Prospects of Using Tree-Ring Earlywood and Latewood Width for Reconstruction of Crops Yield on Example of South Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Grain Crops Yield Data
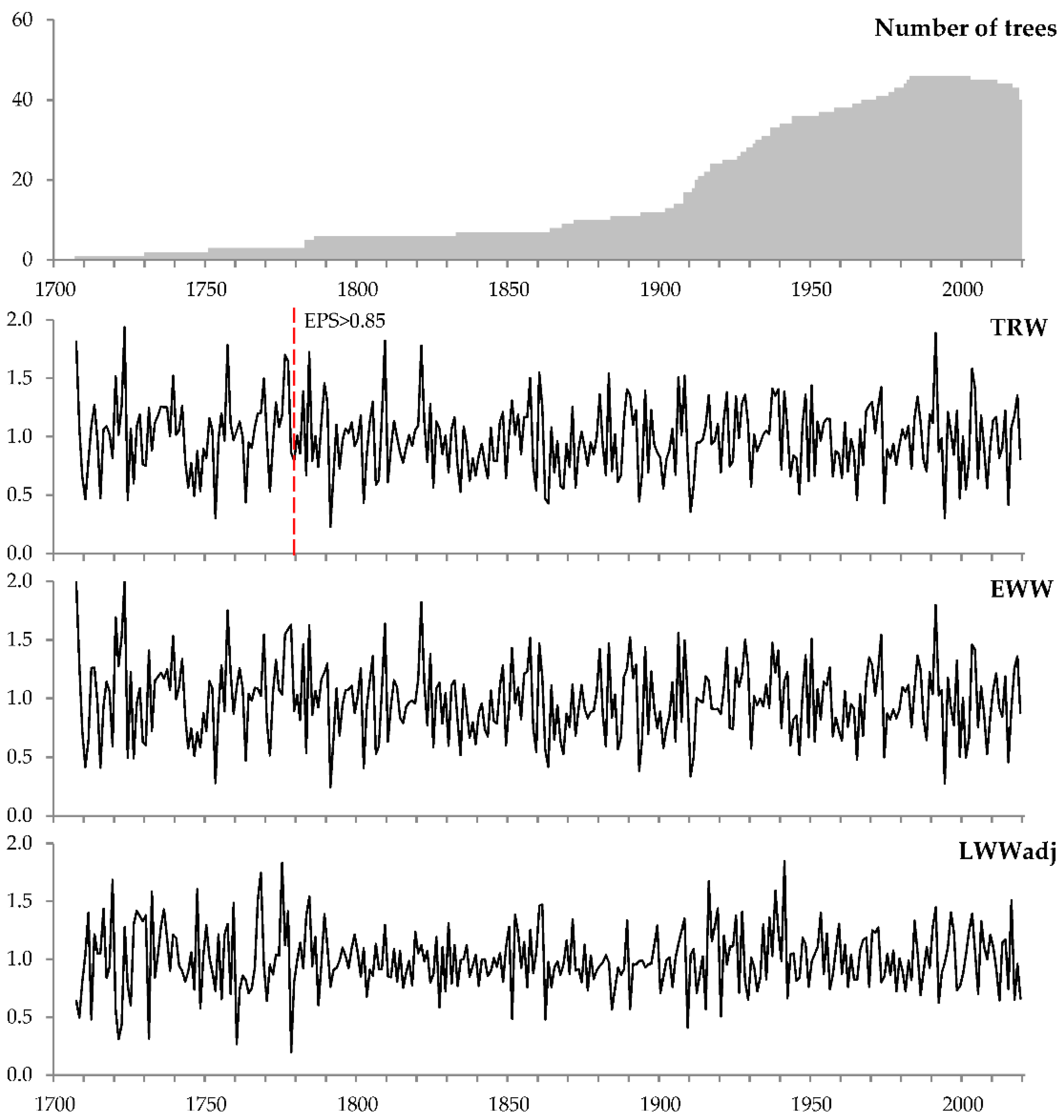
2.3. Tree-Ring Chronologies
2.4. Statistical Analysis
3. Results
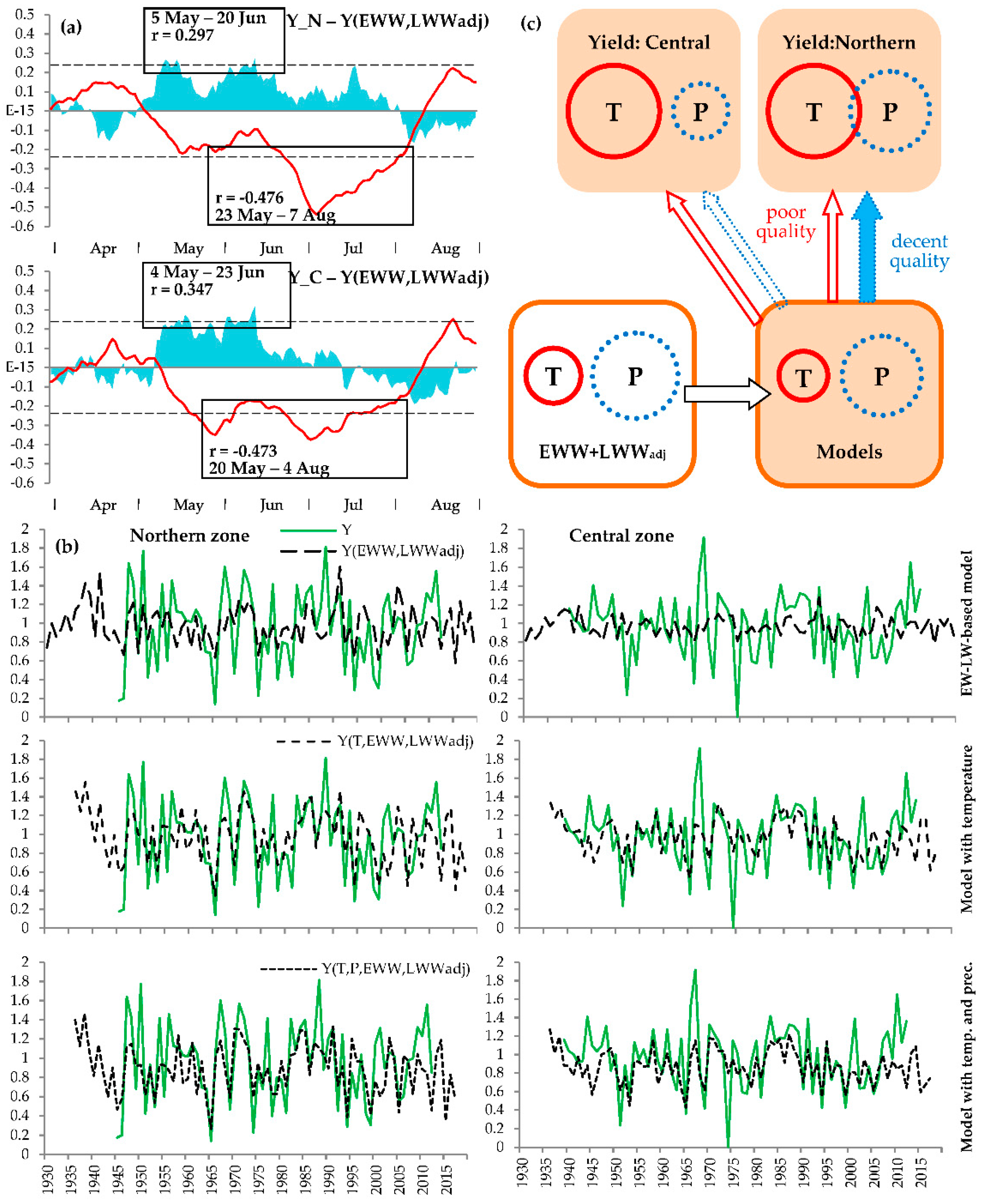
3.1. Relationships between Chronologies
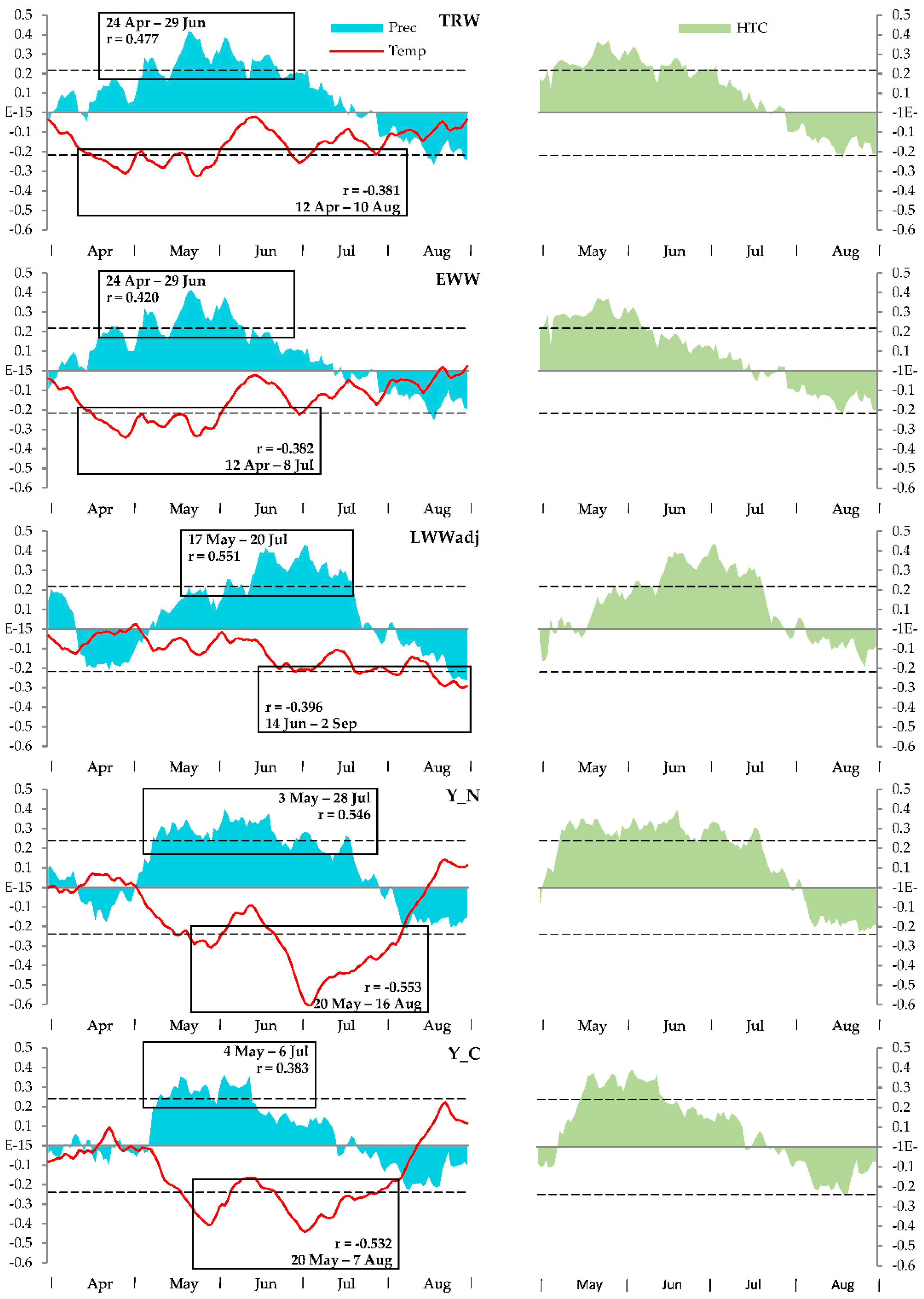
3.2. Climatic Response
3.3. Yield Reconstruction Models
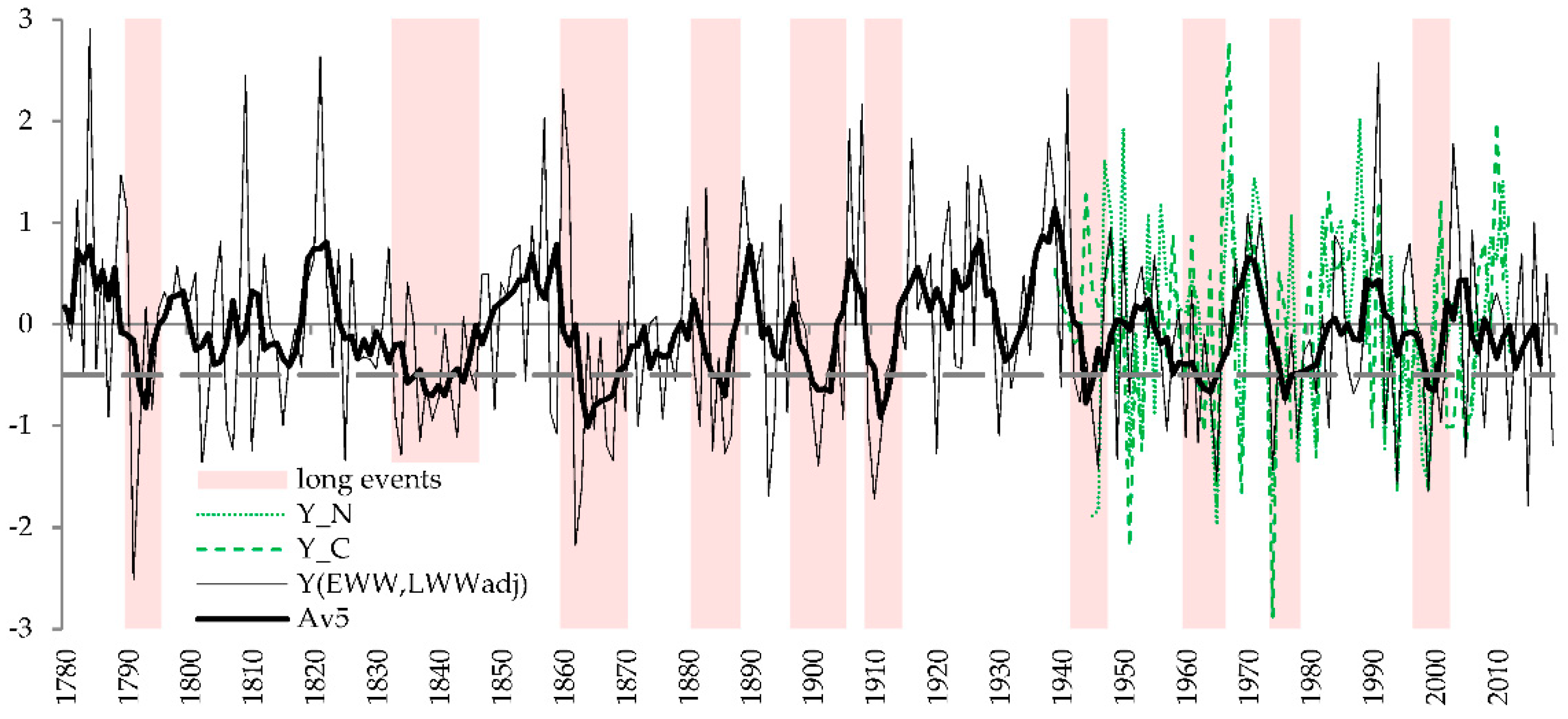
3.4. History of Crop Failures and Low-Yield Periods
4. Discussion
4.1. Plant Behavior Under Climatic Extremes
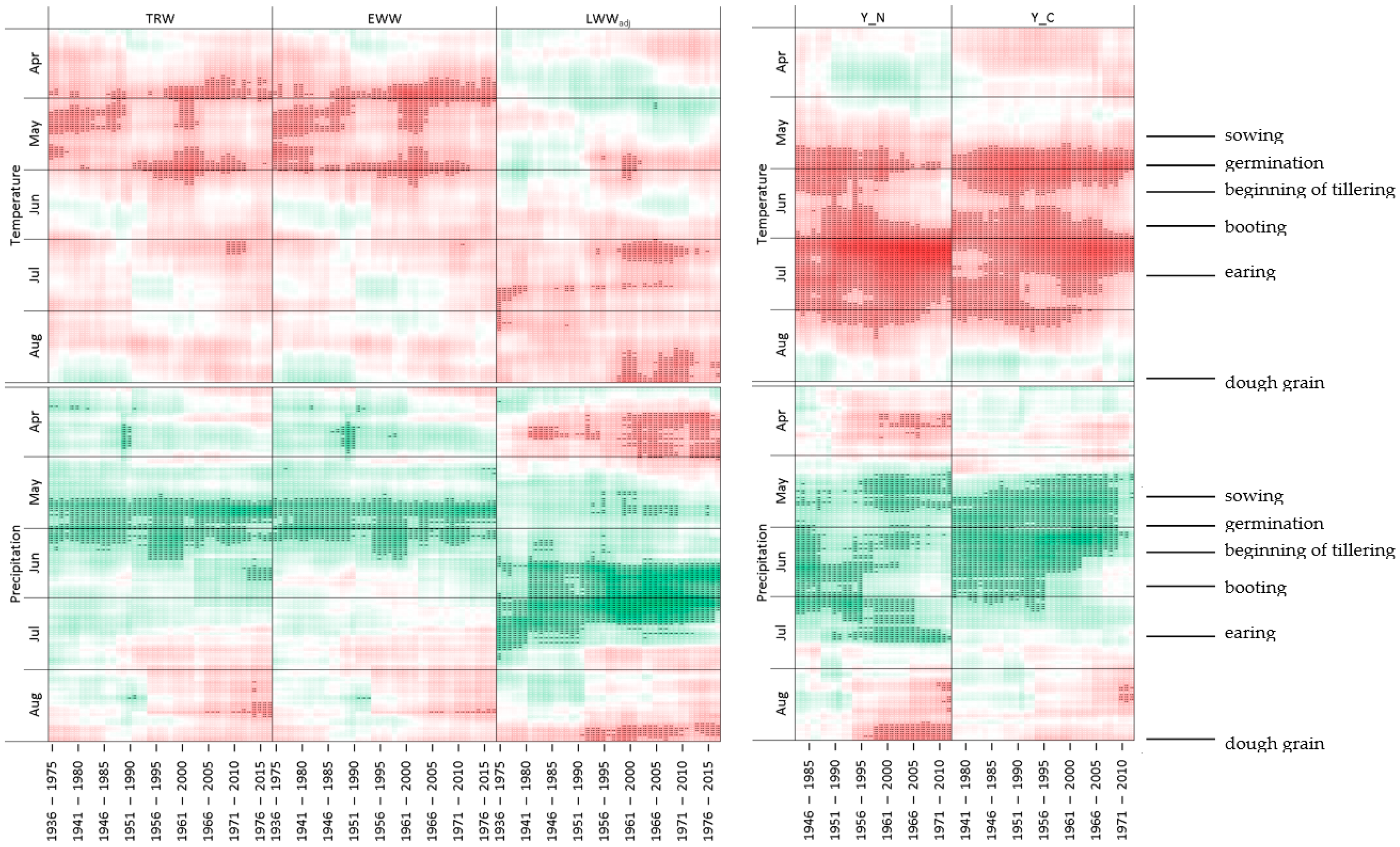
4.2. Seasonality of Climatic Responses and Its Dynamics
4.3. Assessment of the Reconstructed Crop Yield in South Siberia
4.4. History and Prospects of Crops Failures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaganov, E.A. Method of crops yield prediction using dendrochronological data. Soviet J. Ecol. 1989, 3, 15–23. (In Russian) [Google Scholar]
- Wu, X.; Babst, F.; Ciais, P.; Frank, D.; Reichstein, M.; Wattenbach, M.; Zang, C.; Mahecha, M.D. Climate-mediated spatiotemporal variability in terrestrial productivity across Europe. Biogeosciences 2014, 11, 3057–3068. [Google Scholar] [CrossRef]
- Bindi, M.; Olesen, J.E. The responses of agriculture in Europe to climate change. Regi. Environ. Chang. 2011, 11, 151–158. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Jauhiainen, L.; Palosuo, T.; Hakala, K.; Ruosteenoja, K. Rainfed crop production challenges under European high latitude conditions. Reg. Environ. Chang. 2016, 16, 1521–1533. [Google Scholar] [CrossRef]
- Myglan, V.S.; Ovchinnikov, D.V.; Vaganov, E.A.; Zhirnova, D.F. Influence of climatic changes on the economic activities of the population in Southern Siberia during the Little Ice Age. Geogr. Nat. Resour. 2007, 1, 90–96. (In Russian) [Google Scholar]
- Sauchyn, D.; Barrow, E.; Fang, X.; Henderson, N.; Johnston, M.; Pomeroy, J.; Thorpe, J.; Wheaton, E.; Williams, B. Saskatchewan’s Natural Capital in a Changing Climate: An Assessment of Impacts and Adaptation. Report to Saskatchewan Ministry of Environment from the Prairie Adaptation Research Collaborative; PARC: Saskatchewan, Canada, 2009; Available online: https://www.parc.ca/wp-content/uploads/2019/05/Sauchyn_Barrow_Fang-2009-Saskatchewans_natural_capital_in_changing_climate_an_assessment_of_impacts_and_adaptation.pdf (accessed on 1 September 2020).
- Huhtamaa, H.; Helama, S.; Holopainen, J.; Rethorn, C.; Rohr, C. Crop yield responses to temperature fluctuations in 19th century Finland: Provincial variation in relation to climate and tree-rings. Boreal Environ. Res. 2015, 20, 707–723. [Google Scholar] [CrossRef]
- Therrell, M.D.; Stanle, D.W.; Diaz, J.V.; Cornelo Oviedo, E.H.; Cleaveland, M.K. Tree-ring reconstructed maize yield in central Mexico: 1474-2001. Clim. Chang. 2006, 74, 493–504. [Google Scholar] [CrossRef]
- Shi, F.; Yang, B.; Mairesse, A.; von Gunten, L.; Li, J.; Bräuning, A.; Yang, F.; Xiao, X. Northern Hemisphere temperature reconstruction during the last millennium using multiple annual proxies. Clim. Res. 2013, 56, 231–244. [Google Scholar] [CrossRef]
