Mixed Regional Shifts in Conifer Productivity under 21st-Century Climate Projections in Canada’s Northeastern Boreal Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
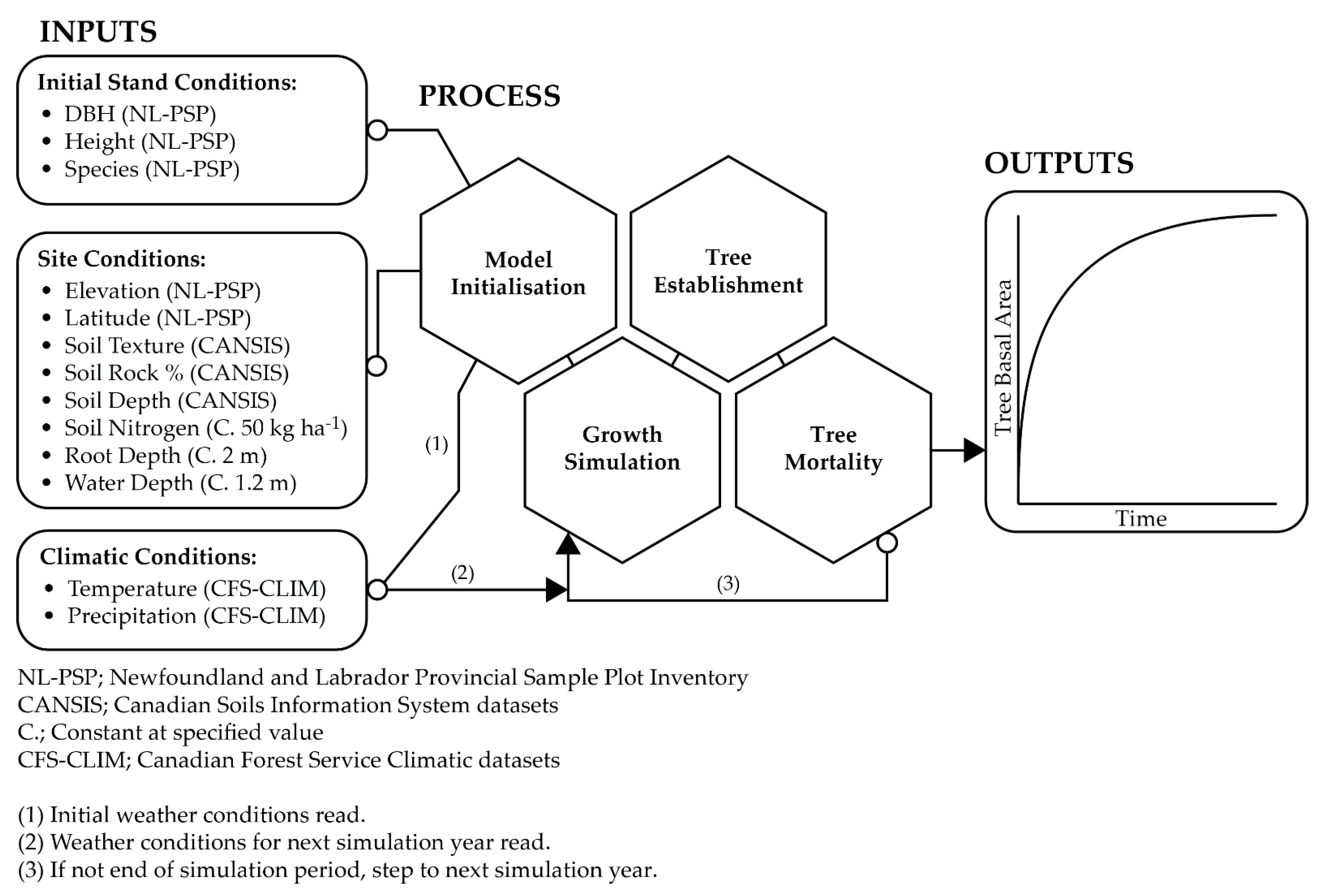
2.2. Model Description
2.3. Model Inputs
2.3.1. Initial Stand Conditions
2.3.2. Site Conditions
2.3.3. Climatic Conditions
2.4. Model Calibration
2.5. Model Validation
2.6. Growth Scenarios
2.7. Growth Modifiers
3. Results
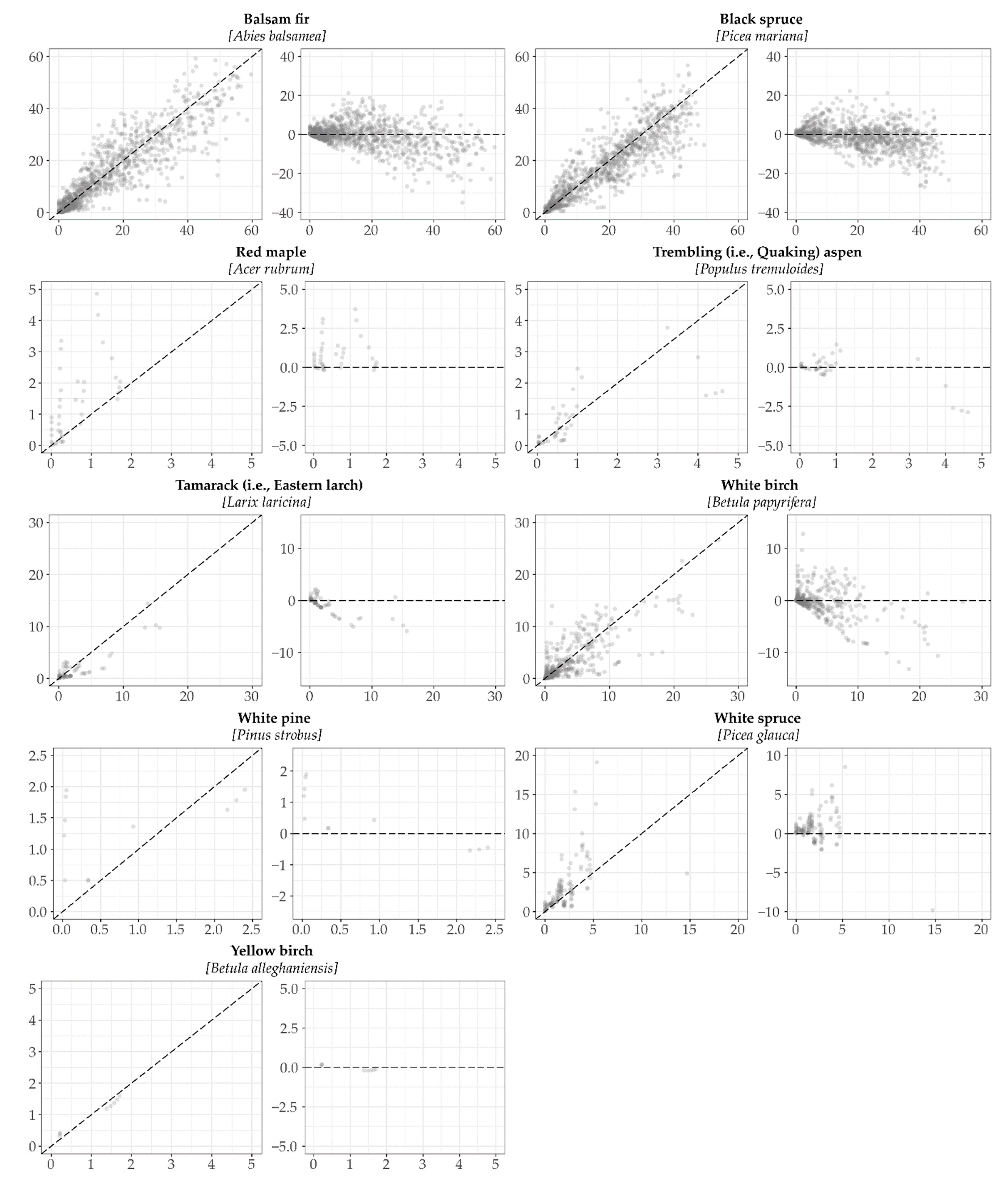
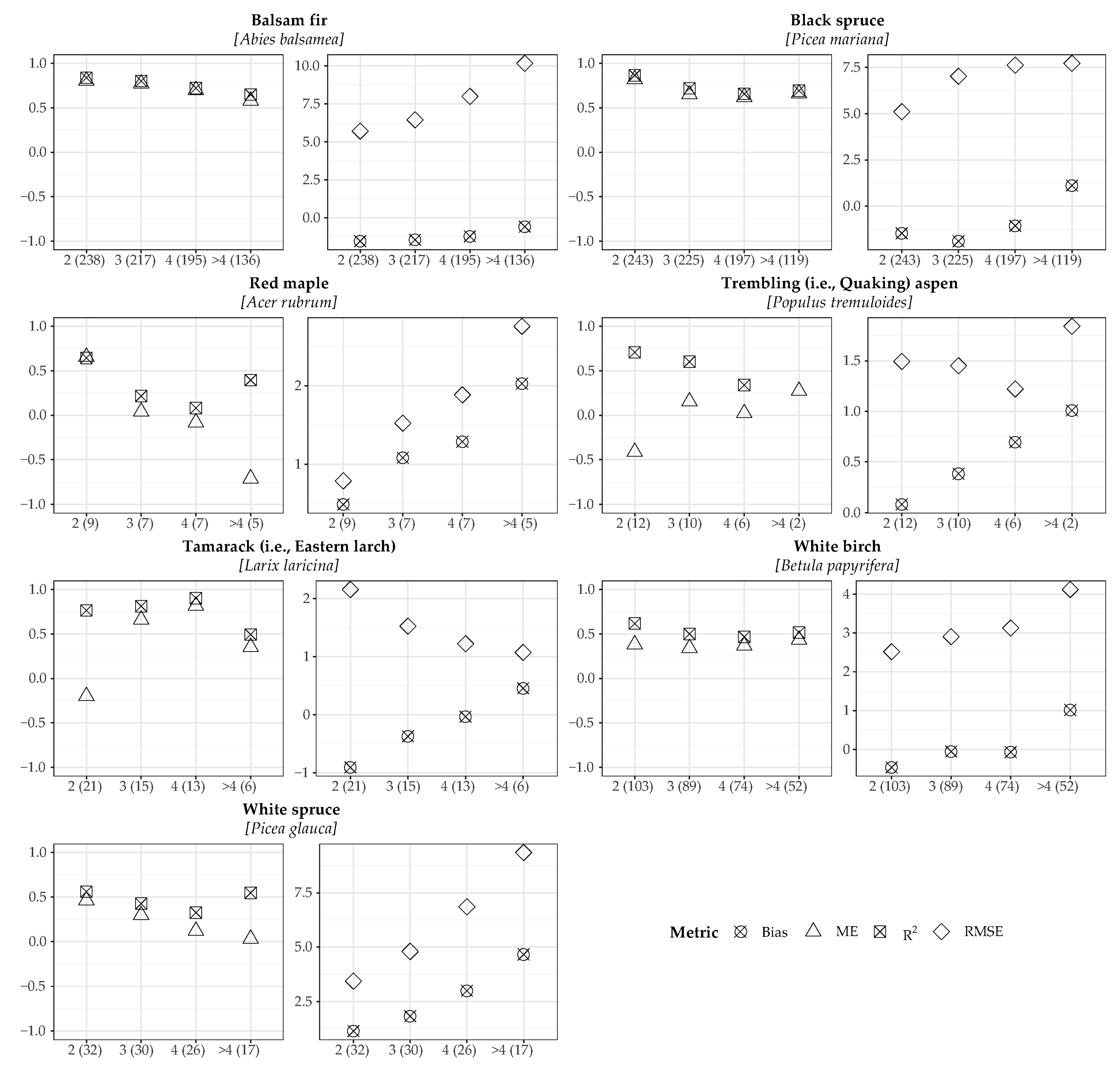
3.1. Model Validation
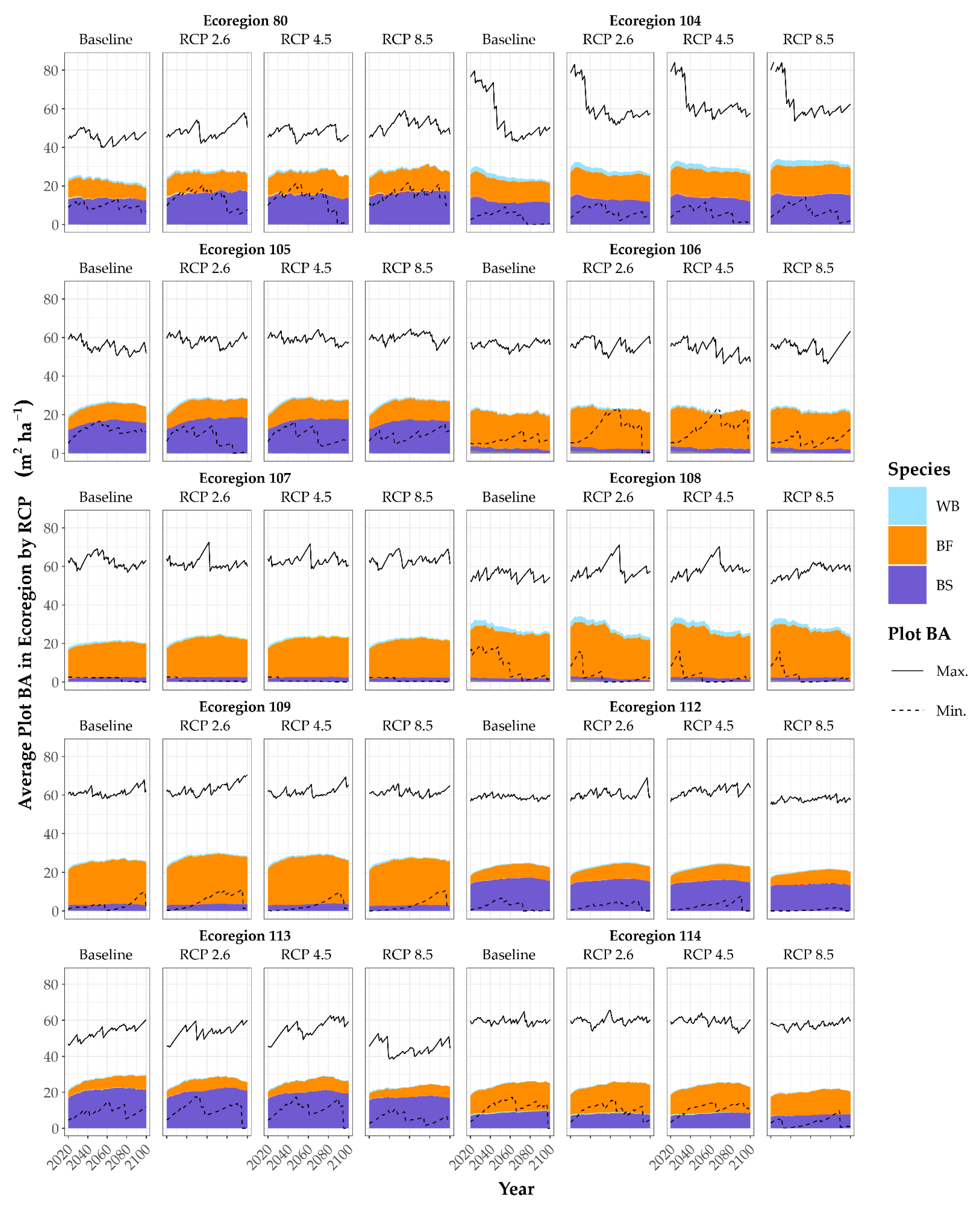
3.2. Growth Scenarios and Modifiers
4. Discussion
4.1. Implications on Sustainable Forest Management
4.2. Caveats and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis; Climate Change 2013; Intergovernmental Panel on Climate Change: New York, NY, USA, 2014; p. 1552. [Google Scholar]
- Morison, J.I.L.; Morecroft, M.D. (Eds.) Plant Growth and Climate Change; Blackwell Publishing Ltd.: Oxford, UK, 2006; ISBN 9780470988695. [Google Scholar]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth Century Redistribution in Climatic Drivers of Global Tree Growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef] [PubMed]
- Oboite, F.O.; Comeau, P.G. Competition and Climate Influence Growth of Black Spruce in Western Boreal Forests. For. Ecol. Manag. 2019, 443, 84–94. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The Representative Concentration Pathways: An Overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Charney, N.D.; Babst, F.; Poulter, B.; Record, S.; Trouet, V.M.; Frank, D.; Enquist, B.J.; Evans, M.E.K. Observed Forest Sensitivity to Climate Implies Large Changes in 21st Century North American Forest Growth. Ecol. Lett. 2016, 19, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, G.D.; Boudreau, S. Black Spruce Regeneration at the Treeline Ecotone: Synergistic Impacts of Climate Change and Caribou Activity. Can. J. For. Res. 2011, 41, 460–468. [Google Scholar] [CrossRef]
- Michaelian, M.; Hogg, E.H.; Hall, R.J.; Arsenault, E. Massive Mortality of Aspen Following Severe Drought along the Southern Edge of the Canadian Boreal Forest. Glob. Chang. Biol. 2011, 17, 2084–2094. [Google Scholar] [CrossRef]
- Hogg, E.H.; Michaelian, M.; Hook, T.I.; Undershultz, M.E. Recent Climatic Drying Leads to Age-Independent Growth Reductions of White Spruce Stands in Western Canada. Glob. Chang. Biol. 2017, 23, 5297–5308. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.W.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional Response of Amazon Forests to Climate Change. Glob. Chang. Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef]
