Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management
Abstract
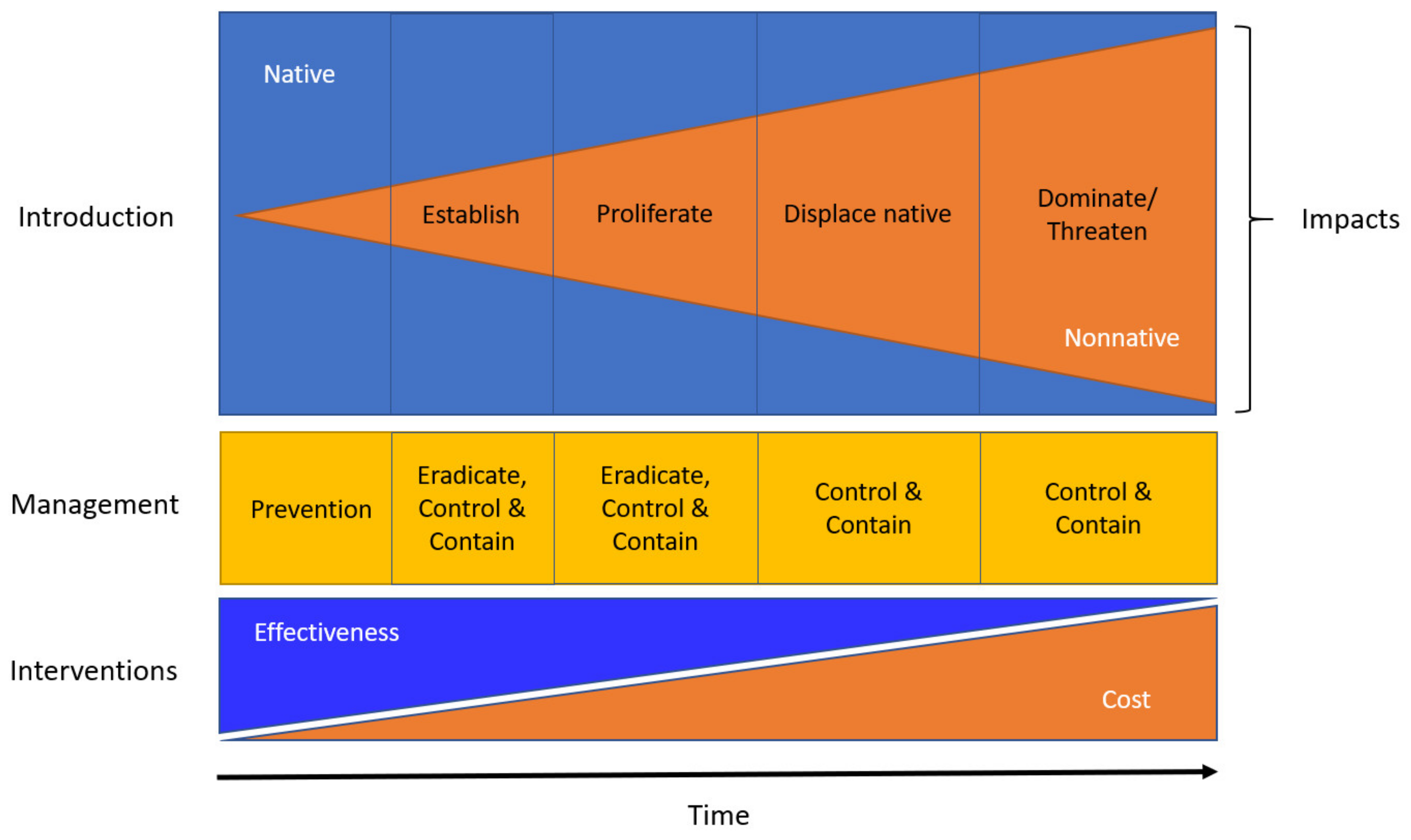
1. Introduction
Xyleborus glabratus and Its Fungal Associates
2. Host Species Susceptibility
2.1. Impacts on Species in the United States
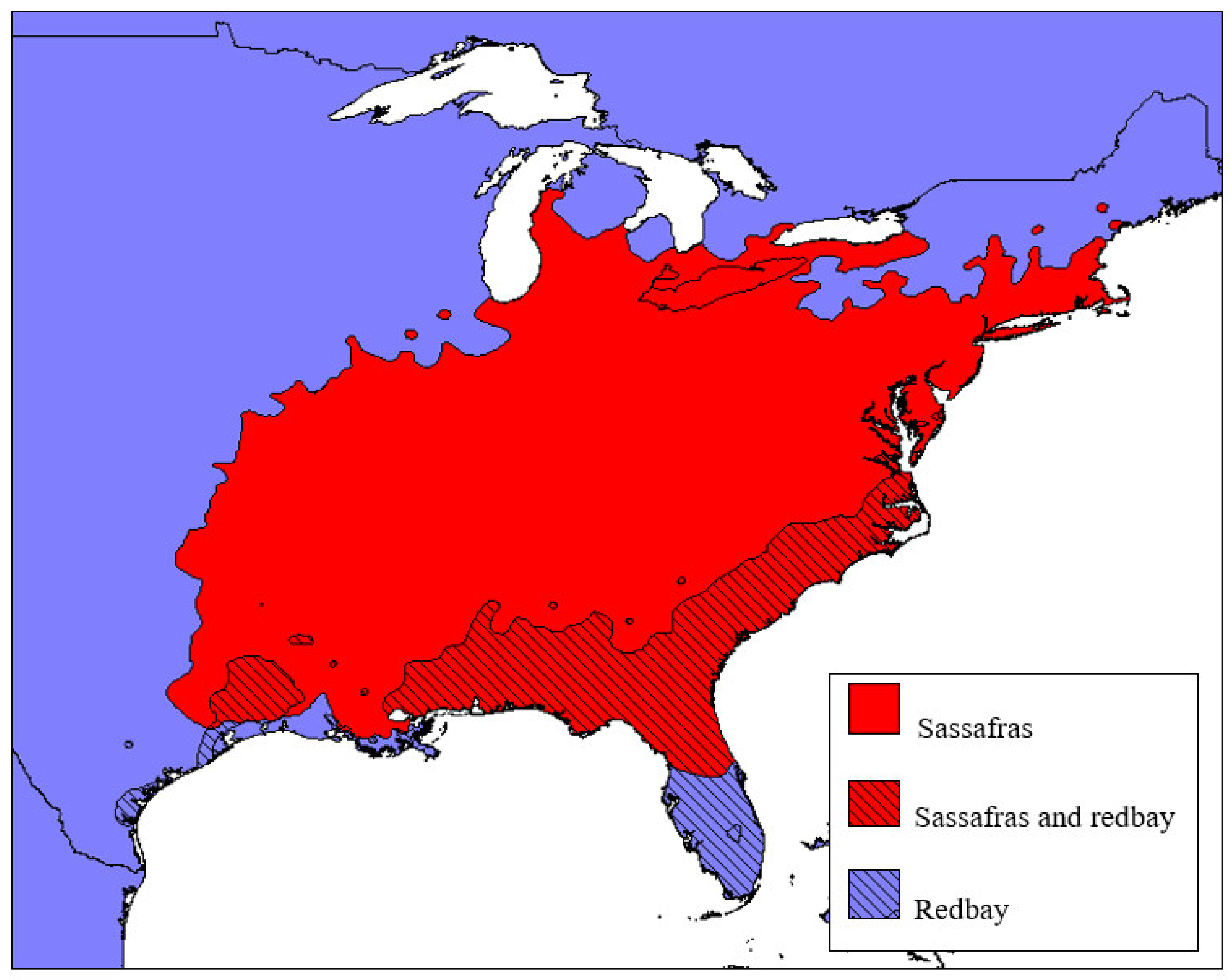
2.1.1. Redbay (Persea borbonia)
2.1.2. Sassafras albidum
2.1.3. Avocado (Persea americana)
2.2. Other Lauraceous Species in the USA
2.3. Potential Impacts on Lauraceae Outside of the US
3. Economic Impacts
4. Ecological Impacts
5. Climatic Conditions
6. Management
6.1. Sanitation
6.2. Public Awareness
6.3. Chemical Control
6.4. Biological Control and Genetic Resistance
6.5. Survey and Detection
6.6. Disrupting Pest Introduction Pathway
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aukema, J.E.; McCullough, D.G.; Von Holle, B.; Liebhold, A.M.; Britton, B.; Frankel, S.J. Historical accumulation of nonindigenous forest pests in the continental US. BioScience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Fei, S.; Morin, R.S.; Oswalt, C.M.; Liebhold, A.M. Biomass losses resulting from insect and disease invasions in US forests. Proc. Nalt. Acad. Sci. USA 2019, 116, 17371–17376. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Olatinwo, R.; Guo, Q.; Fei, S.; Otrosina, W.; Klepzig, K.D.; Streett, D. Climate-induced changes in vulnerability to biological threats in the Southern United States. In Climate Change Adaptation and Mitigation Management Options; Routledge in Association with GSE Research; CRC Press: Boca Raton, FL, USA, 2014; Volume 127, pp. 127–172. [Google Scholar]
- Plein, M.; Shine, R. Australia’s Silent Invader. Australian Academy of Science. 2017. Available online: https://www.science.org.au/curious/earth-environment/invasive-species (accessed on 2 December 2020).
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Lodge, D.M.; Williams, S.; MacIsaac, H.J.; Hayes, K.R.; Leung, B.; Reichard, S.; Mack, R.N.; Moyle, P.B.; Smith, M.; Andow, D.A.; et al. Biological invasions: Recommendations for US policy and management. Ecol. Appl. 2006, 16, 2035–2054. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Humphries, S.E. An integrated approach to the ecology and management of plant invasions. Conserv. Biol. 1995, 9, 761–770. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Berec, L.; Brockerhoff, E.G.; Epanchin-Niell, R.S.; Hastings, A.; Herms, D.A.; Kean, J.M.; McCullough, D.G.; Suckling, D.M.; Tobin, P.C.; et al. Eradication of invading insect populations: From concepts to applications. Annu. Rev. Entomol. 2016, 61, 335–352. [Google Scholar] [CrossRef]