- Huhtamaa, H.; Helama, S. Reconstructing crop yield variability in Finland: Long-term perspective of the cultivation history on the agricultural periphery since AD 760. Holocene 2017, 27, 3–11. [Google Scholar] [CrossRef]
- Salzer, M.W.; Kipfmueller, K.F. Reconstructed temperature and precipitation on a millennial timescale from tree-rings in the southern Colorado Plateau, USA. Clim. Chang. 2005, 70, 465–487. [Google Scholar] [CrossRef]
- Stahle, D.W.; Diaz, J.V.; Burnette, D.J.; Paredes, J.C.; Heim, R.R., Jr.; Fye, F.K.; Soto, R.A.; Therrell, M.D.; Cleaveland, M.K.; Stahle, D.K. Major Mesoamerican droughts of the past millennium. Geophys. Res. Lett. 2011, 38, L05703. [Google Scholar] [CrossRef]
- Touchan, R.; Kherchouche, D.; Oudjehih, B.; Touchan, H.; Slimani, S.; Meko, D.M. Dendroclimatology and wheat production in Algeria. J. Arid Environ. 2016, 124, 102–110. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Belokopytova, L.V.; Shah, S.K.; Zhirnova, D.F. Past crops yield dynamics reconstruction from tree-ring chronologies in the forest-steppe zone based on low- and high-frequency components. Int. J. Biometeorol. 2018, 62, 861–871. [Google Scholar] [CrossRef]
- Rygalova, N.V.; Rygalov, E.V.; Bykov, N.I. Reconstruction of grain yield in the steppe zone of the Altai territory (south of Western Siberia) in the XIX-XX centuries on the basis of tree-ring chronologies of tape burs. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on Sustainable Development of Cross-Border Regions, Barnaul, Russia, 19–20 April 2019; IOP Publishing: Bristol, England, 2019; Volume 395, p. 012046. [Google Scholar]
- Kaisermann, A.; de Vries, F.T.; Griffiths, R.I.; Bardgett, R.D. Legacy effects of drought on plant–soil feedbacks and plant–plant interactions. New Phytol. 2017, 215, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Geng, X.; Yin, G.; Hänninen, H.; Hao, F.; Zhang, X.; Fu, Y.H. Legacy effect of spring phenology on vegetation growth in temperate China. Agric. For. Meteorol. 2020, 281, 107845. [Google Scholar] [CrossRef]
- Campelo, F.; Vieira, J.; Nabais, C. Tree-ring growth and intra-annual density fluctuations of Pinus pinaster responses to climate: Does size matter? Trees 2013, 27, 763–772. [Google Scholar] [CrossRef]
- Schuster, R.; Oberhuber, W. Age-dependent climate–growth relationships and regeneration of Picea abies in a drought-prone mixed-coniferous forest in the Alps. Can. J. For. Res. 2013, 43, 609–618. [Google Scholar] [CrossRef]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Ogle, K.; Pacala, S.W. A modeling framework for inferring tree growth and allocation from physiological, morphological and allometric traits. Tree Physiol. 2009, 29, 587–605. [Google Scholar] [CrossRef]
- Piper, F.I.; Fajardo, A. Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. J. Ecol. 2014, 102, 1101–1111. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Application in Environmental Sciences; Kluwer Academic Publishers: Dordrecht, Germany, 1990. [Google Scholar]
