin-layer chromatography |
| C | cytosine |
| CWRnl | Crop Wild Relatives (CWR) in the Netherlands |
| HPLC | high-performance liquid chromatography |
| LN | liquid nitrogen |
| m5C | 5-methylcytosine in DNA |
| R | DNA methylation level expressed in % according to the formula provided in the manuscript |
| T | thymine |
| WC | water content |
References
- Colville, L.; Pritchard, W. Seed life span and food security. New Phytol. 2019, 2, 557–562. [Google Scholar] [CrossRef]
- Pence, V.C.; Ballesteros, D.; Walters, C.; Reed, B.M.; Philpott, M.; Dixon, K.W.; Pritchard, H.W.; Culley, T.M.; Vanhove, A.C. Cryobiotechnologies: Tools for expanding long-term ex situ conservation to all plant species. Biol. Conserv. 2020, 250, 108736. [Google Scholar] [CrossRef]
- Touchell, D.H.; Dixon, K.W. Cryopreservation of seed of Western Australian native species. Biodivers. Conserv. 1993, 2, 594–602. [Google Scholar] [CrossRef]
- Mitchell, J.; Johnston, I.G.; Bassel, G.W. Variability in seeds: Biological, ecological, and agricultural implications. J. Exp. Bot. 2017, 68, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Pritchrd, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 11, 614–621. [Google Scholar] [CrossRef]
- Reed, B.M.; Schwanke, S.; Shala, R. Pear seeds retain viability after liquid nitrogen immersion. HortScience 2001, 36, 1121–1122. [Google Scholar] [CrossRef]
- Walters, C. About the limited benefit of water content and temperature on orthodox seed longevity. S. Afr. J. Bot. 2007, 73, 495–496. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitnik, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Oliver, A.E.; Leprince, O.; Wolkers, W.F.; Hincha, D.K.; Heyer, A.G.; Crowe, J.H. Non disaccharide-based mechanisms of protection during drying. Cryobiology 2001, 43, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Kotlarski, S.; Tomaszewski, D.; Tylkowski, T.; Barciszewski, J.; Chmielarz, P.; Michalak, M. Changes in genomic 5-methylcytosine level mirror the response of orthodox (Acer platanoides L.) and recalcitrant (Acer pseudoplatanus L.) seeds to severe desiccation. Tree Physiol. 2018, 38, 617–629. [Google Scholar] [CrossRef] [PubMed]