- Myers, J.H.; Simberloff, D.; Kuris, A.M.; Carey, J.R. Eradication revisited: Dealing with exotic species. Trends Ecol. Evol. 2000, 15, 316–320. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfield, A.E., III; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Wood Johnson, C.; Menard, R.D.; Harrington, T.C.; Olatinwo, R.; Best, G.S. First report of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) and laurel wilt in Louisiana, USA: The disease continues westward on sassafras. Fla. Entomol. 2015, 98, 1266–1268. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Peña, J.E.; Smith, J.A.; Dreaden, T.J.; Crane, J.H.; Schubert, T.; Dixon, W. Laurel wilt, caused by Raffaelea lauricola, is confirmed in Miami-Dade County, center of Florida’s commercial avocado production. Plant Dis. 2011, 95, 1589. [Google Scholar] [CrossRef] [PubMed]
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring north of Mexico, with an illustrated key. Ann. Entomol. Soc. Am. 2006, 99, 1034–1056. [Google Scholar] [CrossRef]
- Hughes, M.A.; Smith, J.A.; Ploetz, R.C.; Kendra, P.E.; Mayfield, A.E.; Hanula, J.L.; Hulcr, J.; Stelinski, L.L.; Cameron, S.; Riggins, J.J.; et al. Recovery plan for laurel wilt on redbay and other forest species caused by Raffaelea lauricola and disseminated by Xyleborus glabratus. Plant Health Prog. 2015, 16, 173–210. [Google Scholar] [CrossRef]
- Harrington, T.C.; Yun, H.-Y.; Goto, H.; Aghayeva, D.N.; Fraedrich, S.W. Isolations from the redbay ambrosia beetle, Xyleborus glabratus, confirm that the laurel wilt pathogen Raffaelea lauricola originated in Asia. Mycologia 2011, 103, 1028–1036. [Google Scholar] [CrossRef]
- Dreaden, T.J.; Hughes, M.A.; Ploetz, R.C.; Black, A.; Smith, J.A. Genetic Analyses of the Laurel Wilt Pathogen, Raffaelea lauricola, in Asia Provide Clues on the Source of the Clone that is Responsible for the Current USA Epidemic. Forests 2019, 10, 37. [Google Scholar] [CrossRef]
- Wood, S.L.; Bright, D.E. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic index. Great Basin Nat. Mem. 1992, 13, 1–1553. [Google Scholar]
- Hulcr, J.; Lou, Q.Z. The redbay ambrosia beetle (Coleoptera: Curculionidae) prefers Lauraceae in its native range: Records from the Chinese National Insect Collection. Fla. Entomol. 2013, 96, 1595–1596. [Google Scholar] [CrossRef]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I. A monograph of the Xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. ZooKeys 2020, 983, 1–442. [Google Scholar] [CrossRef]
- Gomez, D.F.; Rabaglia, R.J.; Fairbanks, K.E.O.; Hulcr, J. North American Xyleborini north of Mexico: A review and key to genera and species (Coleoptera, Curculionidae, Scolytinae). ZooKeys 2018, 768, 19–68. [Google Scholar] [CrossRef]
- Miller, D.R.; Rabaglia, R.J. Ethanol and (−)-α-pinene: Attractant kairomones for bark and ambrosia beetles in the southeastern US. J. Chem. Ecol. 2009, 35, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Hanula, J.L.; Mayfield, A.E.; Fraedrich, S.W.; Rabaglia, R.J. Biology and host associations of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae), exotic vector of laurel wilt killing redbay trees in the southeastern United States. J. Econ. Entomol. 2008, 101, 1276–1286. [Google Scholar] [CrossRef]
