Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region
Abstract
1. Introduction
2. Materials and Methods
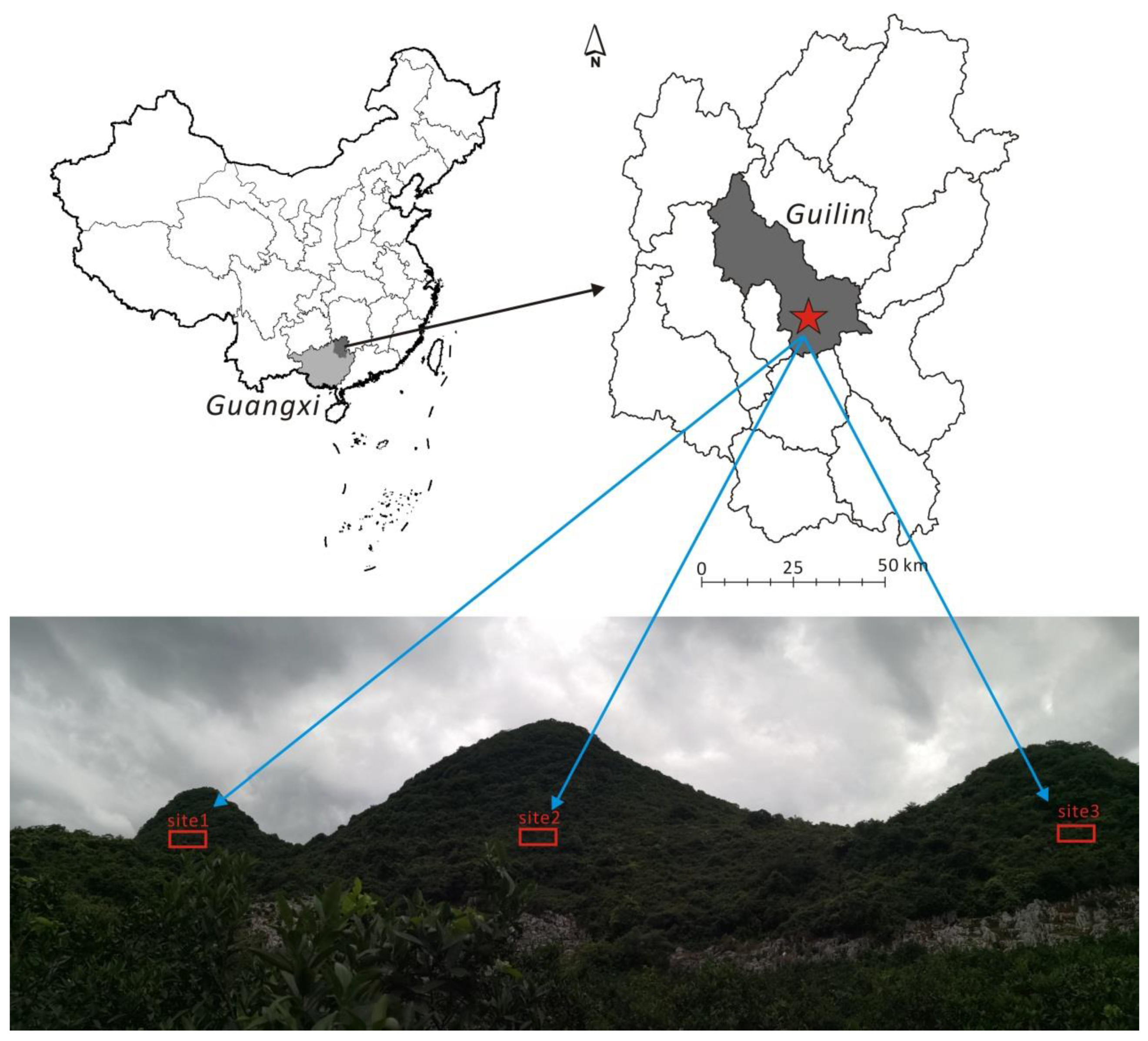
2.1. Study Area and Sample Collection
2.2. Laboratory Analysis
2.2.1. Fractions of Soil Organic C
2.2.2. Chemical Characterization of Soil Organic C
2.2.3. PLFA Extraction and Analysis
2.3. Statistical Analysis
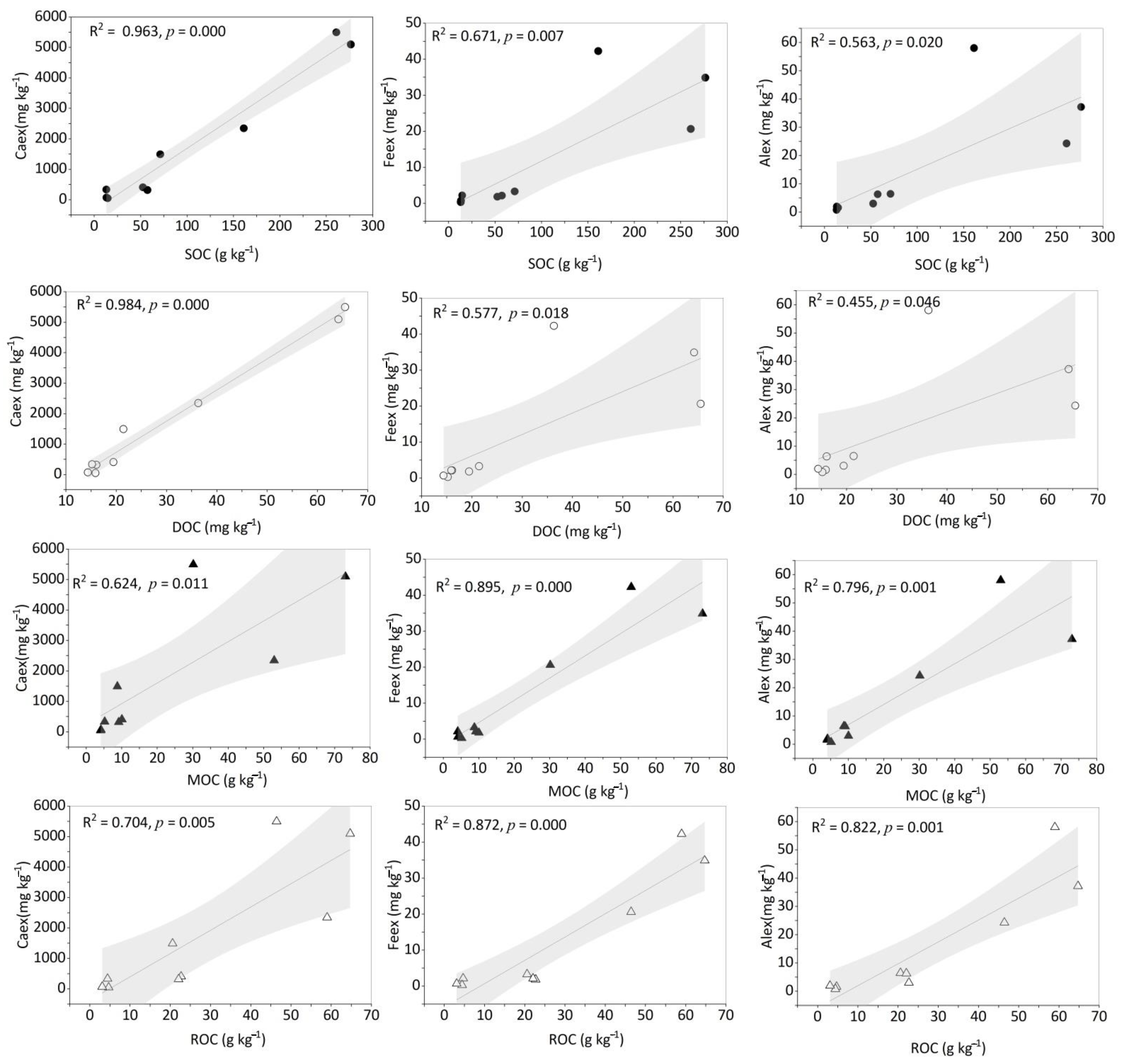
3. Results
3.1. Soil Physico-Chemical Properties
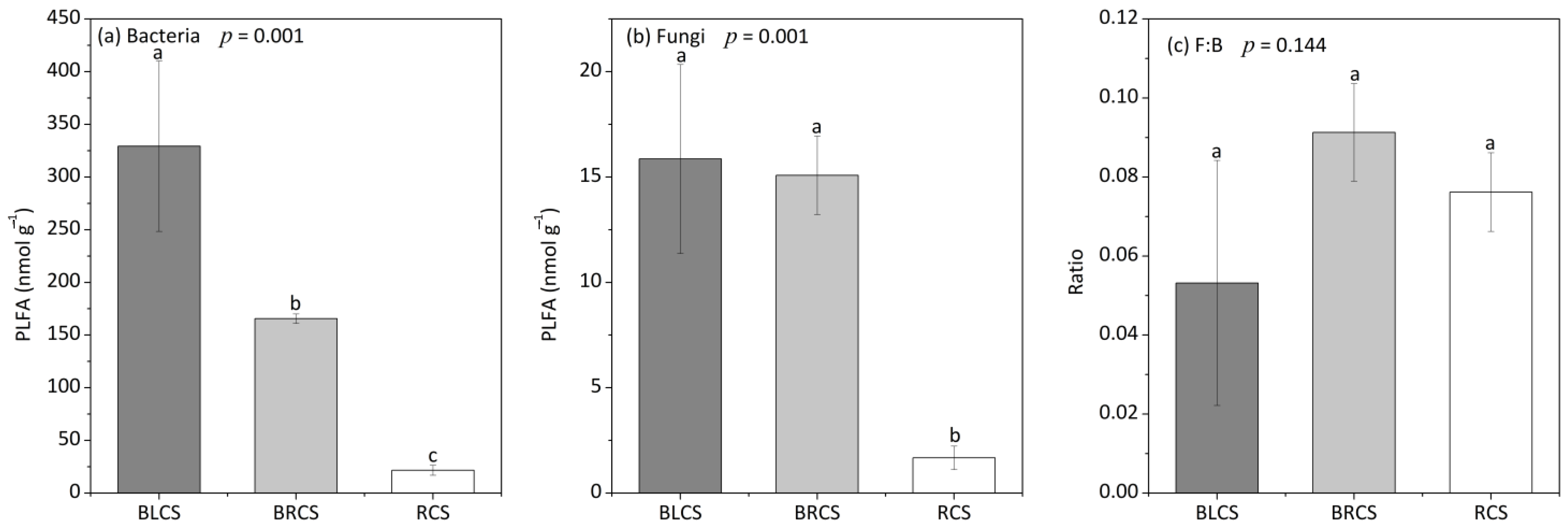
3.2. Soil Microbial Community Composition
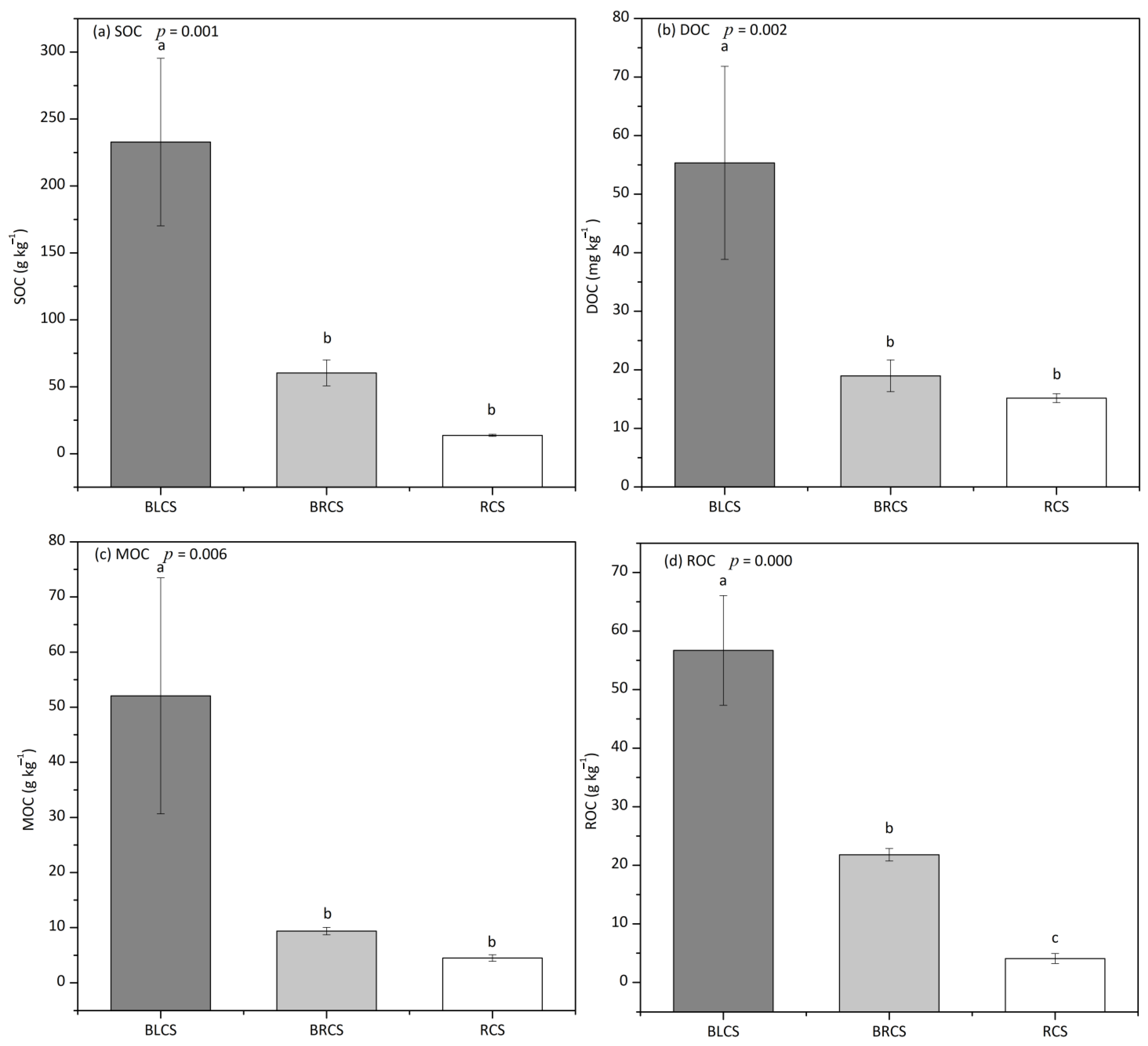
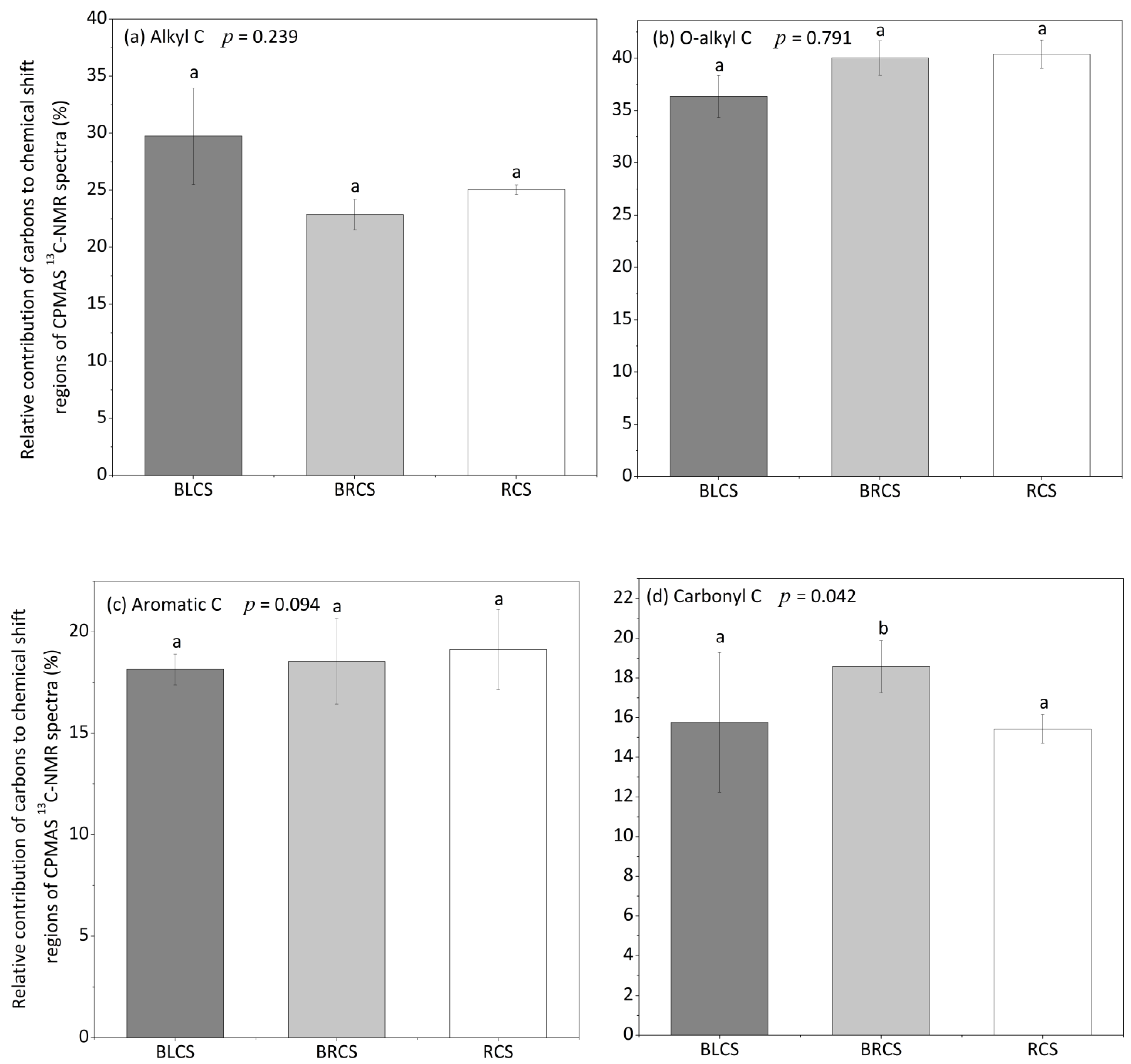
3.3. The Changes in Soil Organic C during the Evolvement of Calcareous Soil
4. Discussion
4.1. The Changes in Microbial Community Composition during the Evolvement of Calcareous Soil
4.2. The Changes in Various SOC Fraction during the Evolvement of Calcareous Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Xu, Y.; Ding, F.; Gao, X.; Li, S.; Sun, L.; An, T.; Pei, J.; Li, M.; Wang, Y.; et al. Process of plant residue transforming into soil organic matter