Intermediate Epicotyl Physiological Dormancy in the Recalcitrant Seed of Quercus chungii F.P.Metcalf with the Elongated Cotyledonary Petiole
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Site and Seed Collection
2.2. Fruit and Seed Size and Mass
2.3. Morphological and Anatomical Analysis
2.4. Cotyledonary Petiole Growth and Root Emergence
2.5. Effect of Temperature on the Release of Shoot Dormancy in Root Emerged Seeds
2.6. Statistical Analysis
3. Results
3.1. Fruit Morphology during Seed Germination
3.2. Quantitative Characteristics of Fruit and Seeds
3.3. Effects of Temperature on Root Emergence
3.4. Effects of Temperature on Epicotyl Dormancy Release in Seeds with the Emerging Radicle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 157–178. [Google Scholar] [CrossRef]
- Keeley, J.E. Seed germination and life history syndromes in the California chaparral. Bot. Rev. 1991, 57, 81–116. [Google Scholar] [CrossRef]
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; De Casas, R.R. The NESCent Germination Working Group The evolution of seed dormancy: Environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol. 2014, 203, 300–309. [Google Scholar] [CrossRef]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An Updated Infrageneric Classification of the Oaks: Review of Previous Taxonomic Schemes and Synthesis of Evolutionary Patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus; Quercus, L., Gil-Pelegrín, E., Peguero-Pina, J.J., Sancho-Knapik, D., Eds.; Springer: Gewerbestrasse, Switzerland, 2017; Volume 7, pp. 13–38. [Google Scholar]
- Fazan, L.; Song, Y.-G.; Kozlowski, G. The Woody Planet: From Past Triumph to Manmade Decline. Plants 2020, 9, 1593. [Google Scholar] [CrossRef]
- Carrero, C.; Jerome, D.; Beckman, E.; Byrne, A.; Coombes, A.J.; Deng, M.; Rodriguez, A.G.; Sam, H.V.; Khoo, E.; Nguyen, N.; et al. The Red List of Oaks 2020; The Morton Arboretum: Lisle, IL, USA, 2020; pp. 11–12. [Google Scholar]
- Royal Botanic Gardens Kew, Seed Information Database (SID). Available online: http://data.kew.org/sid (accessed on 30 April 2020).
- Xia, K.; Daws, M.I.; Hay, F.R.; Chen, W.-Y.; Zhou, Z.-K.; Pritchard, H.W. A comparative study of desiccation responses of seeds of Asian Evergreen Oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. S. Afr. J. Bot. 2012, 78, 47–54. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.I.; Stuppy, W.; Zhou, Z.-K.; Pritchard, H.W. Rates of Water Loss and Uptake in Recalcitrant Fruits of Quercus Species Are Determined by Pericarp Anatomy. PLoS ONE 2012, 7, e47368. [Google Scholar] [CrossRef]