- Pérez-de-Lis, G.; García-González, I.; Rozas, V.; Olano, J.M. Feedbacks between earlywood anatomy and non-structural carbohydrates affect spring phenology and wood production in ring-porous oaks. Biogeosciences 2016, 13, 5499–5510. [Google Scholar] [CrossRef]
- Szejner, P.; Wright, W.E.; Belmecheri, S.; Meko, D.; Leavitt, S.W.; Ehleringer, J.R.; Monson, R.K. Disentangling seasonal and interannual legacies from inferred patterns of forest water and carbon cycling using tree-ring stable isotopes. Glob. Chang. Biol. 2018, 24, 5332–5347. [Google Scholar] [CrossRef] [PubMed]
- Meko, D.M.; Baisan, C.H. Pilot study of latewood width of conifers as an indicator of variability of summer rainfall in the North American monsoon region. Int. J. Climatol. J. R. Meteorol. Soc. 2001, 21, 697–708. [Google Scholar] [CrossRef]
- Vedrov, N.G. Breeding and Seed Production of Spring Wheat in Extreme Conditions; Krasnoyarsk University: Krasnoyarsk, Russia, 1984. (In Russian) [Google Scholar]
- Vedrov, N.G.; Dmitriev, V.E.; Nesterenko, E.M.; Nikitina, V.I.; Sannikova, L.G.; Frolov, I.N.; Halipsky, A.N. Spring Wheat in Eastern Siberia (Biology, Ecology, Breeding and Seed Production, Cultivation Technology); Krasnoyarsk State Agrarian University: Krasnoyarsk, Russia, 1998. (In Russian) [Google Scholar]
- Tsybenov, B.B.; Biltuev, A.S. Connection of yielding capacity of spring wheat with elements of productivity in arid conditions of Buryatia. Bull. North. Trans-Ural State Agric. Univers. 2016, 2, 87–93. (In Russian) [Google Scholar]
- Brinton, J.; Uauy, C. A reductionist approach to dissecting grain weight and yield in wheat. J. Integr. Plant Biol. 2019, 61, 337–358. [Google Scholar] [CrossRef]
- Acevedo, E.; Silva, P.; Silva, H. Wheat growth and physiology. In Bread Wheat, Improvement and Production; Curtis, B.C., Rajaram, S., Gómez Macpherson, H., Eds.; FAO: Rome, Italy, 2002; pp. 39–70. [Google Scholar]
- Majumder, D.A.N.; Shamsuddin, A.K.M.; Kabir, M.A.; Hassan, L. Genetic variability, correlated response and path analysis of yield and yield contributing traits of spring wheat. J. Bangladesh Agric. Univ. 2008, 6, 227–234. [Google Scholar] [CrossRef]
- Prasad, P.V.; Bheemanahalli, R.; Jagadish, S.K. Field crops and the fear of heat stress–opportunities, challenges and future directions. Field Crop. Res. 2017, 200, 114–121. [Google Scholar] [CrossRef]
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma functionality and fertility are reduced by heat and drought co-stress in wheat. Front. Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef]
- Zargar, M.; Bodner, G.; Tumanyan, A.; Tyutyuma, N.; Plushikov, V.; Pakina, E.; Shcherbakova, N.; Bayat, M. Productivity of various barley (Hordeum vulgare L.) cultivars under semi-arid conditions in southern Russia. Agron. Res. 2018, 16, 2242–2253. [Google Scholar] [CrossRef]
- Ivanova, I.; Ilina, S. Variability of morphological features of spring soft wheat Moskovskaya 35. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International AgroScience Conference (AgroScience-2019), Cheboksary, Russia, 1–2 June 2019; IOP Publishing: Bristol, UK, 2020; Volume 433, p. 012016. [Google Scholar]