- Hanula, J.L.; Sullivan, B. Manuka Oil and Phoebe Oil are Attractive Baits for Xyleborus glabratus (Coleoptera Scolytinae), the Vector of Laurel Wilt. Environ. Entomol. 2008, 37, 1403–1409. [Google Scholar] [CrossRef]
- Hanula, J.L.; Sullivan, B.T.; Wakarchuk, D. Variation in Manuka Oil Lure Efficacy for Capturing Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), and Cubeb Oil as an Alternative Attractant. Environ. Entomol. 2013, 42, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Deyrup, M.A.; Wakarchuk, D. Improved lure for redbay ambrosia beetle developed by enrichment of α-copaene content. J. Pest. Sci. 2016, 89, 427–438. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Brownie, C. The redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) uses stem silhouette diameter as a visual host-finding cue. Environ. Entomol. 2013, 42, 743–750. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Bates, C.A.; Johnson, J.; Reid, L.S.; Best, G.S.; Leininger, T.D.; Hawkins, T.S. Susceptibility to laurel wilt and disease incidence in two rare plant species, pondberry and pondspice. Plant Dis. 2011, 95, 1056–1062. [Google Scholar] [CrossRef][Green Version]
- Harrington, T.C.; Fraedrich, S.; Aghayeva, D.N. Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Harrington, T.C.; Aghayeva, D.N.; Fraedrich, S.W. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 2010, 111, 337–361. [Google Scholar] [CrossRef]
- Harrington, T.C.; Fraedrich, S.W. Quantification of Propagules of the Laurel Wilt Fungus and Other Mycangial Fungi from the Redbay Ambrosia Beetle, Xyleborus glabratus. Phytopathology 2010, 100, 1118–1123. [Google Scholar] [CrossRef]
- Campbell, A.S.; Ploetz, R.C.; Dreaden, T.J.; Kendra, P.E.; Montgomery, W.S. Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia 2016, 108, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, T.J.; Campbell, A.S.; Gonzalez-Benecke, C.A.; Ploetz, R.C.; Smith, J.A. Response of swamp bay, Persea palustris, and redbay, P. borbonia, to Raffaelea spp. isolated from Xyleborus glabratus. For. Pathol. 2017, 47, e12288. [Google Scholar] [CrossRef]
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat. Mem. 1982, 6, 1–1359. [Google Scholar]
- Inch, S.A.; Ploetz, R.C. Impact of laurel wilt, caused by Raffaelea lauricola, on xylem function in avocado, Persea americana. For. Pathol. 2012, 42, 239–245. [Google Scholar] [CrossRef]
- Inch, S.; Ploetz, R.; Held, B.; Blanchette, R. Histological and anatomical responses in avocado, Persea americana, induced by the vascular wilt pathogen, Raffaelea lauricola. Botany 2012, 90, 627–635. [Google Scholar] [CrossRef]
- Maner, M.L.; Hanula, J.L.; Horn, S. Population trends of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae): Does utilization of small diameter redbay trees allow populations to persist? Fla. Entomol. 2014, 97, 208–216. [Google Scholar] [CrossRef]