- Dey, D.C. Sustaining Oak Forests in Eastern North America: Regeneration and Recruitment, the Pillars of Sustainability. For. Sci. 2014, 60, 926–942. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.M.; Liu, G.Q.; Liu, Y.; Hou, L.Y.; Li, G.L. Inhibitory mechanism of seed germination of Quercus acutissima. Sci. Silva. Sin. 2012, 48, 164–170. [Google Scholar]
- Xia, K.; Daws, M.I.; Zhou, Z.-K.; Pritchard, H.W. Habitat-linked temperature requirements for fruit germination in Quercus species: A comparative study of Quercus subgenus Cyclobalanopsis (Asian evergreen oaks) and Quercus subgenus Quercus. S. Afr. J. Bot. 2015, 100, 108–113. [Google Scholar] [CrossRef]
- Merouani, H.; Branco, C.; Almeida, M.H.; Pereira, J.S. Effects of acorn storage duration and parental tree on emergence and physiological status of Cork oak (Quercus suber L.) seedlings. Ann. For. Sci. 2001, 58, 543–554. [Google Scholar] [CrossRef]
- Li, Q.S.; Chen, J.; Deng, M.; Zhou, C.L.; Shen, J. Seed germination and seedling establishment of the endangered oak Quercus austrocochinchinensis. Seed 2016, 35, 4–12. [Google Scholar]
- Huang, Y.R.; Zhuang, K.; Wu, P.F.; Ma, X.Q.; Lai, X.L.; Tang, W.M. Seed germination and growth characteristics of Cyclobalanopsis chungii. Chinese J. Ecol. 2017, 36, 1251–1258. [Google Scholar]
- Zhou, Y. Seed germination of Cyclobalanopsis glaucoides. J. Plant Physiol. 2003, 39, 325–326. [Google Scholar]
- Chen, X.B.; Zhang, X. Effects of storage temperature on seed germination and emergence of Quercus aquifolioides. Guizhou Agric. Sci. 2015, 43, 129–131. [Google Scholar]
- Rao, P.B. Effects of Environmental Factors on Germination and Seedling Growth in Quercus floribunda and Cupressus torulosa, Tree Species of Central Himalaya. Ann. Bot. 1988, 61, 531–540. [Google Scholar] [CrossRef]
- Rao, P.B. Effect of temperature on seed germination of certain woody species of central Himalaya. Adv. Biosci. 1992, 11, 41–52. [Google Scholar]
- Viswanath, S.; Singh, R.P.; Thapliyal, R.C. Seed germination patterns in a Himalayan moist temperate forest. Trop. Ecol. 2002, 43, 265–273. [Google Scholar]
- Saklani, K.P.; Singh, B.; Bhatt, B.P. Influence of altitude on seed and seedling characteristics in Quercus leucotrichophora A. Camus. ex. Bahadur. Silvae Genet. 2012, 61, 36–43. [Google Scholar] [CrossRef]