- Plaut, Z.; Butow, B.J.; Blumenthal, C.S.; Wrigley, C.W. Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crop. Res. 2004, 86, 185–198. [Google Scholar] [CrossRef]
- Hay, R.K.M. Harvest index: A review of its use in plant breeding and crop physiology. Ann. Appl. Biol. 1995, 126, 197–216. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A.J. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef] [PubMed]
- Babushkina, E.A.; Belokopytova, L.V.; Zhirnova, D.F.; Shah, S.K.; Kostyakova, T.V. Climatically driven yield variability of major crops in Khakassia (South Siberia). Int. J. Biometeorol. 2018, 62, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Alisov, B.P. Climate of the USSR; Moscow State University: Moscow, Russia, 1956. (In Russian) [Google Scholar]
- USSR. Hydrometeorological Service. In Agroclimatic Resources of the Krasnoyarsk Krai and of the Tuva ASSR; Hydrometeoizdat: Leningrad, Russia, 1974. (In Russian) [Google Scholar]
- Mistryukov, A.A. Geomorphological Zoning of the Nazarovsko-Minusinsk Intermountain Depression; A.A. Trofimuk Joint Institute of Geology, Geophysics and Mineralogy SB RAS: Novosibirsk, Russia, 1991. (In Russian) [Google Scholar]
- Russian Federation. Government of the Republic of Khakassia. Territorial Planning Scheme of the Republic of Khakassia; Approved by Resolution No 763 from 14 November 2011. Available online: http://www.pravo.gov.ru/proxy/ips/?docbody=&nd=167019881 (accessed on 1 September 2020). (In Russian)
- Likhenko, I.E.; Artemova, G.V.; Khristov, Y.A. Establishment and development of plant breeding in Siberia. Sib. Bull. Agric. Sci. 2014, 5, 28–35. (In Russian) [Google Scholar]
- Cook, E.R.; Krusic, P.J. Program ARSTAN: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Lamont-Doherty Earth Observatory, Columbia University: New York, NY, USA, 2005. [Google Scholar]
- Ozturk, A.; Aydin, F. Effect of water stress at various growth stages on some quality characteristics of winter wheat. J. Agron. Crop Sci. 2004, 190, 93–99. [Google Scholar] [CrossRef]
- Meng, Z.; Duan, A.; Dassanayake, K.B.; Chen, D.; Gao, Y.; Wang, X.; Shen, X. Effects of regulated deficit irrigation on grain yield and quality traits in winter wheat. Trans. ASABE 2016, 59, 897–907. [Google Scholar] [CrossRef]
- Wigley, T.; Briffa, K.; Jones, P. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Selyaninov, G.T. Principles of agroclimatic regional planning in USSR. In Questions of Agroclimatic Zoning of the USSR; Davitaya, F.F., Shulgina, A.I., Eds.; Ministry of Agriculture of the USSR: Moscow, Russia, 1958; pp. 18–26. (In Russian) [Google Scholar]
- Kharuk, V.I.; Petrov, I.A.; Dvinskaya, M.L.; Im, S.T.; Shushpanov, A.S. Comparative reaction of larch (Larix sibirica Ledeb.) radial increment on climate change in the forest steppe and highlands of Southern Siberia. Contemp. Probl. Ecol. 2018, 11, 388–395. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef]
- Chenlemuge, T.; Schuldt, B.; Dulamsuren, C.; Hertel, D.; Leuschner, C.; Hauck, M. Stem increment and hydraulic architecture of a boreal conifer (Larix sibirica) under contrasting macroclimates. Trees 2015, 29, 623–636. [Google Scholar] [CrossRef]