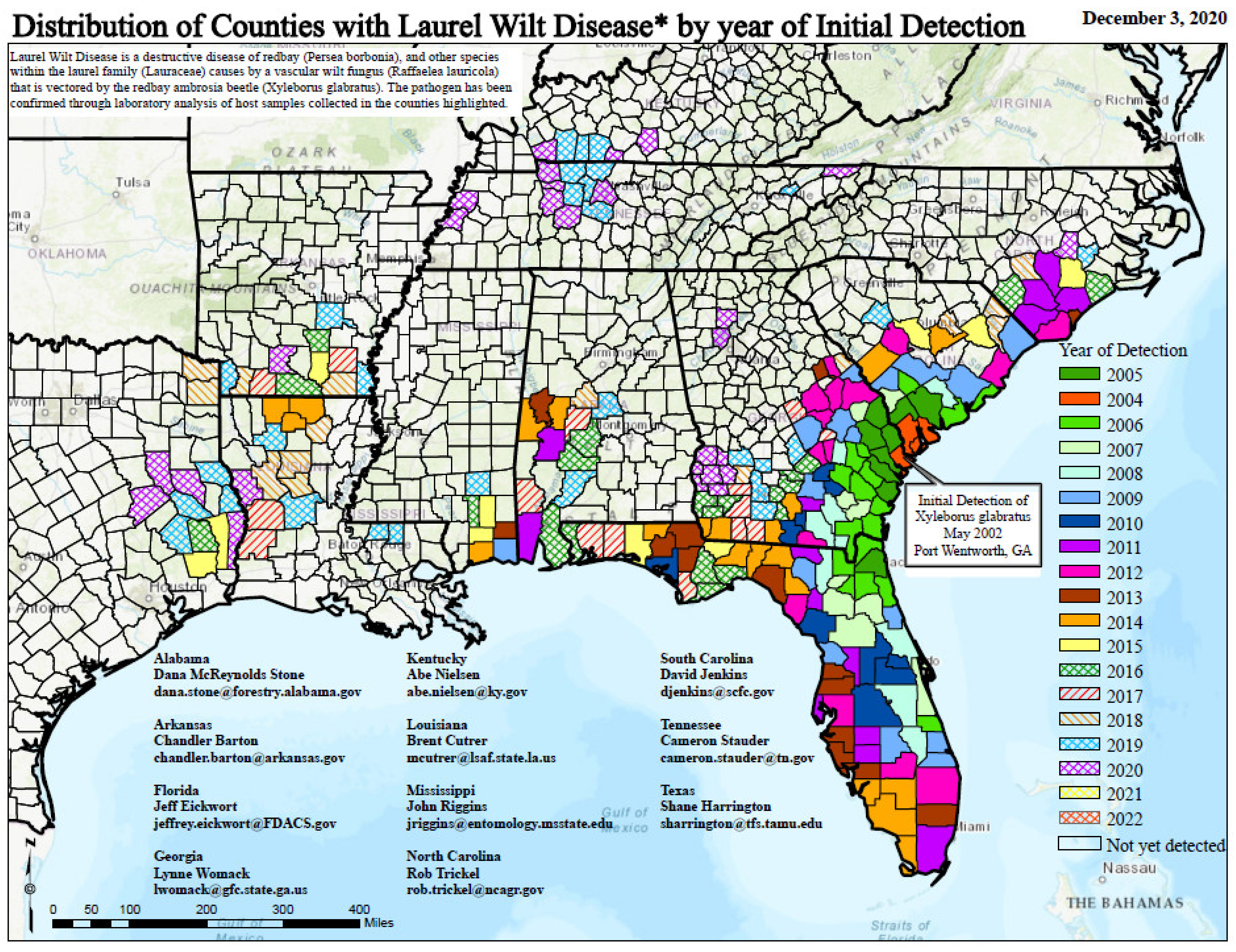
- Cameron, R.S.; Bates, C.; Johnson, J. Distribution and Spread of Laurel Wilt in Georgia: 2006-08 Survey and Field Observations. In Georgia Forestry Commission Report; Georgia Forestry Commission: Dry Branch, GA, USA, 2008; p. 28. [Google Scholar]
- Wuest, C.E.; Harrington, T.C.; Fraedrich, S.W.; Yun, H.Y.; Lu, S.S. Genetic variation in native populations of the laurel wilt pathogen, Raffaelea lauricola, in Taiwan and Japan and the introduced population in the United States. Plant Dis. 2017, 101, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.A.; Riggins, J.J.; Koch, F.H.; Cognato, A.I.; Anderson, C.; Formby, J.P.; Dreaden, T.J.; Ploetz, R.C.; Smith, J.A. No rest for the laurels: Symbiotic invaders cause unprecedented damage to southern USA forests. Biol. Invasions 2017, 19, 2143–2157. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; van der Werff, H.; Renner, S.S. Phylogeny and historical biogeography of Lauraceae: Evidence from the chloroplast and nuclear genomes. Ann. Mo. Bot. Gard. 2001, 88, 104–134. [Google Scholar] [CrossRef]
- Little, E.L., Jr. Checklist of United States Trees (Native and Naturalized). In Agriculture Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1979; 375p. [Google Scholar]
- Gramling, J.M. Potential effects of Laurel Wilt on the flora of North America. Southeast. Nature 2010, 9, 827–836. [Google Scholar]
- Brendemuehl, R.H. Persea borbonia (L.) Spreng. Redbay. In Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; Hardwoods. U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2, pp. 503–510. [Google Scholar]
- Prasad, A.M.; Iverson, L.R. Little’s Range and FIA Importance Value Database for 135 Eastern US Tree Species. Northeastern Research Station, USDA Forest Service: Delaware, OH, USA, 2003. Available online: http://www.fs.fed.us/ne/delaware/4153/global/littlefia/index.html (accessed on 2 December 2020).
- Coder, K.D. Redbay Wilt Risk Assessment Map for Georgia. University of Georgia, Warnell School of Forestry and Natural Resources, Outreach Publication, Wilt Risk Assessment Map for Georgia 2006. SFNR06-8. Available online: https://urbanforestrysouth.org/resources/library/citations/redbay-wilt-risk-assessment-map-for-georgia-map/at_download/file/29a1a48dfc2dffb005ccaed84d178e2e-Redbay (accessed on 2 December 2020).
- Hall, D.W.; Butler, J.F. Palamedes Swallowtail, Papilio palamedes (Drury). University of Florida, Institute of Food and Agricultural Sciences Extension, Document. 2005. Available online: https://edis.ifas.ufl.edu/in217 (accessed on 2 December 2020).
- Riggins, J. Modeling the Spread of Laurel Wilt in Sassafras: Are Additional Ecosystems at Risk? Presentation at Conference on Laurel wilt & Natural Ecosystems, Coral Springs, FL, USA. June 2015. Available online: https://conference.ifas.ufl.edu/laurelwilt/agenda.html (accessed on 2 December 2020).