and mechanism of its stabilization: A review. Acta Pedol. Sin. 2019, 56, 528–540. (In Chinese) [Google Scholar]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.; Ranalli, M.; Haddix, M.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Gentsch, N.; Wild, B.; Mikutta, R.; Capek, P.; Diakova, K.; Schrumpf, M.; Turner, S.; Minnich, C.; Schaarschmidt, F.; Shibistova, O.; et al. Temperature response of permafrost soil carbon is attenuated by mineral protection. Glob. Chang. Biol. 2018, 24, 3401–3415. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Sun, Y.; Cui, X. Distribution of mineral-bonded organic carbon and black carbon in forest soils of great Xing’an mountains, China and carbon sequestration potential of the soils. Acta Pedol. Sin. 2018, 55, 236–246. (In Chinese) [Google Scholar]
- Ye, C.; Hall, S.; Hu, S. Controls on mineral-associated organic matter formation in a degraded Oxisol. Geoderma 2019, 338, 383–392. [Google Scholar] [CrossRef]
- Wei, X.; Deng, X.; Xiang, W.; Lei, P.; Ouyang, S.; Wen, H.; Chen, L. Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan, China. Biogeosciences 2018, 15, 2991–3002. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Cao, J.; Yang, H.; Kang, Z. Preliminary regional estimation of carbon sink flux by carbonate rock corrosion: A case study of the Pearl River Basin. Chin. Sci. Bull. 2011, 56, 3766–3773. [Google Scholar] [CrossRef]
- Wu, L. Soil Fertilizer Science; China Agricultural Publishing House: Beijing, China, 2004. (In Chinese) [Google Scholar]
- Wei, Q.; Chen, H.; Wu, Z.; Huang, Y.; Jiao, J. The geochemical characteristics of limestome soils in Longgang area, Guangxi. Acta Pedol. Sin. 1983, 20, 30–42. [Google Scholar]
- Yang, H.; Prelovsek, M.; Huang, F.; Zhang, C.; Cao, J.; Ravbar, N. Quantifcation and evaluation of soil organic carbon and its fractions: Case study from the Classical Karst, SW Slovenia. Acta Carsologica. 2019, 48, 295–311. [Google Scholar] [CrossRef]
- Huang, C. Soil Science; China Agricultural Publishing House: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Yang, H.; Zhang, P.; Zhu, T.; Li, Q.; Cao, J. The characteristics of soil C, N, and P stoichiometric ratios as affected by geological background in a karst graben area, Southwest China. Forests 2019, 10, 601. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, E.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Yang, S.; Cammeraat, E.; Jansen, B.; Cerli, C.; Kalbitz, K. Organic carbon stabilization of soils formed on acidic and calcareous bedrocks in Neotropical Alpine grassland, Peru. EGU Gen. Assem. 2016, 18, EGU2016-2646. [Google Scholar]
- Whittinghill, K.A.; Hobbie, S.E. Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra. Biogeochemistry 2012, 111, 569–581. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Hopkins, D.W.; Gregory, A.S.; Whitmore, A.P. Soil organic matter turnover is governed by accessibility not recalcitrance. Glob. Chang. Biol. 2012, 18, 1781–1796. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J.; Qin, S.; Liu, L.; Fang, K.; Xu, Y.; Ding, J.; Li, F.; Luo, Y.; Yang, Y. Determinants of carbon release from the active layer and permafrost deposits on the Tibetan Plateau. Nat. Commun. 2016, 7, 13046. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, K.K.; Hobbie, S.E. Comparison of labile soil organic matter fractionation techniques. Soil Sci. Soc. Am. J. 2004, 68, 1616–1625. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Qi, Z.; Han, J.; Zhu, Y. Land-use impacts on profile distribution of labile and recalcitrant carbon in the Ili River Valley, northwest China. Sci. Total Environ. 2017, 586, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Ye, T.; Lu, Y.; Chen, W.; Cai, P.; Huang, Q. Iron oxides selectively stabilize plant-derived polysaccharides and aliphatic compounds in agricultural soils. Eur. J. Soil Sci. 2019, 70, 1153–1163. [Google Scholar] [CrossRef]
- Zong, M.; Lin, C.; Li, S.; Li, H.; Duan, C.; Peng, C.; Guo, Y.; An, R. Tillage activates iron to prevent soil organic carbon loss following forest conversion to cornfields in tropical acidic red soils. Sci. Total Environ. 2020, 761, 143253. [Google Scholar] [CrossRef]
- Schneider, M.P.W.; Scheel, T.; Mikutta, R.; Van, H.; Kaiser, K.; Kalbitz, K. Sorptive stabilization of organic matter by amorphous Al hydroxide. Geochim Cosmochim Acta 2010, 74, 1606–1619. [Google Scholar] [CrossRef]
- Jilling, A.; Keiluweit, M.; Contosta, A.R.; Frey, S.; Schimel, J.; Schnecker, J.; Smith, R.G.; Tiemann, L.; Grandy, A.S. Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 2018, 139, 103–122. [Google Scholar] [CrossRef]
- Guangxi Soil and Fertilizer Workstation; Guangxi Science and Technology Press: Nanning, China, 1994. (In Chinese)
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef]
- Grover, M.; Maheswari, M.; Desai, S.; Gopinath, K.A.; Venkateswarlu, B. Elevated CO2: Plant associated microorganisms and carbon sequestration. Appl. Soil Ecol. 2015, 95, 73–85. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, L.; Zhu, T.; Yang, H.; Zhang, J.; Yang, J.; Cao, J.; Bai, B.; Jiang, Z.; Liang, Y.; et al. Rapid recovery of nitrogen retention capacity in a subtropical acidic soil following afforestation. Soil Biol. Biochem. 2018, 120, 171–180. [Google Scholar] [CrossRef]
- IUSS Working Group. WRB World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014.