- Griffin, J.R. Oak Regeneration in the Upper Carmel Valley, California. Ecology 1971, 52, 862–868. [Google Scholar] [CrossRef]
- Matsuda, K.; McBride, J.R. Germination Characteristics of Selected California Oak Species. Am. Midl. Nat. 1989, 122, 66. [Google Scholar] [CrossRef]
- Nyandiga, C.O.; McPherson, G.R. Germination of two warm-temperate oaks, Quercusemoryi and Quercusarizonica. Can. J. For. Res. 1992, 22, 1395–1401. [Google Scholar] [CrossRef]
- Peterson, J.K. Mechanisms Involved in Delayed Germination of Quercus nigra L. Seeds. Ann. Bot. 1983, 52, 81–92. [Google Scholar] [CrossRef]
- Hawkins, T.S. Regulating acorn germination and seedling emergence in Quercus pagoda (Raf.) as it relates to natural and artificial regeneration. New For. 2019, 50, 425–436. [Google Scholar] [CrossRef]
- Hawkins, T.S. The Influence of Dormancy Break Requirements on Germination and Viability Responses to Winter Submergence in Acorns of Three Bottomland Red Oak (Sect. Lobatae) Species. For. Sci. 2019, 65, 556–561. [Google Scholar] [CrossRef]
- Hopper, G.M.; Smith, D.W.; Parrish, D.J. Germination and seedling growth of northern red oak: Effects of stratification and pericarp removal. For. Sci. 1985, 31, 31–39. [Google Scholar]
- Olson, D.F., Jr.; Quercus, L. Oak. In Seeds of Woody Plants in the United States; Agriculture Handbook No., 450; Schopmeyer, C.S., Ed.; Forest Service: Washington, DC, USA, 1974; pp. 692–703. [Google Scholar]
- Chandler, A.F. Glacier point restoration project. Comb. Proc. Int. Plant. Prop. Soc. 2000, 50, 585–587. [Google Scholar]
- Farmer, R.E., Jr. Epicotyl dormancy in white and chestnut oaks. For. Sci. 1977, 23, 329–332. [Google Scholar]
- Liu, Q.; Lan, Q.Y.; Tan, Y.H.; Shen, Y.X.; Wen, B. A preliminary study on seed germination of dominant plants from a Karst landscape in Bijie, Guizhou. Acta Botanical Yunnanica 2010, 32, 539–546. [Google Scholar]
- Sopp, D.F.; Salac, S.S.; Sutton, P.K. Germination of Gambel oak seed. Tree Planter’s Notes 1977, 28, 4–5. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier/Academic Press: San Diego, CA, USA, 2014; pp. 41–863. [Google Scholar]
- Garrison, W.J.; Augspurger, C.K. Double- and single-seeded acorns of Bur oak (Quercus macrocarpa): Frequency and some ecologial consequences. Bull. Torrey Bot. Club 1983, 110, 154–160. [Google Scholar] [CrossRef]