- Shields, J.; Jose, S.; Freeman, J.; Bunyan, M.; Celis, G.; Hagan, D.; Morgan, M.; Pieterson, E.C.; Zak, J. Short-Term Impacts of Laurel Wilt on Redbay (Persea borbonia [L.] Spreng.) in a Mixed Evergreen-Deciduous Forest in Northern Florida. J. For. Res. 2011, 109, 82–88. [Google Scholar]
- Spiegel, K.S.; Leege, L.M. Impacts of laurel wilt on redbay (Persea borbonia (L.) Spreng.) population structure and forest communities in the coastal plain of Georgia, USA. Biol. Invasions 2013, 15, 2467–2487. [Google Scholar] [CrossRef]
- Brown, C.L.; Kirkman, L.K. Trees of Georgia and Adjacent States; Timber Press: Portland, OR, USA, 1990. [Google Scholar]
- Wunderlin, R.P.; Hansen, B.F. Guide to the Vascular Plants of Florida, 2nd ed.; University Press of Florida: Gainesville, FL, USA, 2003; 796p. [Google Scholar]
- Hughes, M.A.; Shin, K.; Eickwort, J.; Smith, J.A. First report of laurel wilt caused by Raffaelea lauricola on silk bay in Florida. Plant Dis. 2012, 96, 910. [Google Scholar] [CrossRef]
- Hughes, M.A.; Inch, S.A.; Ploetz, R.C.; Er, H.L.; van Bruggen, A.H.C.; Smith, J.A. Responses of swamp bay, Persea palustris, and avocado, Persea americana, to various concentrations of the laurel wilt pathogen, Raffaelea lauricola. For. Pathol. 2015, 45, 111–119. [Google Scholar] [CrossRef]
- Rodgers, L.; Derksen, A.; Pernas, T. Expansion and impact of laurel wilt in the Florida Everglades. Fla. Entomol. 2014, 97, 1247–1250. [Google Scholar] [CrossRef]
- Snyder, J.R. Ecological Implications of Laurel Wilt Infestation on Everglades Tree Islands, Southern Florida; US Department of the Interior, US Geological Survey: Reston, VI, USA, 2014.
- Choudhury, R.A.; Hong, L.E.; Hughes, M.; Smith, J.A.; Pruett, G.E.; Konkol, J.; Ploetz, R.C.; Marois, J.J.; Garrett, K.A.; van Bruggen, A.H. Host density dependence and environmental factors affecting laurel wilt invasion. bioRxiv 2019, 642827. [Google Scholar] [CrossRef]
- Cassen, D.L. Sassafras. Purdue University Extension, Hardwood Lumber and Veneer Series, 2007; FNR-289-W; Purdue University: West Lafayette, IN, USA, 2007. [Google Scholar]
- Griggs, M.M. Sassafras albidum (Nutt.); Nees. Burns, R.M.; Honkala, B.H. Technical coordinators. Silv. N. Am. 1990, 2, 773–777. [Google Scholar]
- Duncan, W.H.; Duncan, M.B. Trees of the southeastern United States; The University of Georgia Press: Athens, GA, USA, 1988; 322p. [Google Scholar]
- Vines, R.A. Trees, Shrubs, and Woody Vines of the Southwest; University of Texas Press: Austin, TX, USA, 1960; 1104p. [Google Scholar]
- Carey, A.B.; Gill, J.D. Firewood and Wildlife. Res. Note 299; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Broomall, PA, USA, 1980; 5p.
- Randolph, K.C. Status of Sassafras albidum (Nutt.) Nees in the Presence of Laurel wilt and Throughout the Eastern United States. Southeast. Nat. 2017, 16, 37–58. [Google Scholar] [CrossRef]
- Gilman, E.F.; Watson, D.G. Sassafras albidum Sassafras. Fact Sheet ST-584, Environmental Horticulture Department, Florida Cooperative Extension Service, University of Florida. 1994. Available online: http://hort.ufl.edu/trees/SASALBA.pdf (accessed on 2 December 2020).
- Olatinwo, R.; Barton, C.; Fraedrich, S.W.; Johnson, W.; Hwang, J. First report of laurel wilt, caused by Raffaelea lauricola, on sassafras (Sassafras albidum) in Arkansas. Plant Dis. 2016, 100, 2331. [Google Scholar] [CrossRef]
- Mayfield, A.E.; Villari, C.; Hamilton, J.L.; Slye, J.; Langston, W.; Oten, K.; Fraedrich, S. First report of laurel wilt disease caused by Raffaelea lauricola on sassafras in North Carolina. Plant Dis. 2019, 103, 155–156. [Google Scholar] [CrossRef]
- Loyd, A.L.; Chase, K.D.; Nielson, A.; Hoover, N.; Dreaden, T.J.; Mayfield, A.E.; Crocker, E.; Fraedrich, S.W. First Report of Laurel Wilt Caused by Raffaelea lauricola on Sassafras albidum in Tennessee and Kentucky. Plant Dis. 2019, 104, 567. [Google Scholar] [CrossRef]
- Smith, J.A.; Dreaden, T.J.; Mayfield, A.E., III; Boone, A.; Fraedrich, S.W.; Bates, C. First report of laurel wilt caused by Raffaelea lauricola on sassafras in Florida and South Carolina. Plant Dis. 2009, 93, 1079. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.S.; Hanula, J.; Fraedrich, S.; Bates, C. Progression and impact of laurel wilt within redbay and sassafras populations in southeast Georgia. Southeast. Nat. 2015, 14, 650–674. [Google Scholar] [CrossRef]
- Scora, R.W.; Bergh, B.O. The origin and taxonomy of avocado (Persea americana) Mill. Lauraceae. Acta Hort. 1990, 275, 387–394. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Peña, J.E.; Crane, J.H.; Smith, J.A.; Branch, C.L.; Ottoson, E.D.; Hughes, M. Ability of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) to bore into young avocado (Lauraceae) plants and transmit the laurel wilt pathogen (Raffaelea sp.). Fla. Entomol. 2008, 91, 485–487. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Pérez-Martínez, J.M.; Evans, E.A.; Inch, S.A. Toward fungicidal management of laurel wilt of avocado. Plant Dis. 2011, 95, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C.; Pérez-Martínez, J.M.; Smith, J.A.; Hughes, M.; Dreaden, T.J.; Inch, S.A.; Fu, Y. Responses of avocado to laurel wilt, caused by Raffaelea lauricola. Plant Pathol. 2012, 61, 801–808. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Kendra, P.E.; Choudhury, R.A.; Rollins, J.A.; Campbell, A.; Garrett, K.; Hughes, M.; Dreaden, T. Laurel wilt in natural and agricultural ecosystems: Understanding the drivers and scales of complex pathosystems. Forests 2017, 8, 48. [Google Scholar] [CrossRef]
- Carrillo, D.; Duncan, R.E.; Peña, J.E. Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 2012, 95, 573–579. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Hanula, J.L. Effect of tree species and end seal on attractiveness and utility of cut bolts to the redbay ambrosia beetle and granulate ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2012, 105, 461–470. [Google Scholar] [CrossRef][Green Version]
- Peña, J.E.; Carrillo, D.; Duncan, R.E.; Capinera, J.L.; Brar, G.; McLean, S.; Arpaia, M.L.; Focht, E.; Smith, J.A.; Hughes, M.; et al. Susceptibility of Persea spp. and other Lauraceae to attack by redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2012, 95, 783–787. [Google Scholar] [CrossRef]
- Menocal, O.; Kendra, P.E.; Montgomery, W.S.; Crane, J.H.; Carrillo, D. Vertical distribution and daily flight periodicity of ambrosia beetles (Coleoptera: Curculionidae) in Florida avocado orchards affected by laurel wilt. J. Econ. Entomol. 2018, 111, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.; Dunlap, C.A.; Avery, P.B.; Navarrete, J.; Duncan, R.E.; Jackson, M.A.; Behle, R.W.; Cave, R.D.; Crane, J.; Rooney, A.P.; et al. Entomopathogenic fungi as biological control agents for the vector of the laurel wilt, the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae). Biol. Control 2015, 81, 44–50. [Google Scholar] [CrossRef]
- USFWS (US Fish and Wildlife Service). Pondspice (Litsea aestivalis). ECOS Environmental Conservation Online System. 2020. Available online: https://ecos.fws.gov/ecp/species/177 (accessed on 6 November 2020).