- Klos, R.J.; Wang, G.G.; Bauerle, W.L.; Rieck, J.R. Drought Impact on Forest Growth and Mortality in the Southeast USA: An Analysis Using Forest Health and Monitoring Data. Ecol. Appl. 2009, 19, 699–708. [Google Scholar] [CrossRef]
- Liu, H.; Park Williams, A.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid Warming Accelerates Tree Growth Decline in Semi-Arid Forests of Inner Asia. Glob. Chang. Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Boulanger, Y.; Taylor, A.R.; Price, D.T.; Cyr, D.; McGarrigle, E.; Rammer, W.; Sainte-Marie, G.; Beaudoin, A.; Guindon, L.; Mansuy, N. Climate Change Impacts on Forest Landscapes along the Canadian Southern Boreal Forest Transition Zone. Landsc. Ecol. 2017, 32, 1415–1431. [Google Scholar] [CrossRef]
- Taylor, A.R.; Boulanger, Y.; Price, D.T.; Cyr, D.; McGarrigle, E.; Rammer, W.; Kershaw, J.A. Rapid 21st Century Climate Change Projected to Shift Composition and Growth of Canada’s Acadian Forest Region. For. Ecol. Manag. 2017, 405, 284–294. [Google Scholar] [CrossRef]
- Berner, L.T.; Beck, P.S.A.; Bunn, A.G.; Goetz, S.J. Plant Response to Climate Change along the Forest-Tundra Ecotone in Northeastern Siberia. Glob. Change Biol. 2013, 3449–3462. [Google Scholar] [CrossRef]
- Price, D.T.; Alfaro, R.I.; Brown, K.J.; Flannigan, M.D.; Fleming, R.A.; Hogg, E.H.; Girardin, M.P.; Lakusta, T.; Johnston, M.; McKenney, D.W.; et al. Anticipating the Consequences of Climate Change for Canada’s Boreal Forest Ecosystems. Environ. Rev. 2013, 21, 322–365. [Google Scholar] [CrossRef]
- Searls, T.; Zhu, X.; McKenney, D.W.; Mazumder, R.; Steenberg, J.; Yan, G.; Meng, F.-R. Assessing the Influence of Climate on the Growth Rate of Boreal Tree Species in Northeastern Canada through Long Term Permanent Sample Plot Datasets. Can. J. For. Res. 2020. [Google Scholar] [CrossRef]
- Noseworthy, J.; Beckley, T.M. Borealization of the New England—Acadian Forest: A Review of the Evidence. Environ. Rev. 2020, 28, 284–293. [Google Scholar] [CrossRef]
- Bouchard, M.; Pothier, D.; Gauthier, S. Fire Return Intervals and Tree Species Succession in the North Shore Region of Eastern Quebec. Can. J. For. Res. 2008, 38, 1621–1633. [Google Scholar] [CrossRef]
- Régnière, J.; St-Amant, R.; Duval, P. Predicting Insect Distributions under Climate Change from Physiological Responses: Spruce Budworm as an Example. Biol. Invasions 2012, 14, 1571–1586. [Google Scholar] [CrossRef]