- Corbineau, F.; Dacher, F.; Come, D. Effects of cold storage duration of acorns and of germination temperature on seedling development in sessile oak. Rev. For. 2001, 53, 32–43. [Google Scholar]
- Jastrzębowski, S.; Ukalska, J. Dynamics of epicotyl emergence of Quercus robur from different climatic regions is strongly driven by post-germination temperature and humidity conditions. Dendrobiology 2019, 81, 73–85. [Google Scholar] [CrossRef]
- Center, A.; Etterson, J.R.; Deacon, N.J.; Cavender-Bares, J. Seed production timing influences seedling fitness in the tropical live oak Quercus oleoides of Costa Rican dry forests. Am. J. Bot. 2016, 103, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Lewis, I.M. The seedling of Querucus virginiana. Plant World 1911, 14, 119–123. [Google Scholar]
- Atwater, B.R. Germination, dormancy and morphology of the seeds of herbaceous ornamental plants. Seed Sci. Technol. 1980, 8, 523–573. [Google Scholar]
- Joley, D.B.; Maddox, D.M.; Schoenig, S.E.; Mackey, B.E. Parameters afferting germinability and seed bank dynamics in dimorphic achenes of Centaurea solstitialis in California. Can. J. Bot. 2003, 81, 993–1007. [Google Scholar] [CrossRef]
- Timmermans, B.G.H.; Vos, J.; Van Nieuwburg, J.; Stomph, T.J.; Van Der Putten, P.E.L. Germination rates of Solanum sisymbriifolium: Temperature response models, effects of temperature fluctuations and soil water potential. Seed Sci. Res. 2007, 17, 221–231. [Google Scholar] [CrossRef]
- Huang, C.C.; Chang, Y.T.; Bartholomew, B. Fagaceae. In Flora of China; Wu, C.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; Volume 4, pp. 380–400. (In English) [Google Scholar]
- Lai, H.Y.; Wang, Y.F.; Wu, K.; Ma, X.Q.; Wu, P.F. Effect of exogenous hormones on germination and antioxidant enzyme activity of seeds of Cyclobalanopsis chungii. Chinese J. Ecol. 2017, 36, 382–388. [Google Scholar]
- Huang, Y.R.; Ma, X.Q.; Zhuang, K.; Liu, M.X.; Huang, D.D. Seed Rain and Soil Seed Bank of Cyclobalanopsis chungii Forest in Minqing, Fujian Province. J. Trop. Subtrop. Bot. 2010, 18, 68–74. [Google Scholar]
- Wang, Y.F.; Wu, P.F.; Wang, R.W.; Ma, X.Q.; Zhou, X.H. Community characteristics of Cyclobalanopsis chungii forest in Mingqing nature reserve. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2011, 1, 39–44. [Google Scholar]