- FAO/IPGRI. Genebank standards. In Food and Agriculture Organization of the United Nations; International Plant Genetic Resources Institute: Rome, Italy, 1994; pp. 24–29. [Google Scholar]
- Pritchard, H.W.; Dickie, J.B. Predicting seed longevity: The use and abuse of seed viability equations. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., Eds.; Royal Botanic Gardens: London, UK, 2003; pp. 655–721. [Google Scholar]
- Walters, C.; Wheeler, L.; Stanwood, P.C. Longevity of cryogenically stored seeds. Cryobiology 2004, 48, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Stanwood, P.C. Cryopreservation of seed germplasm for genetic conservation. In Cryopreservation of Plant Cells and Organs; Kartha, K.K., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 199–226. [Google Scholar]
- Wang, B.S.P.; Charest, P.J.; Downie, B. Ex situ Storage of Seeds, Pollen and In Vitro Cultures of Perennial Woody Plant Species; FAO Forestry Paper: Rome, Italy, 1993. [Google Scholar]
- Pence, V.C. Germination, desiccation and cryopreservation of seeds of Populus deltoides Bartr. Seed Sci. Technol. 1996, 24, 151–157. [Google Scholar]
- Pritchard, H.W.; Nadarajan, J. Cryopreservation of orthodox (desiccation tolerant) seeds. In Plant Cryopreservation: A Practical Guide; Reed, B., Ed.; Springer Publishing: New York, NY, USA, 2008; pp. 485–501. [Google Scholar]
- Kaczmarczyk, A.; Funnekotter, B.; Menon, A.; Phang, P.Y.; Al-Hanbali, A.; Bunn, E.; Ricardo, L.M. Current Issues in Plant Cryopreservation. In Current Frontiers in Cryobiology; Katkov, I.I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 417–438. [Google Scholar]
- Pritchard, H.W. Cryopreservation of Desiccation-Tolerant Seeds. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacy, G.N., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 185–202. [Google Scholar]
- Michalak, M.; Plitta, B.P.; Tylkowski, T.; Chmielarz, P.; Suszka, J. Desiccation tolerance and cryopreservation of seeds of black poplar (Populus nigra L.), a disappearing tree species in Europe. Eur. J. For. Res. 2014, 134, 53–60. [Google Scholar] [CrossRef]