- Boulanger, Y.; Gauthier, S.; Burton, P.J. A Refinement of Models Projecting Future Canadian Fire Regimes Using Homogeneous Fire Regime Zones. Can. J. For. Res. 2014, 44, 365–376. [Google Scholar] [CrossRef]
- Vanclay, J.K.; Skovsgaard, J.P. Evaluating Forest Growth Models. Ecol. Model. 1997, 98, 1–12. [Google Scholar] [CrossRef]
- Porté, A.; Bartelink, H.H. Modelling Mixed Forest Growth: A Review of Models for Forest Management. Ecol. Model. 2002, 150, 141–188. [Google Scholar] [CrossRef]
- Korzukhin, M.D.; Ter-Mikaelian, M.T.; Wagner, R.G. Process versus Empirical Models: Which Approach for Forest Ecosystem Management? Can. J. For. Res. 1996, 26, 879–887. [Google Scholar] [CrossRef]
- Paulsen, J.C. Kurze Praktische Anleitung zun Forstwesen. Verfasst von Einem Forstmanne; Verfasst Von Einem Forstmanne: Detmold, Germany, 1795. [Google Scholar]
- Von Cotta, H. Hülfstafeln für Forstwirte und Forsttaxatoren; Arnoldische Buchhandlung: Dresden, Germany, 1821. [Google Scholar]
- Sullivan, A.D.; Clutter, J.L. A Simultaneous Growth and Yield Model for Loblolly Pine. For. Sci. 1972, 18, 76–86. [Google Scholar] [CrossRef]
- Subedi, N.; Sharma, M. Individual-Tree Diameter Growth Models for Black Spruce and Jack Pine Plantations in Northern Ontario. For. Ecol. Manag. 2011, 261, 2140–2148. [Google Scholar] [CrossRef]
- Battaglia, M.; Sands, P.J. Process-Based Forest Productivity Models and Their Application in Forest Management. For. Ecol. Manag. 1998, 102, 13–32. [Google Scholar] [CrossRef]
- Johnsen, K.; Samuelson, L.; Teskey, R.; McNulty, S.; Fox, T. Process Models as Tools in Forestry Research and Management. For. Sci. 2001, 47, 2–8. [Google Scholar]
- Vanclay, J.K. Modelling Forest Growth and Yield: Applications to Mixed Tropical Forests; CAB International: Wallingford, UK, 1994; ISBN 9780851989136. [Google Scholar]
- Yaussy, D.A. Comparison of an Empirical Forest Growth and Yield Simulator and a Forest Gap Simulator Using Actual 30-Year Growth from Two Even-Aged Forests in Kentucky. For. Ecol. Manag. 2000, 126, 385–398. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecology: A Foundation for Sustainable Forest Management and Environmental Ethics in Forestry, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2004; ISBN 9780130662583. [Google Scholar]
- Gustafson, E.J. When Relationships Estimated in the Past Cannot Be Used to Predict the Future: Using Mechanistic Models to Predict Landscape Ecological Dynamics in a Changing World. Landsc. Ecol. 2013, 28, 1429–1437. [Google Scholar] [CrossRef]