- Khansaritoreh, E.; Schuldt, B.; Dulamsuren, C. Hydraulic traits and tree-ring width in Larix sibirica Ledeb. as affected by summer drought and forest fragmentation in the Mongolian forest steppe. Ann. For. Sci. 2018, 75, 30. [Google Scholar] [CrossRef]
- Koehler, A.-K.; Challinor, A.J.; Hawkins, E.; Asseng, S. Influences of increasing temperature on Indian wheat: Quantifying limits to predictability. Environ. Res. Lett. 2013, 8, 034016. [Google Scholar] [CrossRef]
- Gibbs, J.G.; Patten, D.T. Plant temperatures and heat flux in a Sonoran Desert ecosystem. Oecol. 1970, 5, 165–184. [Google Scholar] [CrossRef]
- Hesslerová, P.; Pokorný, J.; Brom, J.; Rejšková–Procházková, A. Daily dynamics of radiation surface temperature of different land cover types in a temperate cultural landscape: Consequences for the local climate. Ecol. Eng. 2013, 54, 145–154. [Google Scholar] [CrossRef]
- Altergot, V.F.; Mordkovich, S.S. Heat Damage to Wheat under Conditions of Sufficient Moisture; Science: Novosibirsk, Russia, 1977. (In Russian) [Google Scholar]
- Friend, D.J.C. Ear length and spikelet number of wheat grown at different temperatures and light intensities. Can. J. Bot. 1965, 43, 345–353. [Google Scholar] [CrossRef]
- Hossain, A.; da Silva, J.A.T.; Lozovskaya, M.V.; Zvolinsky, V.P. High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi J. Biol. Sci. 2012, 19, 473–487. [Google Scholar] [CrossRef]
- Kistner, E.; Kellner, O.; Andresen, J.; Todey, D.; Morton, L.W. Vulnerability of specialty crops to short-term climatic variability and adaptation strategies in the Midwestern USA. Clim. Chang. 2018, 146, 145–158. [Google Scholar] [CrossRef]
- Vanhanen, H.; Veteli, T.O.; Päivinen, S.; Kellomäki, S.; Niemelä, P. Climate change and range shifts in two insect defoliators: Gypsy moth and nun moth—A model study. Silva Fenn. 2007, 41, 621–638. [Google Scholar] [CrossRef]
- Nazimova, D.I.; Polikarpov, N.P. Forest zones of Siberia as determined by climatic zones and their possible transformation trends under global change. Silva Fenn. 1996, 30, 201–208. Available online: http://hdl.handle.net/1975/9232 (accessed on 1 September 2020). [CrossRef][Green Version]
- Abaimov, A.P.; Lesinski, J.A.; Martinsson, O.; Milyutin, L.I. Variability and Ecology of Siberian Larch Species; Reports, 43; Swedish University of Agricultural Sciences, Department of Silviculture: Umea, Sweden, 1998. [Google Scholar]
- Antonova, G.F.; Stasova, V.V. Effects of environmental factors on wood formation in larch (Larix sibirica Ldb.) stems. Trees 1997, 11, 462–468. [Google Scholar] [CrossRef]
- Bazhenova, O.I.; Tyumentseva, E.M. The structure of contemporary denudation in the steppes of the Minusinskaya depression. Geogr. Nat. Resour. 2010, 4, 362–369. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Zhirnova, D.F.; Belokopytova, L.V.; Tychkov, I.I.; Vaganov, E.A.; Krutovsky, K.V. Response of four tree species to changing climate in a moisture-limited area of South Siberia. Forests 2019, 10, 999. [Google Scholar] [CrossRef]