- Plitta, B.P.; Michalak, M.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. DNA methylation of Quercus robur L. plumules following cryo-pretreatment and cryopreservation. Plant Cell Tiss. Org. 2014, 117, 31–37. [Google Scholar] [CrossRef]
- Walters, C.; Pence, V.C. The unique role of seed banking and cryobiotechnologies in plant conservation. Plants People Planet 2021, 3, 83–91. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Nery, J.R.; Castanon, R.; Ecker, J.R. Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol. 2017, 18, 171. [Google Scholar] [CrossRef]
- Chen, M.; Lin, J.Y.; Hur, J.; Pelletier, J.M.; Baden, R.; Pellegrini, M.; Harada, J.J.; Goldberg, R.B. Seed genome hypomethylated regions are enriched in transcription factor genes. Proc. Natl. Acad. Sci. USA 2018, 115, E8315–E8322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.L.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide High Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef]
- Dowen, R.H.; Pelizzola, M.; Schmitz, R.J.; Lister, R.; Dowen, J.M.; Nery, J.R.; Dixon, J.E.; Ecker, J.R. Widespread dynamic DNA methylation in response to biotic stress. Proc. Natl. Acad. Sci. USA 2012, 109, E2183–E2191. [Google Scholar] [CrossRef]
- Sørensen, M.B. Methylation of B-hordein genes in barley endosperm is inversely correlated with gene activity and affected by the regulatory gene Lys3. Proc. Natl. Acad. Sci. USA 1992, 89, 4119–4123. [Google Scholar] [CrossRef]
- Song, Q.X.; Lu, X.; Li, Q.T.; Chen, H.; Hu, X.Y.; Ma, B.; Zhang, W.K.; Chen, S.Y.; Zhang, J.S. Genome-wide analysis of DNA methylation in soybean. Mol. Plant 2013, 6, 961–974. [Google Scholar] [CrossRef]
- Portis, E.; Acqyardo, A.; Comino, C.; Lanteri, S. Analysis of DNA methylation during germination of pepper (Capsicum annum L.) seeds using methylation-sensitive amplification polymorphism (MSAP). Plant Sci. 2004, 166, 169–178. [Google Scholar] [CrossRef]
- Kenchanmane Raju, S.K.; Ritter, E.J.; Niederhuth, C.E. Establishment, maintenance, and biological roles of non-CG methylation in plants. Essays Biochem. 2019, 63, 743–755. [Google Scholar]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef] [PubMed]