- Kimmins, J.P. Modelling the Sustainability of Forest Production and Yield for a Changing and Uncertain Future. For. Chron. 1990, 66, 271–280. [Google Scholar] [CrossRef][Green Version]
- Monserud, R.A. Evaluating Forest Models in a Sustainable Forest Management Context. For. Biometry Model. Inf. Sci. 2003, 1, 35–47. [Google Scholar]
- Pinjuv, G.; Mason, E.G.; Watt, M. Quantitative Validation and Comparison of a Range of Forest Growth Model Types. For. Ecol. Manag. 2006, 236, 37–46. [Google Scholar] [CrossRef]
- Bugmann, H. A Review of Forest Gap Models. Clim. Chang. 2001, 51, 259–305. [Google Scholar] [CrossRef]
- Ashraf, M.I.; Bourque, C.P.-A.; MacLean, D.A.; Erdle, T.; Meng, F.-R. Using JABOWA-3 for Forest Growth and Yield Predictions under Diverse Forest Conditions of Nova Scotia, Canada. For. Chron. 2012, 88, 708–721. [Google Scholar] [CrossRef][Green Version]
- Landsberg, J. Modelling Forest Ecosystems: State of the Art, Challenges, and Future Directions. Can. J. For. Res. 2003, 33, 385–397. [Google Scholar] [CrossRef]
- Botkin, D.B. Forest Dynamics: An Ecological Model; Oxford University Press: Oxford, UK, 1993; ISBN 9780195065558. [Google Scholar]
- Botkin, D.B.; Janak, J.F.; Wallis, J.R. Some Ecological Consequences of a Computer Model of Forest Growth. J. Ecol. 1972, 60, 849. [Google Scholar] [CrossRef]
- Bossel, H.; Krieger, H. Simulation Model of Natural Tropical Forest Dynamics. Ecol. Model. 1991, 59, 37–71. [Google Scholar] [CrossRef]
- Larocque, G.R.; Archambault, L.; Delisle, C. Development of the Gap Model ZELIG-CFS to Predict the Dynamics of North American Mixed Forest Types with Complex Structures. Ecol. Model. 2011, 222, 2570–2583. [Google Scholar] [CrossRef]
- Shugart, H.H.; West, D.C. Development of an Appalachian Deciduous Forest Succession Model and Its Application to Assessment of the Impact of the Chestnut Blight. J. Environ. Manag. 1977, 5, 161–179. [Google Scholar]
- Shugart, H.H. A Theory of Forest Dynamics: The Ecological Implications of Forest Succession Models; Blackburn Press: Caldwell, NJ, USA, 2003; ISBN 9781930665750. [Google Scholar]
- Aber, J.D.; Botkin, D.B.; Melillo, J.M. Predicting the Effects of Different Harvesting Regimes on Forest Floor Dynamics in Northern Hardwoods. Can. J. For. Res. 1978, 8, 306–315. [Google Scholar] [CrossRef]
- Urban, D.L.; Bonan, G.B.; Smith, T.M.; Shugart, H.H. Spatial Applications of Gap Models. For. Ecol. Manag. 1991, 42, 95–110. [Google Scholar] [CrossRef]