- Surdick, J.A.; Jenkins, A.M. Pondspice (Litsea aestivalis) Population Status and Response to Laurel Wilt in Northeast Florida; Florida Natural Areas Inventory: Tallahassee, FL, USA, 2009. [Google Scholar]
- Hughes, M.; Smith, J.A.; Mayfield, A.E., III; Minno, M.C.; Shin, K. First report of laurel wilt caused by Raffaelea lauricola on pondspice in Florida. Plant Dis. 2011, 95, 1588. [Google Scholar] [CrossRef] [PubMed]
- USFWS (US Fish and Wildlife Service). Pondberry (Lindera melissifolia). ECOS Environmental Conservation Online System. 2020. Available online: https://ecos.fws.gov/ecp/species/1279 (accessed on 6 November 2020).
- Best, G.S.; Fraedrich, S.W. An assessment of the potential impact of laurel wilt on clonal populations of Lindera melissifolia (Pondberry). Southeast. Nat. 2018, 17, 616–628. [Google Scholar] [CrossRef]
- Woffard, B.E. Lindera. Flora of North America Editorial Committee. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=118626 (accessed on 6 November 2020).
- Fraedrich, S.W.; Harrington, T.C.; McDaniel, B.A.; Best, G.S. First report of laurel wilt, caused by Raffaelea lauricola, on spicebush (Lindera benzoin) in South Carolina. Plant Dis. 2016, 100, 2330. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Pruett, G.E.; Mayfield, A.E., III; MacKenzie, M.; Deyrup, M.A.; Bauchan, G.R.; Ploetz, R.C.; Epsky, N.D. North American Lauraceae: Terpenoid emissions, relative attraction and boring preferences of redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2014, 9, e102086. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; MacKenzie, M.; Cannon, P.G.; Oak, S.W.; Horn, S.; Hwang, J.; Kendra, P.E. Suitability of California bay laurel and other species as hosts for the non-native redbay ambrosia beetle and granulate ambrosia beetle. Agric. For. Entomol. 2013, 15, 227–235. [Google Scholar] [CrossRef]
- Wunderlin, R.P.; Hansen, B.F.; Franck, A.R.; Essig, F.B. Atlas of Florida Plants; Institute for Systematic Botany, University of South Florida: Tampa, FL, USA, 2020; Available online: https://florida.plantatlas.usf.edu/ (accessed on 2 December 2020).
- Ploetz, R.C.; Konkol, J. First report of gulf licaria, Licaria trianda, as a suscept of laurel wilt. Plant Dis. 2013, 97, 1248. [Google Scholar] [CrossRef]
- Stein, W.I. Umbellularia californica (Hook. & Arn.) Nutt. Silv. N. Am. 1990, 2, 826–834. [Google Scholar]
- Fraedrich, S.W. California laurel is susceptible to laurel wilt caused by Raffaelea lauricola. Plant Dis. 2008, 92, 1469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van der Werff, H. A key to the genera of Lauraceae in the New World. Ann. Mo. Bot. Gard. 1991, 78, 377. [Google Scholar] [CrossRef]
- Elchibegoff, I.M. The Forests, Lumber Industry, and Timber Trade of Chile. J. For. Res. 1941, 39, 357–361. [Google Scholar] [CrossRef]
- Krainovic, P.M.; Almeida, D.R.A.D.; Desconci, D.; Veiga-Júnior, V.F.D.; Sampaio, P.D.T.B. Sequential management of commercial rosewood (Aniba rosaeodora ducke) plantations in central Amazonia: Seeking sustainable models for Essential oil production. Forests 2017, 8, 438. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef]
- Kostermans, A.J.G.H. Lauraceae. Reinwardtia 1957, 4, 193–256. [Google Scholar]
- Shih, H.H.; Wuest, C.E.; Fraedrich, S.W.; Harrington, T.C.; Chen, C.Y. Assessing the susceptibility of Asian species of Lauraceae to the laurel wilt pathogen Raffaelea lauricola. Taiwan Lin Ye Ke Xue 2018, 33, 173–184. Available online: https://www.cabi.org/ISC/FullTextPDF/2018/20183348056.pdf (accessed on 2 December 2020).