- He, X.J.; Chen, T.; Zhu, J.K. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Mirouze, M.; Paszkowski, J. Epigenetic contribution to stress adaptation in plants. Curr. Opin. Plant Biol. 2011, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Barciszewska, M.Z.; Barciszewski, J.; Plitta, B.P.; Chmielarz, P. Global changes in DNA methylation in seeds and seedlings of Pyrus communis after seed desiccation and storage. PLoS ONE 2013, 8, e70693. [Google Scholar] [CrossRef]
- Li, J.-W.; Ozudogru, E.A.; Li, J.; Wang, M.R.; Bi, W.L.; Lambardi, M.; Wang, Q.C. Cryobiotechnology offorest trees: Recent advances and future prospects. Biodivers. Conserv. 2018, 27, 795–814. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Gonzalez-Benito, M.E.; Ibanez, M.A.; Pirredda, M.; Mira, S.; Martin, C. Application of the MSAP Technique to Evaluate Epigenetic Changes in Plant Conservation. Int. J. Mol. Sci. 2020, 21, 7459. [Google Scholar] [CrossRef]
- Harding, K. Genetic integrity of cryopreserved plant cells: A review. CryoLetters 2004, 25, 3–22. [Google Scholar]
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop Sci. 2011, 5, 778–800. [Google Scholar]
- Perez, J.; Araya-Valverde, E.; Carro, G.; Abdelnour-Esquivel, A. Analysis of Stress Indicators During Cryopreservation of Seeds of Landrace Maize (Zea mays). CryoLetters 2017, 38, 445–454. [Google Scholar]
- Lu, J.; Greene, S.; Reid, S.; Cruz, V.M.V.; Dierig, D.A.; Byrne, P. Phenotypic changes and DNA methylation status in cryopreserved seeds of rye (Secale cereale L.). Cryobiology 2018, 82, 8–14. [Google Scholar] [CrossRef]
- van Treuren, R.; Hoekstra, R.; van Hintum, T.J.L. Inventory and prioritization for the conservation of crop wild relatives in The Netherlands under climate change. Biol. Conserv. 2017, 216, 123–139. [Google Scholar] [CrossRef]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- Khoury, C.; Laliberté, B.; Guarino, L. Trends in ex situ conservation of plant genetic resources: A review of global crop and regional conservation strategies. Genet. Resour. Crop Evol. 2010, 57, 625–639. [Google Scholar] [CrossRef]
- CWRnl. Pyrus communis L. Available online: https://www.cwrnl.nl/en/CWRnl-1/CWRbybotanicalname/Pyrus-communis-L.-1.html (accessed on 22 February 2021).