- Lexer, M.J.; Hönninger, K. A Modified 3D-Patch Model for Spatially Explicit Simulation of Vegetation Composition in Heterogeneous Landscapes. For. Ecol. Manag. 2001, 144, 43–65. [Google Scholar] [CrossRef]
- Bugmann, H.K.M. A Simplified Forest Model to Study Species Composition Along Climate Gradients. Ecology 1996, 77, 2055–2074. [Google Scholar] [CrossRef]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A.; Kobe, R.K.; Ribbens, E. Forest Models Defined by Field Measurements: Estimation, Error Analysis and Dynamics. Ecol. Monogr. 1996, 66, 1–43. [Google Scholar] [CrossRef]
- Köhler, P.; Ditzer, T.; Huth, A. Concepts for the Aggregation of Tropical Tree Species into Functional Types and the Application to Sabah’s Lowland Rain Forests. J. Trop. Ecol. 2000, 16, 591–602. [Google Scholar] [CrossRef]
- Köhler, P.; Huth, A. The Effects of Tree Species Grouping in Tropical Rainforest Modelling: Simulations with the Individual-Based Model Formind. Ecol. Model. 1998, 109, 301–321. [Google Scholar] [CrossRef]
- Ngugi, M.R.; Botkin, D.B. Validation of a Multispecies Forest Dynamics Model Using 50-Year Growth from Eucalyptus Forests in Eastern Australia. Ecol. Model. 2011, 222, 3261–3270. [Google Scholar] [CrossRef]
- Ecological Stratification Working Group (Canada). A National Ecological Framework for Canada; The Group: Ottawa, ON, Canada, 1996; ISBN 9780662241072. [Google Scholar]
- Ashraf, M.I. A Framework for Predicting Forest Growth and Yield under Projected Climate Change. Ph.D. Thesis, University of New Brunswick, Fredericton, NB, Canada, 2013. [Google Scholar]
- Miao, Z.; Li, C. Predicting Tree Growth Dynamics of Boreal Forest in Response to Climate Change. In Landscape Ecology in Forest Management and Conservation; Li, C., Lafortezza, R., Chen, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 176–205. ISBN 9783642127533. [Google Scholar]
- Government of Newfoundland and Labrador, Department of Natural Resources. Growing Our Renewable and Sustainable Forest Economy: Provincial Sustainable Forest Management Strategy (2014–2024); Government of Newfoundland and Labrador, Department of Natural Resources: St. John’s, NL, Canada, 2014; p. 66.
- Environment and Climate Change Canada. Canadian Climate Normals & Averages; Environment and Climate Change Canada: Ottawa, ON, Canada, 2019. [Google Scholar]
- Environment and Climate Change Canada. Forest Land by Province and Territory; Environment and Climate Change Canada: Ottawa, ON, Canada, 2011. [Google Scholar]
- Agriculture and Agri-Food Canada. National Soil DataBase; Agriculture and Agri-Food Canada, Government of Canada: Ottawa, ON, Canada, 2020.