- Liu, H.; Park Williams, A.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Change Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.-W.; Rathgeber, C.B.K.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Gower, S.T.; Richards, J.H. Larches: Deciduous conifers in an evergreen world. BioScience 1990, 40, 818–826. [Google Scholar] [CrossRef]
- Velisevich, S.N.; Khutornoi, O.V. Effects of climatic factors on radial growth of Siberian stone pine and Siberian larch in sites with different soil humidity in the south of western Siberia. J. Sib. Fed. Univ. Biol. 2009, 12, 11732. (In Russian) [Google Scholar]
- Heide, O.M. Growth and dormancy in Norway spruce ecotypes (Picea abies) I. Interaction of photoperiod and temperature. Physiol. Plant. 1974, 30, 1–12. [Google Scholar] [CrossRef]
- Cartenì, F.; Deslauriers, A.; Rossi, S.; Morin, H.; De Micco, V.; Mazzoleni, S.; Giannino, F. The physiological mechanisms behind the earlywood-to-latewood transition: A process-based modeling approach. Front. Plant Sci. 2018, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Campelo, F.; Ma, Q.; Zhang, Y.; Bergeron, Y.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; et al. Photoperiod plays a dominant and irreplaceable role in triggering secondary growth resumption. Proc. Natl. Acad. Sci. USA 2020, 117, 32865–32867. [Google Scholar] [CrossRef] [PubMed]
- Babushkina, E.A.; Belokopytova, L.V.; Grachev, A.M.; Meko, D.M.; Vaganov, E.A. Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg. Environ. Chang. 2017, 17, 1725–1737. [Google Scholar] [CrossRef]
- Tao, F.; Yokozawa, M.; Xu, Y.; Hayashi, Y.; Zhang, Z. Climate changes and trends in phenology and yields of field crops in China, 1981–2000. Agric. For. Meteorol. 2006, 138, 82–92. [Google Scholar] [CrossRef]
- Estrella, N.; Sparks, T.H.; Menzel, A. Trends and temperature response in the phenology of crops in Germany. Glob. Change Biol. 2007, 13, 1737–1747. [Google Scholar] [CrossRef]
- Ren, S.; Qin, Q.; Ren, H.; Sui, J.; Zhang, Y. Heat and drought stress advanced global wheat harvest timing from 1981–2014. Remote Sens. 2019, 11, 971. [Google Scholar] [CrossRef]
- McMaster, G.; Wilhelm, W. Phenological responses of wheat and barley to water and temperature: Improving simulation models. J. Agric. Sci. 2003, 141, 129–147. [Google Scholar] [CrossRef]
- Lobell, D.B.; Sibley, A.; Ortiz-Monasterio, J.I. Extreme heat effects on wheat senescence in India. Nat. Clim. Chang. 2012, 2, 186–189. [Google Scholar] [CrossRef]
- Belokopytova, L.V.; Babushkina, E.A.; Zhirnova, D.F.; Panyushkina, I.P.; Vaganov, E.A. Pine and larch tracheids capture seasonal variations of climatic signal at moisture-limited sites. Trees 2019, 33, 227–242. [Google Scholar] [CrossRef]
- Kug, J.S.; Jeong, J.H.; Jang, Y.S.; Kim, B.M.; Folland, C.K.; Min, S.K.; Son, S.W. Two distinct influences of Arctic warming on cold winters over North America and East Asia. Nat. Geosci. 2015, 8, 759. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Zhu, X.; Yang, T.; Bai, J.; Sun, Z. Drought evolution and its impact on the crop yield in the North China Plain. J. Hydrol. 2018, 564, 984–996. [Google Scholar] [CrossRef]