- Vandelook, F.; Bolle, N.; Van Assche, J.A. Multiple environmental signals required for embryo growth and germination of seeds of Selinum carvifolia (L.) L. and Angelica sylvestris L. (Apiaceae). Seed Sci. Res. 2007, 17, 283–291. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ma, X.Q.; Liu, A.Q.; Huang, Y.R.; Huang, J.G. Effects of storage time and environmental factors on seed germination of Cyclobalanopsis chungii. J. Southwest For. Univ. 2009, 29, 28–31. [Google Scholar]
- Hou, X.G.; Yi, X.F.; Yang, Y.Q.; Liu, W.J. Acorn germination and seedling survival of Q. variabilis: Effects of cotyledon excision. Ann. For. Sci. 2010, 67, 711. [Google Scholar] [CrossRef]
- Pinheiro, C.U.B. Germination strategies of palms: The case of Schippia concolor Burret in Belize. Brittonia 2002, 53, 519–527. [Google Scholar] [CrossRef]
- Neves, S.D.C.; Ribeiro, L.M.; Da Cunha, I.R.G.; Pimenta, M.A.S.; Mercadante-Simões, M.O.; Lopes, P.S.N. Diaspore structure and germination ecophysiology of the babassu palm (Attalea vitrivir). Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 68–78. [Google Scholar] [CrossRef]
- Fox, J.F. Adaptation of gray squirrel behavior to autumn germination by white oak acorns. Evolution 1982, 36, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.F.; Yang, Y.Q.; Curtis, R.; Bartlow, A.W.; Agosta, S.J.; Steele, M.A. Alternative strategies of seed predator escape by early-germinating oaks in Asia and North America. Ecol. Evol. 2012, 2, 487–492. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. What kind of seed dormancy might palms have? Seed Sci. Res. 2014, 24, 17–22. [Google Scholar] [CrossRef]
- Ribeiro, L.M.; Oliveira, D.M.T.; Garcia, Q.D.S. Structural evaluations of zygotic embryos and seedlings of the macaw palm (Acrocomia aculeata, Arecaceae) during in vitro germination. Trees 2012, 26, 851–863. [Google Scholar] [CrossRef]
- Silva, R.S.; Ribeiro, L.M.; Mercadante-Simões, M.O.; Nunes, Y.R.F.; Lopes, P.S.N. Seed structure and germination in buriti (Mauritia flexuosa), the Swamp palm. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 674–685. [Google Scholar] [CrossRef]
- Yi, X.F.; Curtis, R.; Bartlow, A.W.; Agosta, S.J.; Steele, M.A. Ability of chestnut oak to tolerate acorn pruning by rodents. Naturwissenschaften 2013, 100, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.E.U.; Muller, C.H.; Leao, N.V. Cronologia dos eventos morfologicos associados a germinacao esensibilidade ao dessecamento em sementes de bacuri (Platonia insignis Mart.—Clusiaceae). Rev. Brasil. Semen. 1998, 20, 236–240. [Google Scholar]