- Suszka, B. After-ripening and germination of crab apple (Malus sylvestris) and common pear (Pyrus communis) seeds. Arbor. Kórnickie 1989, 34, 101–112. [Google Scholar]
- Wawrzyniak, M.; Michalak, M.; Chmielarz, P. Effect of different conditions of storage on seed viability and seedling growth of six European wild fruit woody plants. Ann. For. Sci. 2020, 77, 58. [Google Scholar] [CrossRef]
- Suszka, B.; Muller, C.; Bonnet-Masimbert, M. Seeds of Forest Broadleaves from Harvest to Sowing; INRA: Paris, France, 1986. [Google Scholar]
- Tang, Y.; Xiong, J.; Jiang, H.P.; Zheng, S.J.; Feng, Y.Q.; Yuan, B.F. Determination of Oxidation Products of 5-Methylcytosine in Plants by Chemical Derivatization Coupled with Liquid Chromatography/Tandem Mass Spectrometry Analysis. Anal. Chem. 2014, 86, 7764–7772. [Google Scholar] [CrossRef]
- Liu, S.; Dunwell, T.L.; Pfeifer, G.P.; Dunwell, J.M.; Ullah, I.; Wang, Y. Detection of Oxidation Products of 5-Methyl-2’-Deoxycytidine in Arabidopsis DNA. PLoS ONE 2013, 8, e84620. [Google Scholar] [CrossRef] [PubMed]
- Madugundu, G.S.; Cadet, J.; Wagner, J.R. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Res. 2014, 42, 7450–7460. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ballesteros, D.; Pritchard, H.W.; Walters, C. Dry architecture: Towards the understanding of the variation of longevity in desiccation-tolerant germplasm. Seed Sci. Res. 2020, 30, 142–155. [Google Scholar] [CrossRef]
- Sacandé, M.; Buitink, J.; Hoekstra, F.A. A study of water relations in neem (Azadirachta indica) seed that is characterized by complex storage behaviour. J. Exp. Bot. 2000, 344, 635–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hao, Y.J.; Liu, Q.L.; Deng, X.X. Effect of cryopreservation on apple genetic resources at morphological, chromosomal, and molecular levels. Cryobiology 2001, 43, 46–53. [Google Scholar] [CrossRef]
- Hao, Y.J.; You, C.X.; Deng, X.X. Analysis of ploidy and patterns of amplified fragment length polymorphism and methylation sensitive amplified polymorphism in strawberry plants recovered from cryopreservation. CryoLetters 2002, 23, 37–46. [Google Scholar] [PubMed]