- Ding, J.; Li, F.; Yang, G.; Chen, L.; Zhang, B.; Liu, L.; Fang, K.; Qin, S.; Chen, Y.; Peng, Y.; et al. The permafrost carbon inventory on the Tibetan Plateau: A new evaluation using deep sediment cores. Glob. Chang. Biol. 2016, 22, 2688–2701. [Google Scholar] [CrossRef]
- Ross, D.S.; Ketterings, Q. Recommended methods for determining soil cation exchange capacity. Recomm. Soil Test. Proced. Northeast. United States 1995, 2, 75–86. [Google Scholar]
- Aguilera, N.H.; Jackson, M.L. Iron Oxide Removal from Soils and Clays. Soil Sci. Soc. Am. J. 1953, 17, 359–364. [Google Scholar] [CrossRef]
- Kleber, M.; Mikutta, R.; Torn, M.S.; Jahn, R. Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur. J. Soil Sci. 2005, 56, 717–725. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of Soil Organic Matter: Association with Minerals or Chemical Recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]
- Wagai, R.; Kishimoto-Mo, A.W.; Yonemura, S.; Shirato, Y.; Hiradate, S.; Yagasaki, Y. Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Glob. Chang. Biol. 2013, 19, 1114–1125. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of Carbon and Flooding on Soil Microbial Communities: Phospholipid Fatty Acid Profiles and Substrate Utilization Patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Frostegard, A.; Tunlid, A.; Baath, E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Z.; Tang, M.; Zhu, B. Greater variations of rhizosphere effects within mycorrhizal group than between mycorrhizal group in a temperate forest. Soil Biol. Biochem. 2018, 126, 237–246. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, W.; Zheng, C.; Zhu, B. Nitrogen addition has contrasting effects on particulate and mineral-associated soil organic carbon in a subtropical forest. Soil Biol. Biochem. 2020, 142, 107708. [Google Scholar] [CrossRef]
- Yan, B.; Sun, L.; Li, J.; Liang, C.; Wei, F.; Xue, S.; Wang, G. Change in composition and potential functional genes of soil bacterial and fungal communities with secondary succession in Quercus liaotungensis forests of the Loess Plateau, western China. Geoderma 2020, 364, 114199. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Baath, E. Contrasting Soil pH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.T.; Arias, M.; Guérif, J. Effects of iron and organicmatter on the porosity and structural stability of soil aggregates. Soil Till. Res. 1998, 46, 261–272. [Google Scholar] [CrossRef]
- Wang, L.Y.; Qin, L.; Lü, X.G.; Jiang, M.; Zou, Y.C. Progress in researches on effect of iron promoting accumulation of soil. Acta Pedol. Sin. 2018, 55, 1041–1050. (In Chinese) [Google Scholar]