- Hutchinson, M.F. Interpolating Mean Rainfall Using Thin Plate Smoothing Splines. Int. J. Geogr. Inf. Syst. 1995, 9, 385–403. [Google Scholar] [CrossRef]
- McKenney, D.W.; Hutchinson, M.F.; Papadopol, P.; Lawrence, K.; Pedlar, J.; Campbell, K.; Milewska, E.; Hopkinson, R.F.; Price, D.; Owen, T. Customized Spatial Climate Models for North America. Bull. Am. Meteorol. Soc. 2011, 92, 1611–1622. [Google Scholar] [CrossRef]
- McKenney, D.; Pedlar, J.; Hutchinson, M.; Papadopol, P.; Lawrence, K.; Campbell, K.; Milewska, E.; Hopkinson, R.F.; Price, D. Spatial Climate Models for Canada’s Forestry Community. For. Chron. 2013, 89, 659–663. [Google Scholar] [CrossRef]
- Chylek, P.; Li, J.; Dubey, M.K.; Wang, M.; Lesins, G. Observed and Model Simulated 20th Century Arctic Temperature Variability: Canadian Earth System Model CanESM2. Atmos. Chem. Phys. Discuss. 2011, 11, 22893–22907. [Google Scholar] [CrossRef]
- Chiesi, M.; Maselli, F.; Moriondo, M.; Fibbi, L.; Bindi, M.; Running, S.W. Application of BIOME-BGC to Simulate Mediterranean Forest Processes. Ecol. Model. 2007, 206, 179–190. [Google Scholar] [CrossRef]
- Lindner, M.; Sievänen, R.; Pretzsch, H. Improving the Simulation of Stand Structure in a Forest Gap Model. For. Ecol. Manag. 1997, 95, 183–195. [Google Scholar] [CrossRef]
- Pabst, R.J.; Goslin, M.N.; Garman, S.L.; Spies, T.A. Calibrating and Testing a Gap Model for Simulating Forest Management in the Oregon Coast Range. For. Ecol. Manag. 2008, 256, 958–972. [Google Scholar] [CrossRef]
- Risch, A.C.; Heiri, C.; Bugmann, H. Simulating Structural Forest Patterns with a Forest Gap Model: A Model Evaluation. Ecol. Model. 2005, 181, 161–172. [Google Scholar] [CrossRef]
- Huang, S.; Price, D.; Morgan, D.; Titus, S. Validation of Ecoregion-Based Taper Equations for White Spruce in Alberta. For. Chron. 1999, 75, 281–292. [Google Scholar] [CrossRef]
- Yang, Y.; Monserud, R.A.; Huang, S. An Evaluation of Diagnostic Tests and Their Roles in Validating Forest Biometric Models. Can. J. Forest Res. 2004, 34, 11. [Google Scholar] [CrossRef]
- Moore, D.S.; Notz, W.; Fligner, M.A. The Basic Practice of Statistics, 6th ed.; W.H. Freeman: New York, NY, USA, 2013; ISBN 9781464102547. [Google Scholar]
- Nash, J.E.; Sutcliffe, J.V. River Flow Forecasting through Conceptual Models Part I—A Discussion of Principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Soares, P.; Tomé, M.; Skovsgaard, J.P.; Vanclay, J.K. Evaluating a Growth Model for Forest Management Using Continuous Forest Inventory Data. For. Ecol. Manag. 1995, 71, 251–265. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model Evaluation Guidelines for Systematic Quantification of Accuracy in Watershed Simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Ritter, A.; Muñoz-Carpena, R. Performance Evaluation of Hydrological Models: Statistical Significance for Reducing Subjectivity in Goodness-of-Fit Assessments. J. Hydrol. 2013, 480, 33–45. [Google Scholar] [CrossRef]
- Finnis, J.; Daraio, J. Projected Impacts of Climate Change for the Province of Newfoundland & Labrador: 2018 Update; Memorial University of Newfoundland: St. John’s, NL, Canada, 2018; p. 134. [Google Scholar]
- Rogers, B.M.; Jantz, P.; Goetz, S.J. Vulnerability of Eastern US Tree Species to Climate Change. Glob. Chang. Biol. 2017, 23, 3302–3320. [Google Scholar] [CrossRef]
- Steenberg, J.W.N.; Duinker, P.N.; Bush, P.G. Exploring Adaptation to Climate Change in the Forests of Central Nova Scotia, Canada. For. Ecol. Manag. 2011, 262, 2316–2327. [Google Scholar] [CrossRef]