- Kaity, A.; Ashmore, S.E.; Drew, R.A.; Dulloo, M.E. Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Rep. 2008, 27, 1529–1539. [Google Scholar] [CrossRef]
- Pedro, E.L.; Arroyo-Garcia, R.; Reed, B.M.; Revilla, M.A. Genetic and epigenetic stability of cryopreserved and cold-stored hops (Humulus lupulus L.). Cryobiology 2008, 57, 234–241. [Google Scholar]
- Sisunandar; Rival, A.; Turquay, P.; Samosir, Y.; Adkins, S.W. Cryopreservation of coconut (Cocos nucifera L.) zygotic embryos does not induce morphological, cytological or molecular changes in recovered seedling. Planta 2010, 232, 435–447. [Google Scholar] [CrossRef]
- Nuc, K.; Marszałek, M.; Pukacki, P.M. Cryopreservation changes the DNA methylation of embryonic axes of Quercus robur and Fagus sylvatica seeds during in vitro culture. Trees 2016, 30, 1831–1841. [Google Scholar] [CrossRef]
- Villalobos, A.; Arguedas, M.; Escalante, D.; Martinez, J.; Zevallos, B.E.; Cejas, I.; Yabor, L. Martinez-Montero, M.E.; Sershen; Lorenzo, J.C. Cryopreservation of sorghum seeds modifies germination and seedling growth but not field performance of adult plants. J. Appl. Bot. Food Qual. 2019, 92, 94–99. [Google Scholar]
- Arguedas, M.; Gomez, D.; Hernandez, L.; Engelmann, F.; Garramone, R.; Cejas, I.; Yabor, L.; Martinez-Montero, M.E.; Lorenzo, J.C. Maize seed cryo-storage modifies chlorophyll, carotenoid, protein, aldehyde and phenolics levels during early stages of germination. Acta Physiol. Plant. 2018, 40, 118. [Google Scholar] [CrossRef]
- Walters, C.; Engels, J.M.M. The effects of storing seeds under extremely dry conditions. Seed Sci. Res. 1998, 8, 3–8. [Google Scholar]
- Zevallos, B.; Cejas, I.; Engelmann, F.; Carputo, D.; Aversano, R.; Scarano, M.T.; Yanes, E.; Martinez-Montero, M.; Lorenzo, J.C. Phenotypic and molecular characterization of plants regenerated from non-cryopreserved and cryopreserved wild Solanum lycopersicum mill. Seeds. CryoLetters 2014, 35, 216–225. [Google Scholar] [PubMed]