- Rasmussen, C.; Heckman, K.; Wieder, W.R.; Keiluweit, M.; Lawrence, C.R.; Berhe, A.A.; Blankinship, J.C.; Crow, S.E.; Druhan, J.L.; Hicks, P.C.E.; et al. Beyond clay: Towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 2018, 137, 297–306. [Google Scholar] [CrossRef]
| Parameter | BLCS | BRCS | RCS | |
|---|---|---|---|---|
| Soil quality | SOC (g C kg−1) | 233 ± 62.6 a | 60.4 ± 9.72 b | 13.7 ± 0.83 b |
| TN (g N kg−1) | 19.9 ± 4.80 a | 5.41 ± 0.72 b | 1.69 ± 0.03 b | |
| C/N | 11.6 ± 0.39 a | 11.1 ± 0.36 a | 8.10 ± 0.61 b | |
| CEC (cmol kg−1) | 56.6 ± 8.58 a | 28.4 ± 2.36 b | 14.8 ± 0.69 c | |
| pH | 7.20 ± 0.05 a | 7.14 ± 0.11 a | 7.13 ± 0.11 a | |
| WHC (%) | 165.62 ± 30.43 a | 106.09 ± 2.9 b | 88.03 ± 6.96 b | |
| Nutrient elements | Ca (g kg−1) | 58.4 ± 13.1 a | 18.8 ± 12.9 b | 5.72 ± 3.41 b |
| Mg (g kg−1) | 19.9 ± 1.00 a | 19.2 ± 3.89 a | 11.9 ± 1.18 b | |
| K (g kg−1) | 6.55 ± 0.80 a | 5.94 ± 0.70 a | 7.09 ± 0.25 a | |
| P (mg kg−1) | 2992 ± 751 a | 1206 ± 239 b | 807 ± 59.2 b | |
| Weathering indices | Fe (g kg−1) | 56.0 ± 12.0 c | 81.4 ± 6.59 b | 106 ± 3.95 a |
| Al (g kg−1) | 65.1 ± 23.7 c | 128 ± 10.7 b | 171 ± 2.14 a | |
| Free Fe (g kg−1) | 23.4 ± 7.98 c | 44.2 ± 7.73 b | 69.9 ± 5.02 a | |
| Free Al (g kg−1) | 4.79 ± 1.50 c | 8.19 ± 1.79 b | 13.3 ± 0.28 a | |
| Caex (g kg−1) | 4.31 ± 1.72 a | 0.74 ± 0.65 b | 0.15 ± 0.16 b | |
| Alex (mg kg−1) | 39.8 ± 17.0 a | 5.25 ± 1.95 b | 1.43 ± 0.60 b | |
| Feex (mg kg−1) | 32.6 ± 11.0 a | 2.39 ± 0.77 b | 1.04 ± 0.98 b | |
| Si (g kg−1) | 202 ± 4.80 b | 221 ± 6.69 a | 206 ± 1.80 b | |
| Fed/Fet | 0.41 ± 0.05 c | 0.54 ± 0.05 b | 0.66 ± 0.03 a | |
| Sa SiO2/Al2O3 | 6.44 ± 1.95 a | 3.33 ± 0.35 b | 2.32 ± 0.01 b | |
| Saf SiO2/(Al2O3 + Fe2O3) | 4.47 ± 1.19 a | 2.55 ± 0.25 b | 1.79 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Xie, Y.; Zhu, T.; Zhou, M. Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region. Forests 2021, 12, 221. https://doi.org/10.3390/f12020221
Yang H, Xie Y, Zhu T, Zhou M. Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region. Forests. 2021; 12(2):221. https://doi.org/10.3390/f12020221
Chicago/Turabian StyleYang, Hui, Yincai Xie, Tongbin Zhu, and Mengxia Zhou. 2021. "Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region" Forests 12, no. 2: 221. https://doi.org/10.3390/f12020221
APA StyleYang, H., Xie, Y., Zhu, T., & Zhou, M. (2021). Reduced Organic Carbon Content during the Evolvement of Calcareous Soils in Karst Region. Forests, 12(2), 221. https://doi.org/10.3390/f12020221