- Demorenko, I.F.; Vasiljeva, O.M. Selection and seed growing of the spring wheat in conditions of the south of average Siberia. Achiev. Sci. Technol. AIC 2011, 4, 24–25. (In Russian) [Google Scholar]
- Popov, A.V.; Shatravskii, A.I. Removal of floating timber from the Sayano-Shushenskoe hydrostation reservoir. Hydrotech. Constr. 1994, 28, 204. [Google Scholar] [CrossRef]
- Savkin, V.M. Water reservoirs of Siberia: Consequences of their creation to water ecology and water management facilities. Sib. Ecol. J. 2000, 2, 109–121. (In Russian) [Google Scholar]
- Kosmakov, I.V. Thermal and Ice Regime in the Upper and Lower Reaches of High-Pressure Hydroelectric Power Stations on the Yenisei; Klaretianum: Krasnoyarsk, Russia, 2001. (In Russian) [Google Scholar]


 ) are colored in accordance to agroclimatic classification according to Babushkina et al. [40]: rain fed moderately dry Northern zone (dark shaded area,
) are colored in accordance to agroclimatic classification according to Babushkina et al. [40]: rain fed moderately dry Northern zone (dark shaded area,  ), irrigated dry Central zone (light shaded area,
), irrigated dry Central zone (light shaded area,  ); Karatuz variety-testing station outside of Khakassia used as a source of phenological data (
); Karatuz variety-testing station outside of Khakassia used as a source of phenological data ( ). Locations of Minusinsk climatic station (
). Locations of Minusinsk climatic station ( ) and dendrochronological sampling sites (
) and dendrochronological sampling sites ( ) are marked. (b) Climatic diagram at the Minusinsk climatic station: daily series of air temperature (lines) and precipitation (bars) averaged over 1936–2017 (dark shades) and their standard deviations (light shades). (c) Location of the Republic of Khakassia in the South Siberia.
) are marked. (b) Climatic diagram at the Minusinsk climatic station: daily series of air temperature (lines) and precipitation (bars) averaged over 1936–2017 (dark shades) and their standard deviations (light shades). (c) Location of the Republic of Khakassia in the South Siberia.


 ) are colored in accordance to agroclimatic classification according to Babushkina et al. [40]: rain fed moderately dry Northern zone (dark shaded area,
) are colored in accordance to agroclimatic classification according to Babushkina et al. [40]: rain fed moderately dry Northern zone (dark shaded area,  ), irrigated dry Central zone (light shaded area,
), irrigated dry Central zone (light shaded area,  ); Karatuz variety-testing station outside of Khakassia used as a source of phenological data (
); Karatuz variety-testing station outside of Khakassia used as a source of phenological data ( ). Locations of Minusinsk climatic station (
). Locations of Minusinsk climatic station ( ) and dendrochronological sampling sites (
) and dendrochronological sampling sites ( ) are marked. (b) Climatic diagram at the Minusinsk climatic station: daily series of air temperature (lines) and precipitation (bars) averaged over 1936–2017 (dark shades) and their standard deviations (light shades). (c) Location of the Republic of Khakassia in the South Siberia.