- Burlando, P.; Rosso, R. Extreme Storm Rainfall and Climatic Change. Atmos. Res. 1991, 27, 169–189. [Google Scholar] [CrossRef]
- Dash, C.B.; Fraterrigo, J.M.; Hu, F.S. Land Cover Influences Boreal-Forest Fire Responses to Climate Change: Geospatial Analysis of Historical Records from Alaska. Landsc. Ecol. 2016, 31, 1781–1793. [Google Scholar] [CrossRef]
- Robertson, A. The Centroid of Tree Crowns as an Indicator of Abiotic Processes in a Balsam Fir Wave Forest. Can. J. For. Res. 1987, 17, 746–755. [Google Scholar]
- Ruel, J.-C. Understanding Windthrow: Silvicultural Implications. For. Chron. 1995, 71, 434–445. [Google Scholar]
- Arsenault, A.; LeBlanc, R.; Earle, E.; Brooks, D.; Clarke, B.; Lavigne, D.; Royer, L. Unravelling the Past to Manage Newfoundland’s Forests for the Future. For. Chron. 2016, 92, 487–502. [Google Scholar]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.-B.; Peer, G.; Singer, A.; Bridle, J.R.; Crozier, L.G.; De Meester, L.; Godsoe, W.; et al. Improving the Forecast for Biodiversity under Climate Change. Science 2016, 353, aad8466. [Google Scholar] [CrossRef]
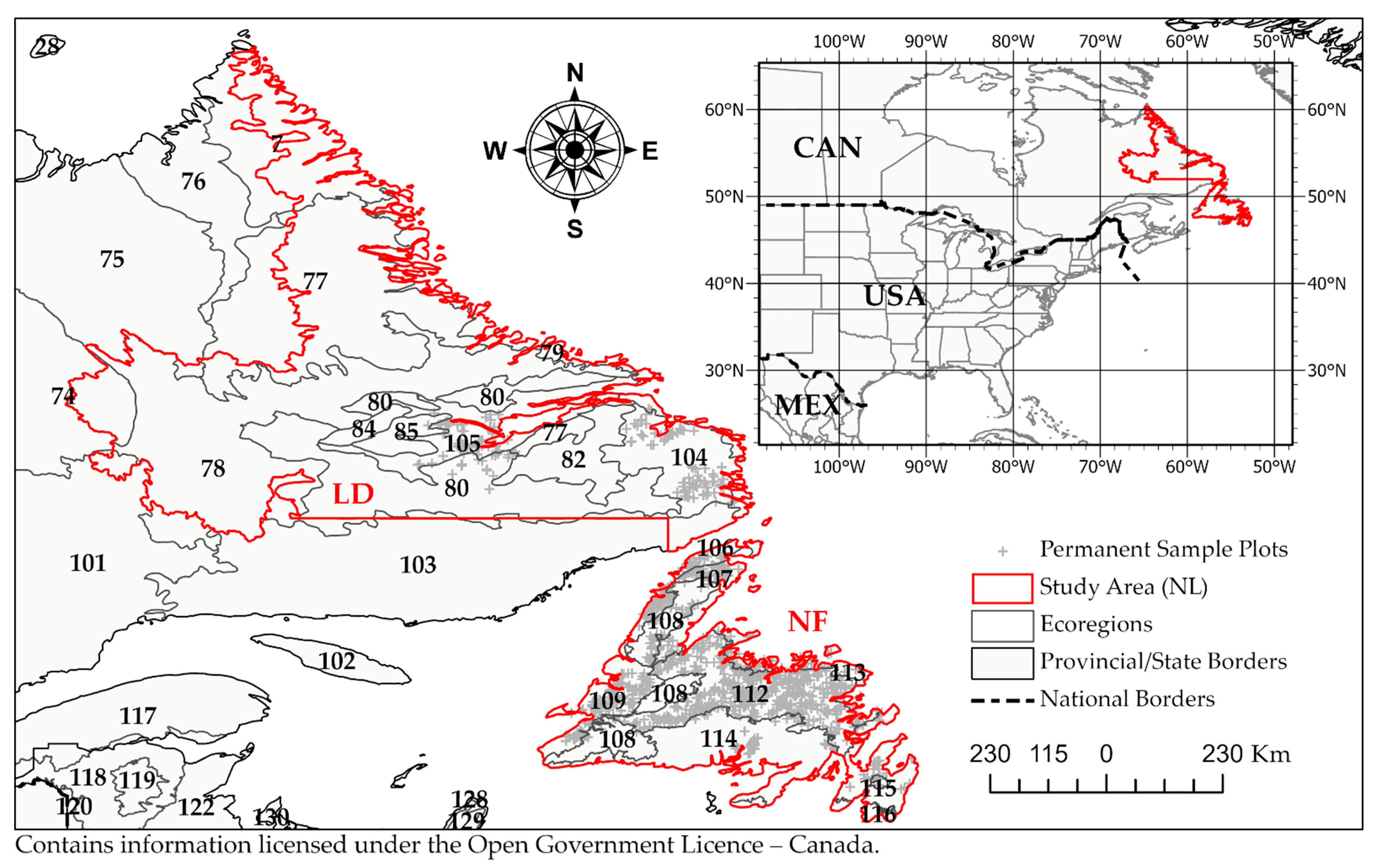

| Ecoregion | Land Area | Permanent Sample Plot Inventory | |||
|---|---|---|---|---|---|
| ID | Common Name | A | AP | n | PP |
| 7 | Torngat Mountains | 18,391 | 4.58 | 0 | 0 |
| 75 | Ungava Bay Basin | 1018 | 0.25 | 0 | 0 |
| 77 | Kingarutuk-Fraser River | 50,612 | 12.59 | 2 | 0.17 |
| 74 | New Quebec Central Plateau | 8091 | 2.01 | 0 | 0 |
| 78 | Smallwood Reservoir-Michikamau | 77,380 | 19.25 | 0 | 0 |
| 79 | Coastal Barrens | 13,690 | 3.41 | 3 | 0.26 |
| 80 | Mecatina River | 52,960 | 13.18 | 16 | 1.4 |
| 82 | Eagle Plateau | 13,320 | 3.31 | 0 | 0 |
| 84 | Harp Lake | 3122 | 0.78 | 0 | 0 |
| 85 | Nipishish Lake | 5134 | 1.28 | 1 | 0.09 |
| 101 | Central Laurentians | 1959 | 0.49 | 0 | 0 |
| 103 | Mecatina Plateau | 6722 | 1.67 | 1 | 0.09 |
| 104 | Paradise River | 19,400 | 4.83 | 83 | 7.24 |
| 105 | Lake Melville | 18,011 | 4.48 | 62 | 5.41 |
| 106 | Strait of Belle Isle | 2485 | 0.62 | 21 | 1.83 |
| 107 | Northern Peninsula | 8118 | 2.02 | 128 | 11.17 |
| 108 | Long Range Mountains | 16,115 | 4.01 | 40 | 3.49 |
| 109 | Southwestern Newfoundland | 10,691 | 2.66 | 149 | 13 |
| 112 | Central Newfoundland | 30,217 | 7.52 | 499 | 43.54 |
| 113 | Northeastern Newfoundland | 5712 | 1.42 | 55 | 4.8 |
| 114 | Maritime Barrens | 36,224 | 9.01 | 78 | 6.81 |
| 115 | Avalon Forest | 493 | 0.12 | 8 | 0.7 |
| 116 | South Avalon-Burin Oceanic Barrens | 2078 | 0.52 | 0 | 0 |
| 401,941 | - | 1146 | - | ||
| Code | Common | Binomial |
|---|---|---|
| BF | Balsam fir | Abies balsamea (L.) Mill |
| BS | Black spruce | Picea mariana (Mill.) Britton et al. |
| WS | White spruce | Picea glauca (Moench) Voss |
| WB | White birch | Betula papyrifera Marshall |
| YB | Yellow birch | Betula alleghaniensisi Britt. |
| RM | Red maple | Acer rubrum L. |
| WP | White pine | Pinus strobus L. |
| TA | Trembling aspen | Populus tremuloides Michx. |
| TL | Tamarack (Eastern larch) | Larix laricina (Du Roi) K. Koch |
| ID | Species Occurrence as % of Total Observations (2000–2017) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BF | BS | RM | TL | TA | WB | WP | WS | YB | Other | |
| 77 | 79.89 | 0.00 | 0.00 | 0.00 | 0.00 | 6.90 | 0.00 | 13.22 | 0.00 | 0.00 |
| 79 | 35.88 | 58.56 | 0.00 | 4.12 | 0.00 | 1.24 | 0.00 | 0.21 | 0.00 | 0.00 |
| 80 | 30.43 | 52.56 | 0.85 | 2.62 | 1.99 | 9.90 | 0.00 | 0.42 | 0.00 | 1.23 |
| 85 | 15.63 | 84.38 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 103 | 73.17 | 26.83 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 104 | 39.99 | 46.56 | 0.00 | 0.14 | 1.36 | 10.62 | 0.00 | 1.15 | 0.00 | 0.17 |