- Carvalho, J.E.U.; Muller, C.H.; Nascimento, W.M.O. Cronologia dos eventos morfologicos associados a germinacao esensibilidade ao dessecamento em sementes de bacuri (Platonia insignis Mart.—Clusiaceae). Rev. Brasil. Semen. 1998, 20, 475–479. [Google Scholar] [CrossRef]
- Jayasuriya, K.M.G.G.; Wijetunga, A.S.T.B.; Baskin, J.M.; Baskin, C.C. Recalcitrancy and a new kind of epicotyl dormancy in seeds of the understory tropical rainforest tree Humboldtia laurifolia(Fabaceae, Ceasalpinioideae). Am. J. Bot. 2010, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, K.M.G.G.; Wijetunga, A.S.T.B.; Baskin, J.M.; Baskin, C.C. Physiological epicotyl dormancy and recalcitrant storage behaviour in seeds of two tropical Fabaceae (subfamily Caesalpinioideae) species. AoB PLANTS 2012, 2012, pls044. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.M. Seed Biology. In Tropical Tree Seed Manual; Vozzo, J.A., Ed.; United States Department of Agriculture, Forest Service: Washington, DC, USA, 2002; Volume 721, pp. 13–124. [Google Scholar]
- Chien, C.-T.; Kuo-Huang, L.-L.; Shen, Y.-C.; Zhang, R.; Chen, S.-Y.; Yang, J.-C.; Pharis, R.P. Storage Behavior of Chionanthus retusus Seed and Asynchronous Development of the Radicle and Shoot Apex during Germination in Relation to Germination Inhibitors, Including Abscisic Acid and Four Phenolic Glucosides. Plant Cell Physiol. 2004, 45, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.C.; Chii, C.T.; Chen, I.Z.; Hsia, I.S. Effect of fruit maturity, seed scarification and medium composition on germination uniformity of Chionanthus retusus seeds. J. Taiwan Soc. Hort. Sci. 2009, 55, 1–12. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. Some considerations for adoption of Nikolaeva’s formula system into seed dormancy classification. Seed Sci. Res. 2008, 18, 131–137. [Google Scholar] [CrossRef]
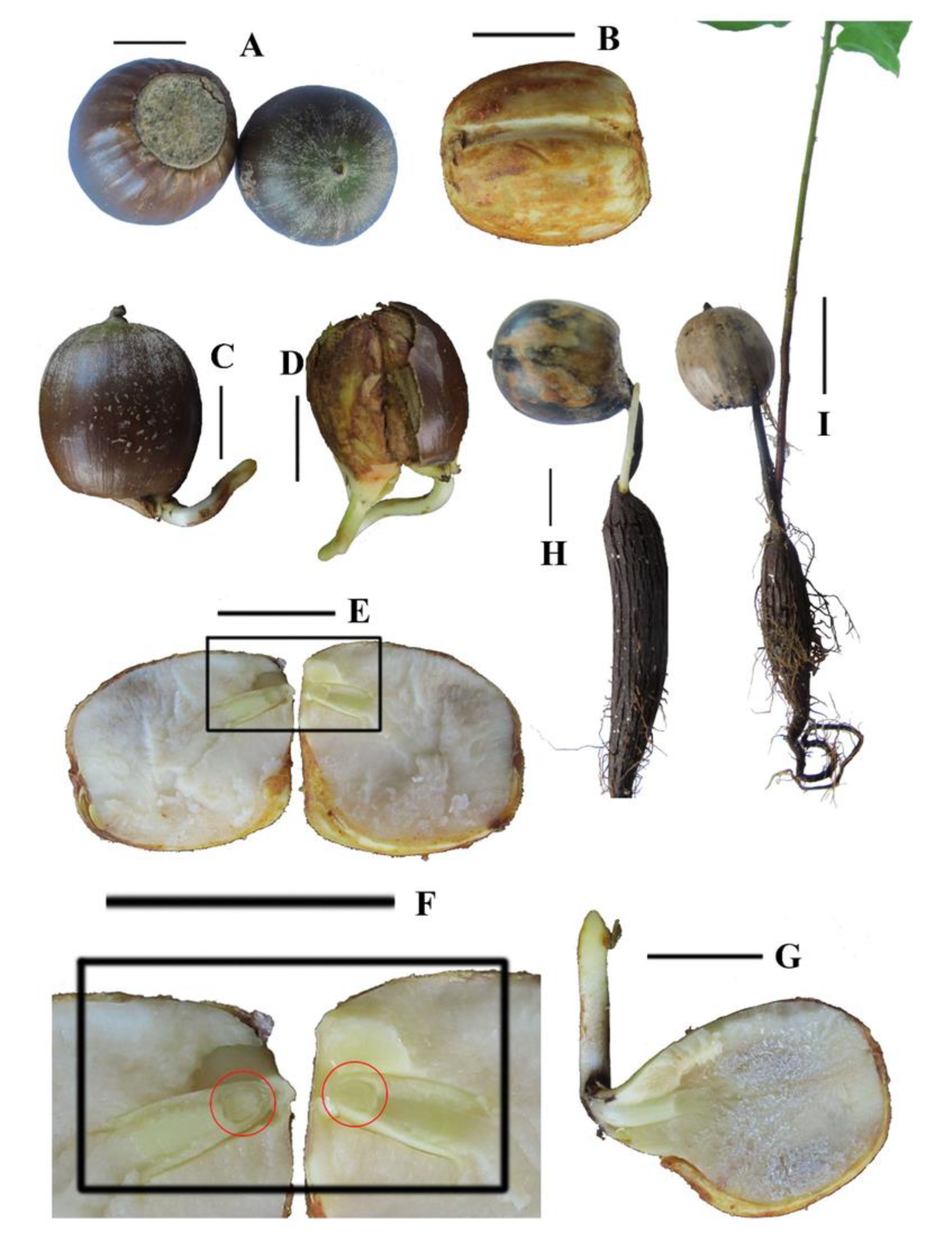

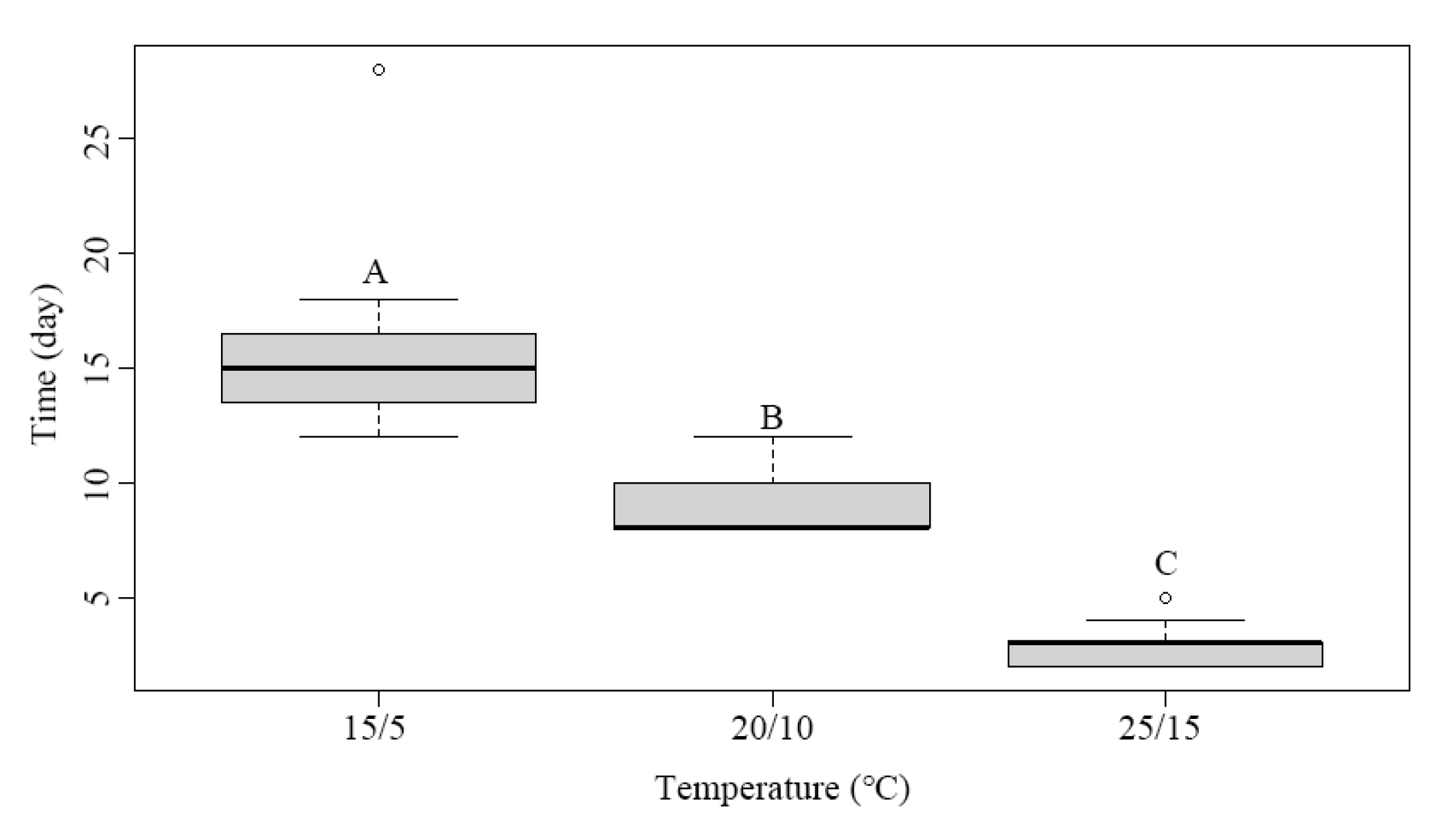
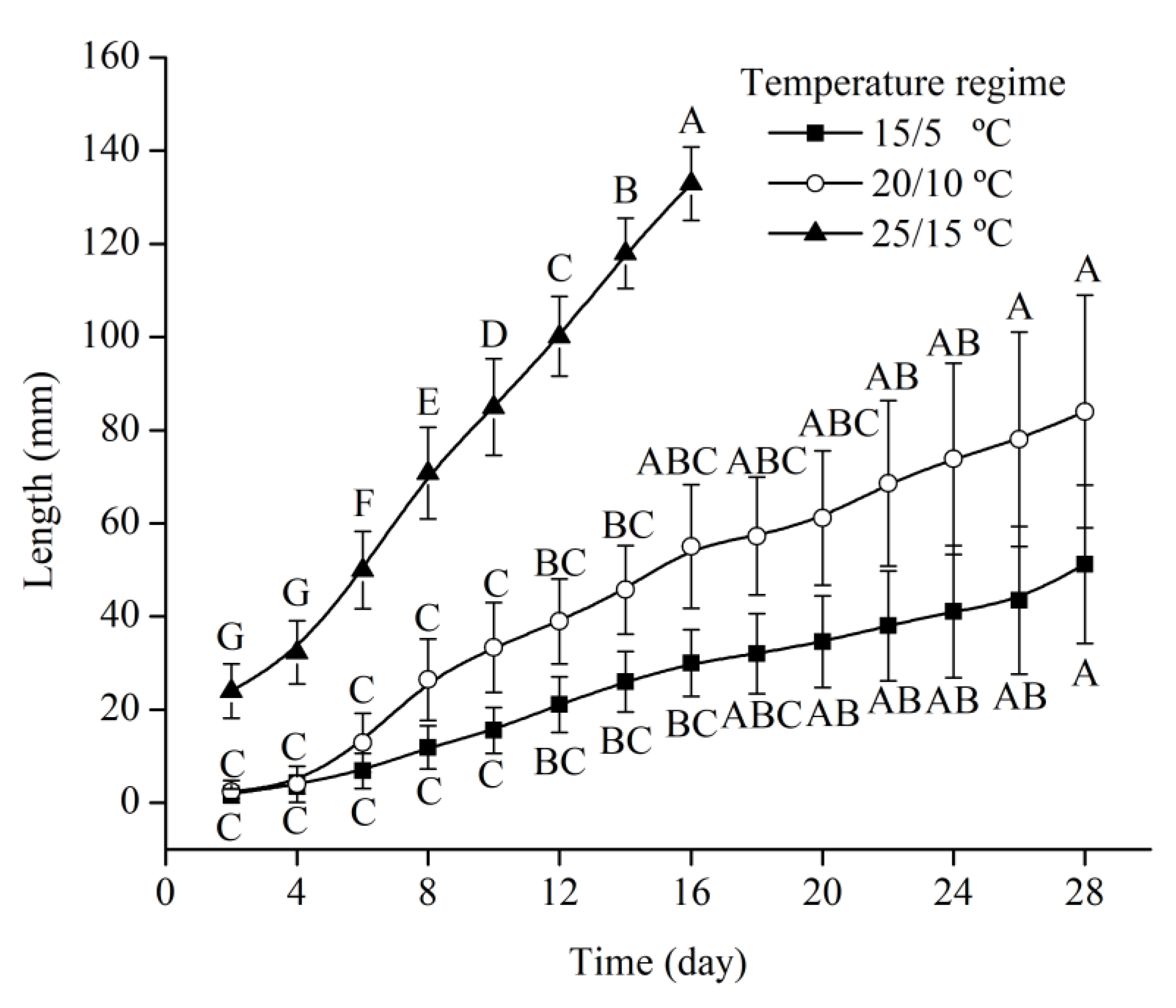
| Scheme | Subgenus (Section) | Root Dormancy | Shoot Dormancy | Dormancy Class |
|---|---|---|---|---|
| Q. acutissima Carruth. [11] | Cerris (Cerris) | PD | PD | |
| Q. cerris L. [12] | Cerris (Cerris) | ND | DD | DD |
| Q. suber L. [12,13] | Cerris (Cerris) | ND | PD | epicotyl PD |
| Q. variabilis Blume [12] | Cerris (Cerris) | ND | DD | DD |
| Q. annulata Sm. [12] | Cerris (Cyclobalanopsis) | ND | DD | DD |