- Raju, R.; Bryant, S.J.; Wilkinson, B.L.; Bryant, G. The need for novel cryoprotectants and cryopreservation protocols: Insights into the importance of biophysical investigation and cell permeability. Biochim. Biophys. Acta 2021, 129749. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Wesley-Smith, J.; Berjak, P.; Pammenter, N.W.; Walters, C. Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: An ultrastructural study of factors affecting cell and ice structures. Ann. Bot. Lond. 2014, 113, 695–709. [Google Scholar] [CrossRef]
- Wesley-Smith, J.; Walters, C.; Pammenter, N.W.; Berjak, P. Why is intracellular ice lethal? A microscopical study showing evidence of programmed cell death in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum. Ann. Bot. Lond. 2015, 115, 991–1000. [Google Scholar] [CrossRef]
- Falk, M.; Falková, I.; Kopečná, O.; Bačíková, A.; Pagáčová, E.; Šimek, D.; Golan, M.; Kozubek, S.; Pekarová, M.; Follett, S.E.; et al. Chromatin Architecture Changes and DNA Replication Fork Collapse Are Critical Features in Cryopreserved Cells That Are Differentially Controlled by Cryoprotectants. Sci. Rep. 2018, 8, 14694. [Google Scholar] [CrossRef]
- Wesley-Smith, J.; Walters, C.; Berjak, P.; Pammenter, N.W. Non-equilibrium cooling of Poncirus trifoliate (L.) embryonic axes at various water content. CryoLetters 2004, 25, 121–128. [Google Scholar] [PubMed]
- Len, J.S.; Koh, W.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef] [PubMed]
- Okotrub, K.A.; Surovtsev, N.V. Redox State of Cytochromes in Frozen Yeast Cells Probed by Resonance Raman Spectroscopy. Biophys. J. 2015, 109, 2227–2234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, U.; Cossu, T.A.; Davies, R.M.; Forest, F.; Dickie, J.B.; Breman, E. Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: The status of seed collections. Biodivers. Conserv. 2020, 29, 2901–2949. [Google Scholar] [CrossRef]
- Michalak, M.; Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.; Barciszewski, J.; Bujarska-Borkowska, B.; Chmielarz, P. Global 5-methylcytosine alterations in DNA during ageing of Quercus robur seeds. Ann. Bot. Lond. 2015, 116, 369–376. [Google Scholar] [CrossRef]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.; Martín, C. DNA methylation and integrity in aged seeds and regenerated plants. Seed Sci. Res. 2020, 30, 92–100. [Google Scholar] [CrossRef]