| 105 | 35.50 | 55.91 | 0.30 | 0.27 | 0.66 | 4.53 | 0.00 | 0.68 | 0.00 | 2.15 |
| 106 | 86.64 | 4.51 | 0.00 | 0.20 | 0.00 | 1.86 | 0.00 | 6.77 | 0.00 | 0.02 |
| 107 | 81.88 | 8.46 | 0.01 | 0.14 | 0.00 | 6.81 | 0.00 | 2.16 | 0.16 | 0.37 |
| 108 | 80.35 | 10.52 | 0.55 | 0.00 | 0.00 | 5.40 | 0.00 | 3.02 | 0.10 | 0.06 |
| 109 | 77.58 | 13.58 | 0.27 | 0.19 | 0.00 | 5.50 | 0.02 | 2.30 | 0.24 | 0.33 |
| 112 | 33.50 | 57.99 | 0.35 | 0.65 | 0.46 | 4.58 | 0.06 | 1.65 | 0.00 | 0.75 |
| 113 | 20.97 | 75.73 | 0.12 | 0.50 | 0.13 | 2.32 | 0.00 | 0.14 | 0.00 | 0.09 |
| 114 | 57.20 | 37.02 | 0.00 | 2.37 | 0.36 | 2.04 | 0.00 | 0.11 | 0.33 | 0.57 |
| 115 | 87.42 | 0.00 | 0.00 | 0.00 | 0.00 | 12.31 | 0.00 | 0.00 | 0.26 | 0.00 |
| Total | 50.20 | 41.23 | 0.25 | 0.56 | 0.34 | 5.02 | 0.03 | 1.70 | 0.08 | 0.58 |
| Species | n | Bias | R2 | RMSE | ME | Regression Coefficients Y = (b0+b1X) | Slope (b1) = 1 and Intercept (b0) = 0 α = 0.05 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Separate t-Test (df = n-2) | Simultaneous F-Test (df = 2 and n-2) | ||||||||||
| t | p | F | p | ||||||||
| WB | 437 | −0.15 | 0.61 | 2.84 | 0.51 | b0 | 0.80 | 5.09 | < 0.001 | 57.14 | < 0.001 |
| b1 | 0.71 | 26.14 | < 0.001 | ||||||||
| BF | 1057 | −1.10 | 0.79 | 6.81 | 0.76 | b0 | 2.23 | 7.83 | < 0.001 | 93.05 | < 0.001 |
| b1 | 0.83 | 63.80 | < 0.001 | ||||||||
| TL | 77 | −0.52 | 0.78 | 1.73 | 0.39 | b0 | 0.08 | 0.53 | 0.60 | 52.83 | < 0.001 |
| b1 | 0.65 | 16.40 | < 0.001 | ||||||||
| TA | 46 | 0.03 | 0.62 | 2.76 | −0.29 | b0 | 0.42 | 3.83 | < 0.001 | 95.29 | < 0.001 |
| b1 | 0.40 | 8.67 | < 0.001 | ||||||||
| BS | 1063 | −0.95 | 0.78 | 6.13 | 0.75 | b0 | 2.19 | 6.99 | < 0.001 | 95.03 | < 0.001 |
| b1 | 0.83 | 61.36 | < 0.001 | ||||||||
| WS | 142 | 1.86 | 0.24 | 5.29 | 0.24 | b0 | 0.72 | 1.33 | 0.19 | 10.79 | < 0.001 |
| b1 | 1.40 | 6.82 | < 0.001 | ||||||||
| RM | 41 | 0.88 | 0.31 | 1.52 | 0.14 | b0 | 0.66 | 2.99 | 0.005 | 12.87 | < 0.001 |
| b1 | 1.22 | 4.36 | < 0.001 | ||||||||
| YB | 8 | 0.07 | 0.99 | 0.33 | 0.89 | b0 | 0.21 | 5.16 | 0.002 | 30.38 | < 0.001 |
| b1 | 0.76 | 23.29 | < 0.001 | ||||||||
| WP | 13 | 0.19 | 0.26 | 0.78 | −0.41 | b0 | 1.12 | 5.81 | < 0.001 | 19.30 | < 0.001 |
| b1 | 0.29 | 2.29 | 0.04 | ||||||||
| 2884 | −0.67 | 0.83 | 5.79 | 0.80 | b0 | 1.45 | 10.29 | < 0.001 | 223.91 | < 0.001 | |
| b1 | 0.85 | 118.10 | < 0.001 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Searls, T.; Steenberg, J.; Zhu, X.; Bourque, C.P.-A.; Meng, F.-R. Mixed Regional Shifts in Conifer Productivity under 21st-Century Climate Projections in Canada’s Northeastern Boreal Forest. Forests 2021, 12, 248. https://doi.org/10.3390/f12020248
Searls T, Steenberg J, Zhu X, Bourque CP-A, Meng F-R. Mixed Regional Shifts in Conifer Productivity under 21st-Century Climate Projections in Canada’s Northeastern Boreal Forest. Forests. 2021; 12(2):248. https://doi.org/10.3390/f12020248
Chicago/Turabian StyleSearls, Tyler, James Steenberg, Xinbiao Zhu, Charles P.-A. Bourque, and Fan-Rui Meng. 2021. "Mixed Regional Shifts in Conifer Productivity under 21st-Century Climate Projections in Canada’s Northeastern Boreal Forest" Forests 12, no. 2: 248. https://doi.org/10.3390/f12020248
APA StyleSearls, T., Steenberg, J., Zhu, X., Bourque, C. P.-A., & Meng, F.-R. (2021). Mixed Regional Shifts in Conifer Productivity under 21st-Century Climate Projections in Canada’s Northeastern Boreal Forest. Forests, 12(2), 248. https://doi.org/10.3390/f12020248