- Hyland, B.P.M. A revision of the Lauraceae in Australia (excluding Cassytha). Aust. Syst. Bot. 1989, 2, 135–367. [Google Scholar] [CrossRef]
- Van der Werff, H. The genera of Lauraceae in Madagascar with nomenclatural novelties in Cryptocarya. Candollea 2017, 72, 323–328. [Google Scholar]
- Ogundajo, A.L.; Adeniran, L.A.; Ashafa, A.O. Medicinal properties of Ocotea bullata stem bark extracts: Phytochemical constituents, antioxidant and anti-inflammatory activity, cytotoxicity and inhibition of carbohydrate-metabolizing enzymes. J. Integr. Med. 2018, 16, 132–140. [Google Scholar] [CrossRef]
- Pignatti, E.; Pignatti, S.; D’Angeli, D.; De Nicola, C.; Maffei, L.; Testi, A.; Tinelli, A. The Laurisilva as a cultural heritage: Proposal for the protection of the relict of laurel forest near Ponte Renaro. Rend. Lincei 2015, 26, 643–649. [Google Scholar] [CrossRef]
- Filibeck, G. Notes on the distribution of Laurus nobilis L. (Lauraceae) in Italy. Webbia 2006, 61, 45–56. [Google Scholar] [CrossRef]
- Hughes, M.A.; Black, A.; Smith, J.A. First report of laurel wilt caused by Raffaelea lauricola on bay laurel (Laurus nobilis) in the United States. Plant Dis. 2014, 98, 1159. [Google Scholar] [CrossRef]
- Tutin, T.G. The vegetation of the Azores. J. Ecol. 1953, 41, 53–61. [Google Scholar] [CrossRef]
- Kondraskov, P.; Schutz, N.; Schussler, C.; de Sequeira, M.M.; Guerra, A.S.; Caujape-Castells, J.; Jaen-Molina, R.; Marrero-Rodriguez, A.; Koch, M.A.; Linder, P.; et al. Biogeography of Mediterranean Hotspot Biodiversity: Re-Evaluating the ‘Tertiary Relict’ Hypothesis of Macaronesian Laurel Forests. PLoS ONE 2015, 10, e0132091. [Google Scholar] [CrossRef]
- Hughes, M.A.; Brar, G.; Ploetz, R.C.; Smith, J.A. Field and Growth Chamber Inoculation Demonstrate Persea indica as a Newly Recognized Host for the Laurel Wilt Pathogen, Raffaelea lauricola. Plant Health Prog. 2013, 14, 44. [Google Scholar] [CrossRef]
- Coder, K.D. Redbay (Persea borbonia): Drifting toward Oblivion Warnell School of Forestry & Natural Resources Outreach, University of Georgia, Athens. Publ. No. WSFNR07-2. 2012. Available online: https://www.warnell.uga.edu/sites/default/files/publications/WSFNR-20-80C_Coder.pdf (accessed on 23 October 2020).
- Evans, E.A.; Crane, J.; Hodges, A.; Osborne, J.L. Potential economic impact of laurel wilt on the Florida avocado industry. HortTechnology 2010, 20, 234–238. [Google Scholar] [CrossRef]
- Evans, E.A.; Ballen, F.H. An Econometric Demand Model for Florida Green-skin Avocados. HortTechnology 2015, 25, 405–411. [Google Scholar] [CrossRef]
- Goldberg, N.; Heine, J. A comparison of arborescent vegetation pre-(1983) and post-(2008) outbreak of the invasive species the Asian ambrosia beetle Xyleborus glabratus in a Florida maritime hammock. Plant Ecol. Divers. 2009, 2, 77–83. [Google Scholar] [CrossRef]
- Evans, J.P.; Scheffers, B.R.; Hess, M. Effect of laurel wilt invasion on redbay populations in a maritime forest community. Biol. Invasions 2014, 16, 1581–1588. [Google Scholar] [CrossRef]
- Loveless, C.M. A study of the vegetation in the Florida Everglades. Ecology 1959, 40, 1–9. [Google Scholar] [CrossRef]
- Riggins, J.J.; Chupp, A.D.; Formby, J.P.; Dearing, N.A.; Bares, H.M.; Brown, R.L.; Oten, K.F. Impacts of laurel wilt on arthropod herbivores of North American Lauraceae. Biol. Invasions 2019, 21, 493–503. [Google Scholar] [CrossRef]
- Nielsen, A.M.; Rieske, L.K. Potential host and range expansion of an exotic insect-pathogen complex: Simulating effects of sassafras mortality from laurel wilt invasion in the central hardwoods region. J. Torrey Bot. Soc. 2015, 142, 292–301. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Balanyá, J.; Oller, J.M.; Huey, R.B.; Gilchrist, G.W.; Serra, L. Global genetic change tracks global climate warming in Drosophila subobscura. Science 2006, 313, 1773–1775. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Evolutionary response to rapid climate change. Science 2006, 312, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Chang. Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Formby, J.P.; Rodgers, J.C.; Koch, F.H.; Krishnan, N.; Duerr, D.A.; Riggins, J.J. Cold tolerance and invasive potential of the redbay ambrosia beetle (Xyleborus glabratus) in the eastern United States. Biol. Invasions 2017, 20, 995–1007. [Google Scholar] [CrossRef]
- Formby, J.P.; Krishnan, N.; Riggins, J.J. Supercooling in the redbay ambrosia beetle (Coleoptera: Curculionidae). Fla. Entomol. 2013, 96, 1530–1540. [Google Scholar] [CrossRef]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C.; Hassol, S.J. (Eds.) Global Climate Change Impacts in the United States; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Trân, J.K.; Ylioja, T.; Billings, R.F.; Régnière, J.; Ayres, M.P. Impact of minimum winter temperatures on the population dynamics of Dendroctonus frontalis. Ecol. Appl. 2007, 17, 882–899. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.S.; Anderson, K.H.; Bartlein, P.J. Atlas of Relations between Climatic Parameters and Distributions of Important Trees and Shrubs in North America. U.S. Geological Survey Professional, Paper 1650 A&B Online Version 1.0. 1999. Available online: http://pubs.usgs.gov/pp/p1650-a/ (accessed on 2 December 2020).
- Don’t Move Firewood. Firewood Map. 2020. Available online: https://www.dontmovefirewood.org/map/ (accessed on 9 November 2020).