- Pirredda, M.; González-Benito, M.E.; Martín, C.; Mira, S. Genetic and Epigenetic Stability in Rye Seeds under Different Storage Conditions: Ageing and Oxygen Effect. Plants 2020, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.C.; Narsai, R.; Wang, Y.; Berkowitz, O.; Whelan, J.; Lewsey, M.G. Temporal tissue-specific regulation of transcriptomes during barley (Hordeum vulgare) seed germination. Plant J. 2020, 101, 700–715. [Google Scholar] [CrossRef]
- Ogneva, Z.V.; Dubrovina, A.S.; Kiselev, K.V. Age-associated alterations in DNA methylation and expression of methyltransferase and demethylase genes in Arabidopsis thaliana. Biol. Plant. 2016, 60, 628–634. [Google Scholar] [CrossRef]
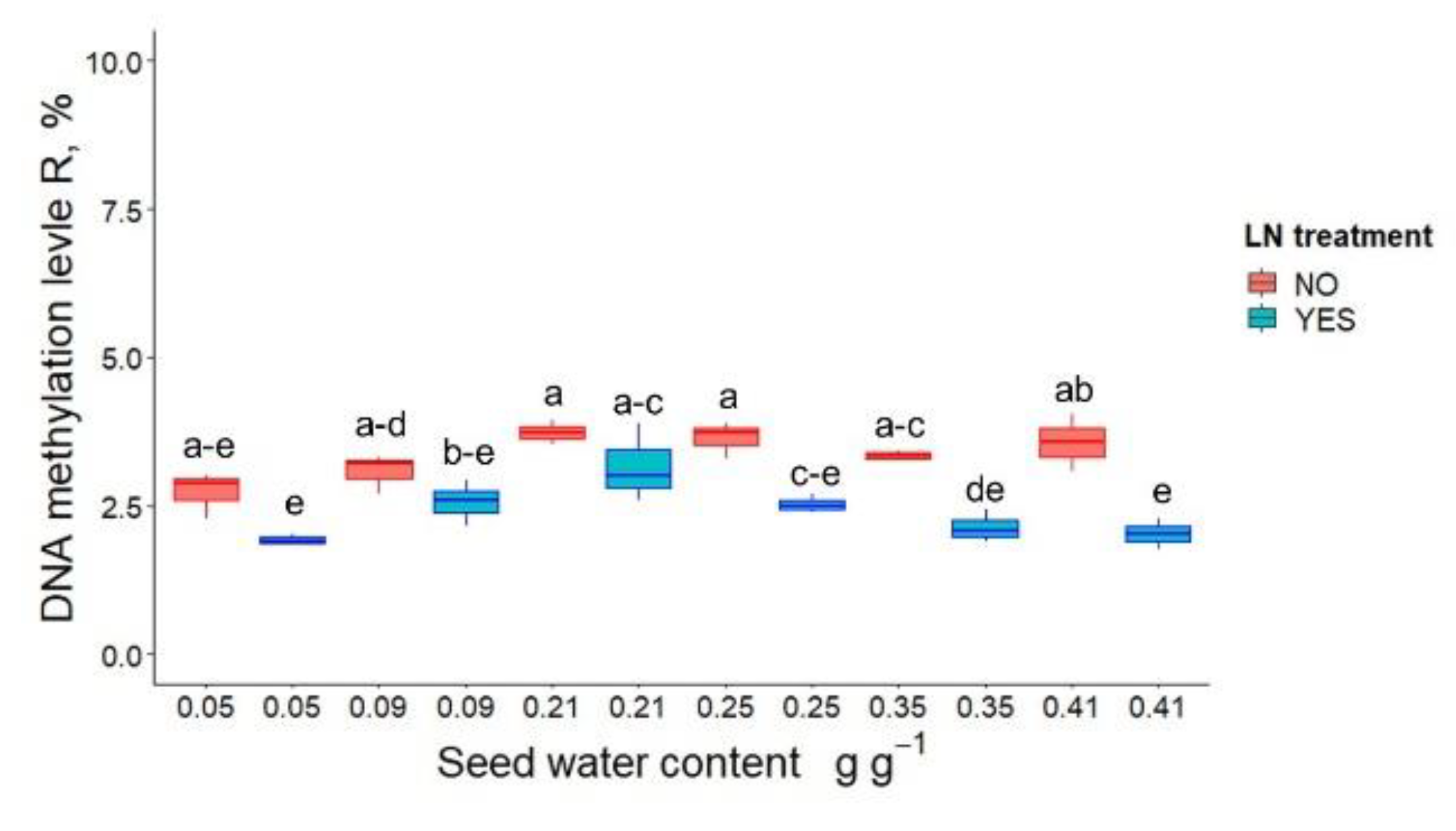
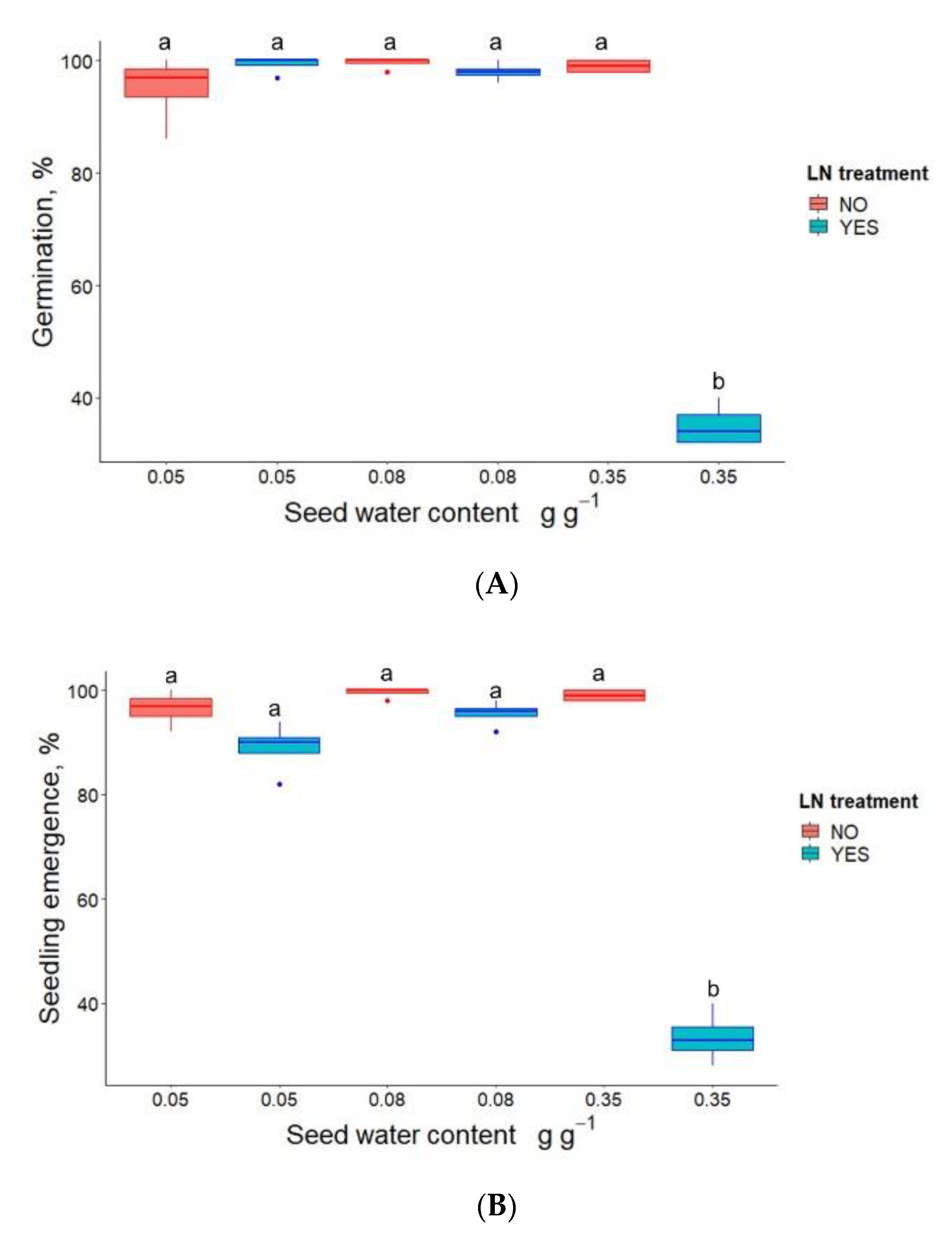

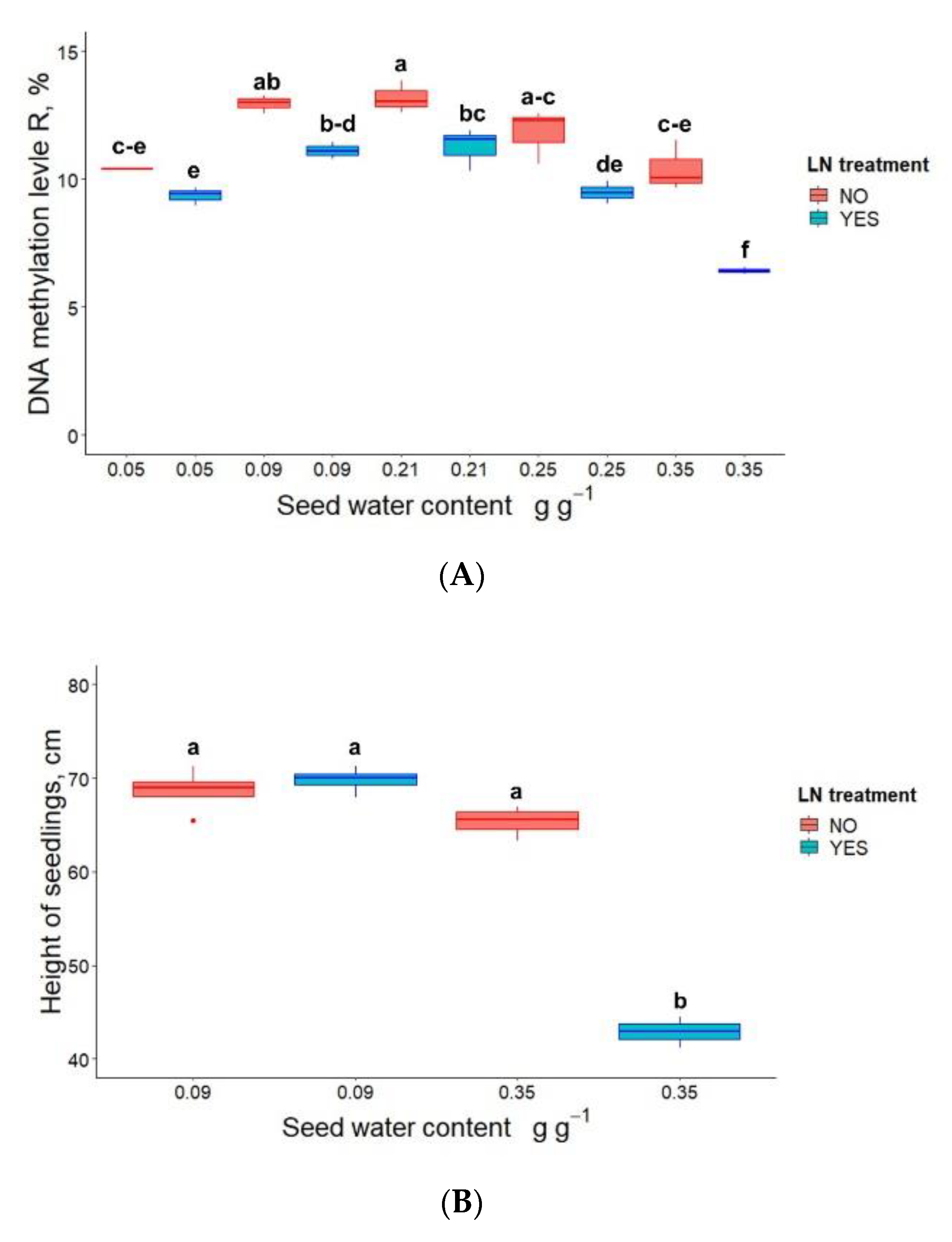
| Seed Water Content, (g g−1) | LN Treatment | Germination (%) | Seedling Emergence (%) |
|---|---|---|---|
| 0.05 | NO | 98 (a) | 99 (a) |
| 0.05 | YES | 99 (a) | 99 (a) |
| 0.09 | NO | 99 (a) | 99 (a) |
| 0.09 | YES | 100 (a) | 99 (a) |
| 0.21 | NO | 97 (a) | 90 (a) |
| 0.21 | YES | 97 (a) | 100 (a) |
| 0.25 | NO | 100 (a) | 99 (a) |
| 0.25 | YES | 97 (a) | 100 (a) |
| 0.35 | NO | 95 (a) | 100 (a) |
| 0.35 | YES | 88 (b) | 77 (b) |
| 0.41 | NO | 97 (a) | 99 (a) |
| 0.41 | YES | 0 (c) | 0 (c) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P.; Michalak, M. Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions. Forests 2021, 12, 288. https://doi.org/10.3390/f12030288
Plitta-Michalak BP, Naskręt-Barciszewska MZ, Barciszewski J, Chmielarz P, Michalak M. Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions. Forests. 2021; 12(3):288. https://doi.org/10.3390/f12030288
Chicago/Turabian StylePlitta-Michalak, Beata P., Mirosława Z. Naskręt-Barciszewska, Jan Barciszewski, Paweł Chmielarz, and Marcin Michalak. 2021. "Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions" Forests 12, no. 3: 288. https://doi.org/10.3390/f12030288
APA StylePlitta-Michalak, B. P., Naskręt-Barciszewska, M. Z., Barciszewski, J., Chmielarz, P., & Michalak, M. (2021). Epigenetic Integrity of Orthodox Seeds Stored under Conventional and Cryogenic Conditions. Forests, 12(3), 288. https://doi.org/10.3390/f12030288