| Q. austrocochinchinenesis Hickel and A.Camus [14] | Cerris (Cyclobalanopsis) | ND | DD | DD |
| Q. camusiae Trel. ex Hickel & A.Camus [12] | Cerris (Cyclobalanopsis) | PD/PY * | DD | PD/PY * |
| Q. chungii F.P.Metcalf [15] | Cerris (Cyclobalanopsis) | ND | DD | DD |
| Q. fleuryi Hickel and Camus [12] | Cerris (Cyclobalanopsis) | PD/PY * | DD | PD/PY * |
| Q. glauca Thunb. [12] | Cerris (Cyclobalanopsis) | PD/PY * | DD | PD/PY * |
| Q. glaucoides M.Martens and Galeotti [16] | Cerris (Cyclobalanopsis) | PD | PD | |
| Q. multinervis Cheng & Hong [12] | Cerris (Cyclobalanopsis) | PD/PY * | DD | PD/PY * |
| Q. schottkyana Rehd. and Wils. [12] | Cerris (Cyclobalanopsis) | PD/PY * | DD | PD/PY * |
| Q. aquifolioides Rehd. and Wils. [17] | Cerris (Ilex) | ND | ND | ND |
| Q. floribunda Wall. [18,19,20] | Cerris (Ilex) | ND | ND | ND |
| Q. ilex L. [12] | Cerris (Ilex) | ND | DD | DD |
| Q. leucotrichophora A.Camus [19,21] | Cerris (Ilex) | ND | PD/ND | |
| Q. semecarpifolia Sm. [20] | Cerris (Ilex) | ND | ND | ND |
| Q. agrifolia Née [22,23] | Quercus (Lobatae) | PD | PD | |
| Q. emoryi Torr. [24] | Quercus (Lobatae) | ND | ND | ND |
| Q. kelloggii Newb. [22,23] | Quercus (Lobatae) | PD | PD | |
| Q. nigra L. [25] | Quercus (Lobatae) | PD | PD | |
| Q. pagoda Raf. [26] | Quercus (Lobatae) | PD | PD | |
| Q. phellos L. [27] | Quercus (Lobatae) | PD | PD | |
| Q. rubra L. [28] | Quercus (Lobatae) | PD | PD | |
| Q. texana Buckley [27] | Quercus (Lobatae) | PD | PD | |
| Q. wislizeni A.DC [23,29] | Quercus (Lobatae) | PD | PD | |
| Q. chrysolepis Liebm. [23,29] | Quercus (Protobalanus) | PD | PD | |
| Q. vaccinifolia Kellogg [30] | Quercus (Protobalanus) | PD | PD | |
| Q. alba L. [31] | Quercus (Quercus) | ND | PD | epicotyl PD |
| Q. arizonica Sarg. [24] | Quercus (Quercus) | ND | ND | ND |
| Q. douglasii Hook. and Arn. [23,29] | Quercus (Quercus) | ND | ND | ND |
| Q. dumosa Nutt. [23,29] | Quercus (Quercus) | ND | ND | ND |
| Q. fabri Hance [12,32] | Quercus (Quercus) | ND | ND | ND |
| Q. gambelii Nutt. [33] | Quercus (Quercus) | ND | ND | ND |
| Q. garryana Dougl. ex Hook. [34] (p. 863) | Quercus (Quercus) | ND | ND | ND |
| Q. lobata Née [22] | Quercus (Quercus) | ND | ND | ND |
| Q. macrocarpa Michx. [35] | Quercus (Quercus) | ND | ND | ND |
| Q. petraea (Matt.) Liebl. [12,36] | Quercus (Quercus) | ND | PD | epicotyl PD |
| Q. prinus L. [31] | Quercus (Quercus) | ND | PD | epicotyl PD |