- Spence, D.J.; Smith, J.A.; Ploetz, R.; Hulcr, J.; Stelinski, L.L. Effect of chipping on emergence of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) and recovery of the laurel wilt pathogen from infested wood chips. J. Econ. Entomol. 2013, 106, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, A.E., III. Laurel wilt: A serious threat to redbay and other related native plants. Palmetto 2007, 24, 8–11. [Google Scholar]
- Riggins, J.J.; Hughes, M.; Smith, J.A.; Mayfield, A.E.; Layton, B.; Balbalian, C.; Campbell, R. First Occurrence of Laurel wilt Caused by Raffaelea lauricola on Redbay Trees in Mississippi. Plant Dis. 2010, 94, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.A.; Fraedrich, S.W.; Harrington, T.C.; Cameron, R.S.; Menard, R.D.; Best, G.S. First report of laurel wilt, caused by Raffaelea lauricola, on sassafras (Sassafras albidum) in Alabama. Plant Dis. 2013, 97, 688. [Google Scholar] [CrossRef]
- Menard, R.D.; Clarke, S.R.; Fraedrich, S.W.; Harrington, T.C. First report of laurel wilt, caused by Raffaelea lauricola, on redbay (Persea borbonia) in Texas. Plant Dis. 2016, 100, 1502. [Google Scholar] [CrossRef]
- Mayfield, A.E., III; Barnard, E.L.; Smith, J.A.; Bernick, S.C.; Eickwort, J.M.; Dreaden, T.J. Effect of propiconazole on laurel wilt development in redbay trees and on the pathogen in vitro. Arboric. Urban For. 2008, 34, 317–324. [Google Scholar]
- Ploetz, R.C.; Konkol, J.L.; Pérez-Martínez, J.M.; Fernandez, R. Management of laurel wilt of avocado caused by Raffaelea lauricola. Eur. J. Plant Pathol. 2017, 149, 133–143. [Google Scholar] [CrossRef]
- Crane, J.H.; Peña, J.E.; Osborne, J.L. Redbay Ambrosia Beetle-Laurel Wilt Pathogen: A Potential Major Problem for the Florida Avocado Industry; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2008. [Google Scholar]
- Pérez-Martínez, J.; Ploetz, R.C.; Konkol, J.L. Significant in vitro antagonism of the laurel wilt pathogen by endophytic fungi from the xylem of avocado does not predict their ability to control the disease. Plant Pathol. 2018, 67, 1768–1776. [Google Scholar] [CrossRef]
- Olatinwo, R.; Fraedrich, S. An Acaromyces species associated with bark beetles from southern pine has inhibitory properties against Raffaelea lauricola, the causal pathogen of laurel wilt of redbay. Plant Health Prog. 2019, 20, 220–228. [Google Scholar] [CrossRef]
- Boekhout, T.; Theelen, B.; Houbraken, J.; Robert, V.; Scorozetti, G.; Gafni, A.; Gerson, U.; Sztejnberg, A. Novel anamorphic mite-associated fungi belonging to the Ustilaginomycetes: Meira geulakonigii gen. nov., sp. nov., Meira argovae sp. nov. and Acaromyces ingoldii gen. nov., sp. nov. Int. J. Syst. Evol. 2003, 53, 1655–1664. [Google Scholar]
- Dunlap, C.A.; Lueschow, S.; Carrillo, D.; Rooney, A.P. Screening of bacteria for antagonistic activity against phytopathogens of avocados. Plant Gene 2017, 11, 17–22. [Google Scholar] [CrossRef]
- Hughes, M.A.; Smith, J.A.; Coyle, D.R. Biology, Ecology, and Management of Laurel Wilt and the Redbay Ambrosia Beetle. Southern Regional Extension Forestry, Forest Health. SREF-FH-006. 2016. Available online: https://sref.info/resources/publications/biology-ecology-and-management-of-laurel-wilt-and-the-redbay-ambrosia-beetle (accessed on 2 December 2020).
- Duerr, D. Laurel wilt/redbay ambrosia beetle. In Major Forest Insect and Disease Conditions in the United States; Man, G., Ed.; USDA Forest Service: Washington, DC, USA, 2009; pp. 35–37. [Google Scholar]
- Sankaran, S.; Ehsani, R.; Inch, S.A.; Ploetz, R.C. Evaluation of visible-near infrared reflectance spectra of avocado leaves as a non-destructive sensing tool for detection of laurel wilt. Plant Dis. 2012, 96, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- De Castro, A.I.; Ehsani, R.; Ploetz, R.C.; Crane, J.H.; Buchanon, S. Detection of laurel wilt in avocado using low altitude aerial imaging. PLoS ONE 2015, 10, e0124642. [Google Scholar] [CrossRef]
- Mendel, J.; Burns, C.; Kallifatidis, B.; Evans, E.; Crane, J.; Furton, K.G.; Mills, D. Agri-dogs: Using canines for earlier detection of laurel wilt disease affecting avocado trees in South Florida. HortTechnology 2018, 28, 109–116. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Keith, L.M.; Heller, W.P.; Hughes, M.A.; Vaughn, N.R.; Hughes, R.F.; Balzotti, C. A spectral mapping signature for the Rapid Ohia Death (ROD) pathogen in Hawaiian forests. Remote Sens. 2018, 10, 404. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatinwo, R.O.; Fraedrich, S.W.; Mayfield, A.E., III. Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management. Forests 2021, 12, 181. https://doi.org/10.3390/f12020181
Olatinwo RO, Fraedrich SW, Mayfield AE III. Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management. Forests. 2021; 12(2):181. https://doi.org/10.3390/f12020181
Chicago/Turabian StyleOlatinwo, Rabiu O., Stephen W. Fraedrich, and Albert E. Mayfield, III. 2021. "Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management" Forests 12, no. 2: 181. https://doi.org/10.3390/f12020181
APA StyleOlatinwo, R. O., Fraedrich, S. W., & Mayfield, A. E., III. (2021). Laurel Wilt: Current and Potential Impacts and Possibilities for Prevention and Management. Forests, 12(2), 181. https://doi.org/10.3390/f12020181