) are marked. (b) Climatic diagram at the Minusinsk climatic station: daily series of air temperature (lines) and precipitation (bars) averaged over 1936–2017 (dark shades) and their standard deviations (light shades). (c) Location of the Republic of Khakassia in the South Siberia.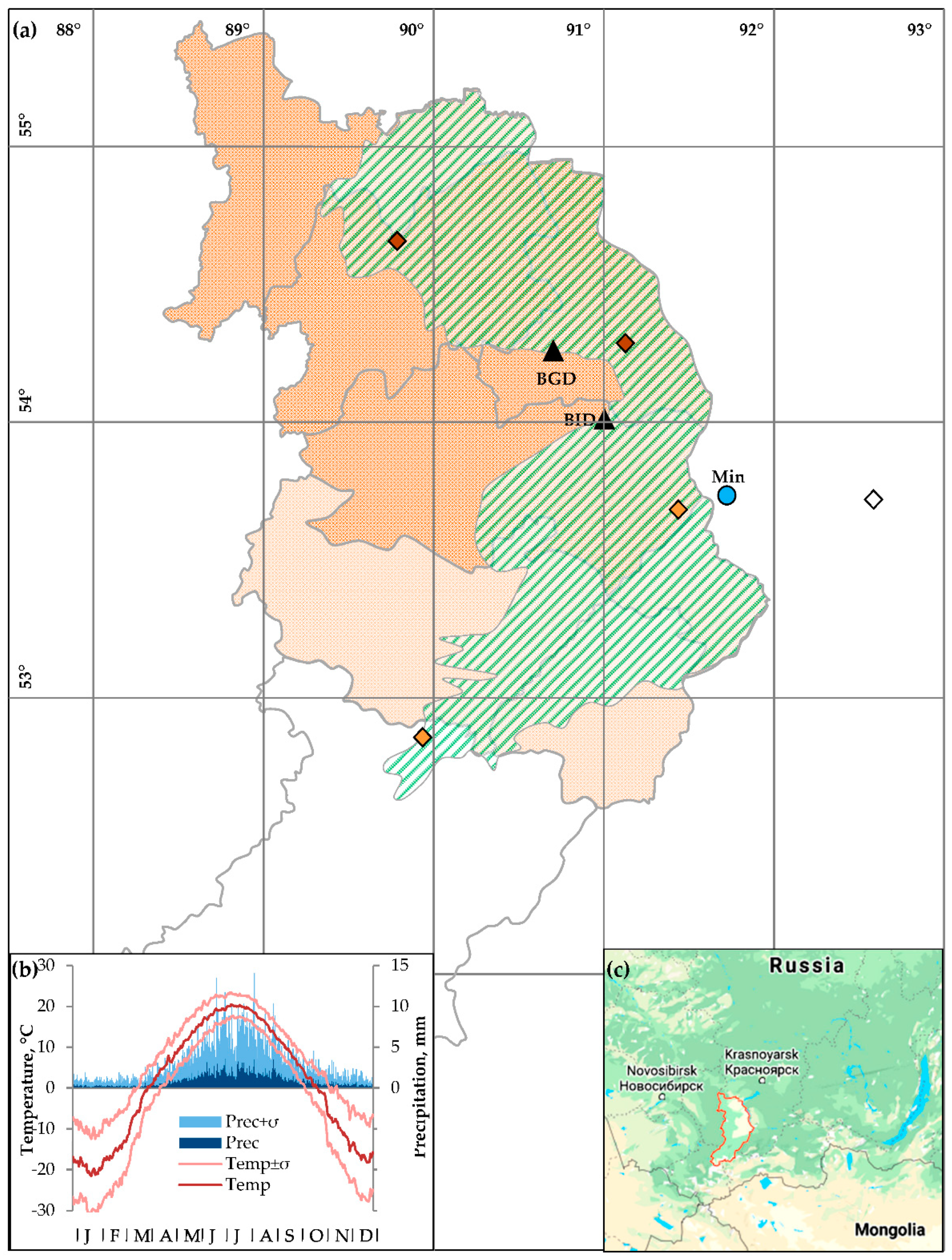
| Variable | Sample Characteristics | Chronology Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length, Years | Cover Period, Years | No. of Series 1 | Mean Value 2 | Standard Deviation (SD) | Mean Inter-Series Correlation Coefficient | Mean Expressed Population Signal | Sensitivity Coefficient | ||
| Yield Northern zone | Y_N | 68 | 1945–2012 | 18 | 1.235 | 0.418 | 0.640 | 0.970 | 0.532 |
| Yield Central zone | Y_C | 74 | 1939–2012 | 15 | 1.296 | 0.339 | 0.485 | 0.934 | 0.438 |
| Tree-ring width | TRW | 314 | 1706–2019 | 46 | 0.735 | 0.304 | 0.452 | 0.974 | 0.372 |
| Earlywood width | EWW | 314 | 1706–2019 | 46 | 0.533 | 0.319 | 0.422 | 0.971 | 0.388 |
| Latewood width 3 | LWW | 314 | 1706–2019 | 46 | 0.184 | 0.337 | 0.292 | 0.950 | 0.403 |
| Model Equation 1 | R | R2 | R2adj | F | p | SE |
|---|---|---|---|---|---|---|
| Northern zone | ||||||
| 0.373 + 0.610·TRW | 0.431 | 0.186 | 0.174 | 15.1 | 0.0002 | 0.380 |
| −0.072 + 0.455·EWW + 0.592·LWWadj | 0.478 | 0.229 | 0.205 | 9.6 | 0.0002 | 0.373 |
| 3.771 − 0.196·Tadd + 0.330·EWW + 0.444·LWWadj | 0.647 | 0.418 | 0.391 | 15.3 | <0.0001 | 0.326 |
| 3.315 − 0.175·Tadd + 0.00187·Padd + 0.264·EWW + 0.373·LWWadj | 0.672 | 0.451 | 0.416 | 12.9 | <0.0001 | 0.319 |
| Central zone | ||||||
| 0.652 + 0.326·TRW | 0.277 | 0.077 | 0.063 | 5.5 | 0.0222 | 0.337 |
| 0.507 + 0.268·EWW + 0.200·LWWadj | 0.278 | 0.077 | 0.049 | 2.7 | 0.0734 | 0.339 |
| 3.935 − 0.177·Tadd + 0.150·EWW + 0.072·LWWadj | 0.553 | 0.306 | 0.274 | 9.4 | <0.0001 | 0.296 |
| 3.425 − 0.153·Tadd + 0.00257·Padd + 0.048·EWW − 0.033·LWWadj | 0.662 | 0.387 | 0.348 | 10.0 | <0.0001 | 0.281 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babushkina, E.A.; Zhirnova, D.F.; Belokopytova, L.V.; Mehrotra, N.; Shah, S.K.; Keler, V.V.; Vaganov, E.A. Prospects of Using Tree-Ring Earlywood and Latewood Width for Reconstruction of Crops Yield on Example of South Siberia. Forests 2021, 12, 174. https://doi.org/10.3390/f12020174
Babushkina EA, Zhirnova DF, Belokopytova LV, Mehrotra N, Shah SK, Keler VV, Vaganov EA. Prospects of Using Tree-Ring Earlywood and Latewood Width for Reconstruction of Crops Yield on Example of South Siberia. Forests. 2021; 12(2):174. https://doi.org/10.3390/f12020174
Chicago/Turabian StyleBabushkina, Elena A., Dina F. Zhirnova, Liliana V. Belokopytova, Nivedita Mehrotra, Santosh K. Shah, Viktoria V. Keler, and Eugene A. Vaganov. 2021. "Prospects of Using Tree-Ring Earlywood and Latewood Width for Reconstruction of Crops Yield on Example of South Siberia" Forests 12, no. 2: 174. https://doi.org/10.3390/f12020174
APA StyleBabushkina, E. A., Zhirnova, D. F., Belokopytova, L. V., Mehrotra, N., Shah, S. K., Keler, V. V., & Vaganov, E. A. (2021). Prospects of Using Tree-Ring Earlywood and Latewood Width for Reconstruction of Crops Yield on Example of South Siberia. Forests, 12(2), 174. https://doi.org/10.3390/f12020174