| Q. robur L. [37] | Quercus (Quercus) | ND | PD | epicotyl PD |
| Q. turbinella Greene [22] | Quercus (Quercus) | ND | ND | ND |
| Q. oleoides Schltdl. and Cham. [38] | Quercus (Quercus) | ND | ND | ND |
| Q. virginiana Mill. [39] | Quercus (Quercus) | ND | ND | ND |
| Incubation under Alternating Temperature Regime (°C) | |||||
|---|---|---|---|---|---|
| Time (month) | 15/5 | 20/10 | 25/15 | 30/20 | 35/25 |
| 1 | 0 ± 0 A | 0 ± 0 A | 0 ± 0 A | 0 ± 0 A | 0 ± 0 A |
| 2 | 0 ± 0 B | 0 ± 0 B | 0 ± 0 B | 0 ± 0 B | 6.67 ± 2.89 A |
| 3 | 0 ± 0 B | 0 ± 0 B | 1.67 ± 2.89 B | 31.67 ± 12.58 A | 46.67 ± 10.40 A |
| 4 | 0 ± 0 B | 0 ± 0 B | 60.00 ± 13.22 A | 68.33 ± 16.07 A | 80.00 ± 10.00 A |
| 5 | 0 ± 0 B | 0 ± 0 B | 68.33 ± 7.64 A | 80.00 ± 5.00 A | 83.33 ± 5.77 A |
| 6 | 0 ± 0 B | 0 ± 0 B | 76.67 ± 2.89 A | 80.00 ± 5.00 A | 83.33 ± 5.77 A |
| 7 | 0 ± 0 B | 0 ± 0 B | 78.33 ± 2.89 A | 80.00 ± 5.00 A | 83.33 ± 5.77 A |
| 8 | 0 ± 0 B | 0 ± 0 B | 78.33 ± 2.89 A | 80.00 ± 5.00 A | 83.33 ± 5.77 A |
| Time (month) | Incubation Alternating Temperature Regime (°C) | |
|---|---|---|
| 15/5 °C to 30/20 °C | 20/10 °C to 30/20 °C | |
| 1 | 0 ± 0 B | 26.67 ± 7.64 A |
| 2 | 31.67 ± 10.41 A | 51.67 ± 10.41 A |
| 3 | 63.33 ± 12.58 A | 85.00 ± 8.66 A |
| 4 | 66.67 ± 7.64 A | 85.00 ± 8.66 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.-Q.; Song, Y.-G.; Ge, B.-J.; Dai, X.-L.; Kozlowski, G. Intermediate Epicotyl Physiological Dormancy in the Recalcitrant Seed of Quercus chungii F.P.Metcalf with the Elongated Cotyledonary Petiole. Forests 2021, 12, 263. https://doi.org/10.3390/f12030263
Sun X-Q, Song Y-G, Ge B-J, Dai X-L, Kozlowski G. Intermediate Epicotyl Physiological Dormancy in the Recalcitrant Seed of Quercus chungii F.P.Metcalf with the Elongated Cotyledonary Petiole. Forests. 2021; 12(3):263. https://doi.org/10.3390/f12030263
Chicago/Turabian StyleSun, Xi-Qing, Yi-Gang Song, Bin-Jie Ge, Xi-Ling Dai, and Gregor Kozlowski. 2021. "Intermediate Epicotyl Physiological Dormancy in the Recalcitrant Seed of Quercus chungii F.P.Metcalf with the Elongated Cotyledonary Petiole" Forests 12, no. 3: 263. https://doi.org/10.3390/f12030263
APA StyleSun, X.-Q., Song, Y.-G., Ge, B.-J., Dai, X.-L., & Kozlowski, G. (2021). Intermediate Epicotyl Physiological Dormancy in the Recalcitrant Seed of Quercus chungii F.P.Metcalf with the Elongated Cotyledonary Petiole. Forests, 12(3), 263. https://doi.org/10.3390/f12030263










