Responses of Soil Organic Carbon Mineralization and Microbial Communities to Leaf Litter Addition under Different Soil Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Litter Properties
2.3. Soil Incubation and CO2 Analysis
2.4. PLFA and 13C PLFA Analysis
2.5. Analyses of Other Soil Properties
2.6. PE Calculation
2.7. Statistical Analyses
3. Results
3.1. Soil Organic Carbon Pool
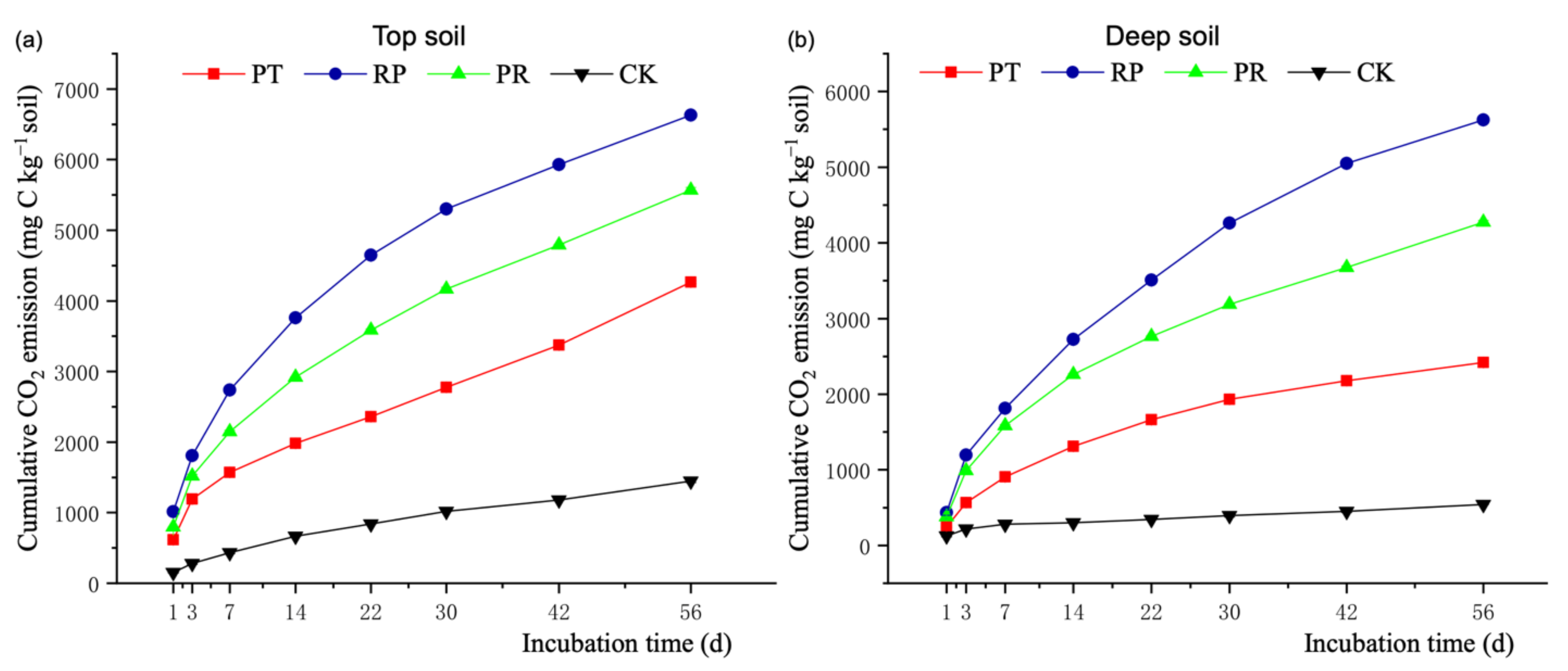
3.2. Soil Organic Matter Mineralization
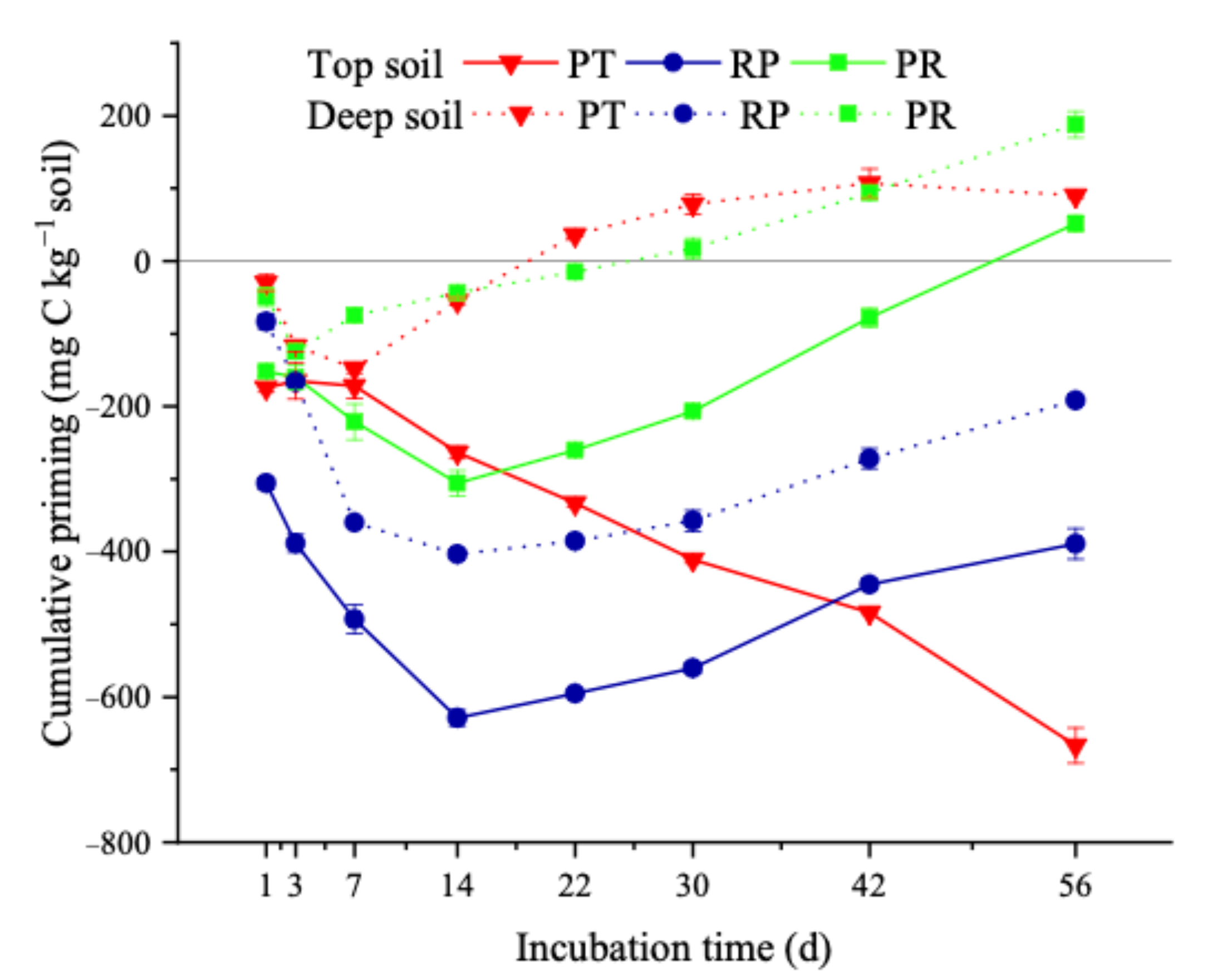
3.3. Soil- and Litter-Derived CO2-C and the PE
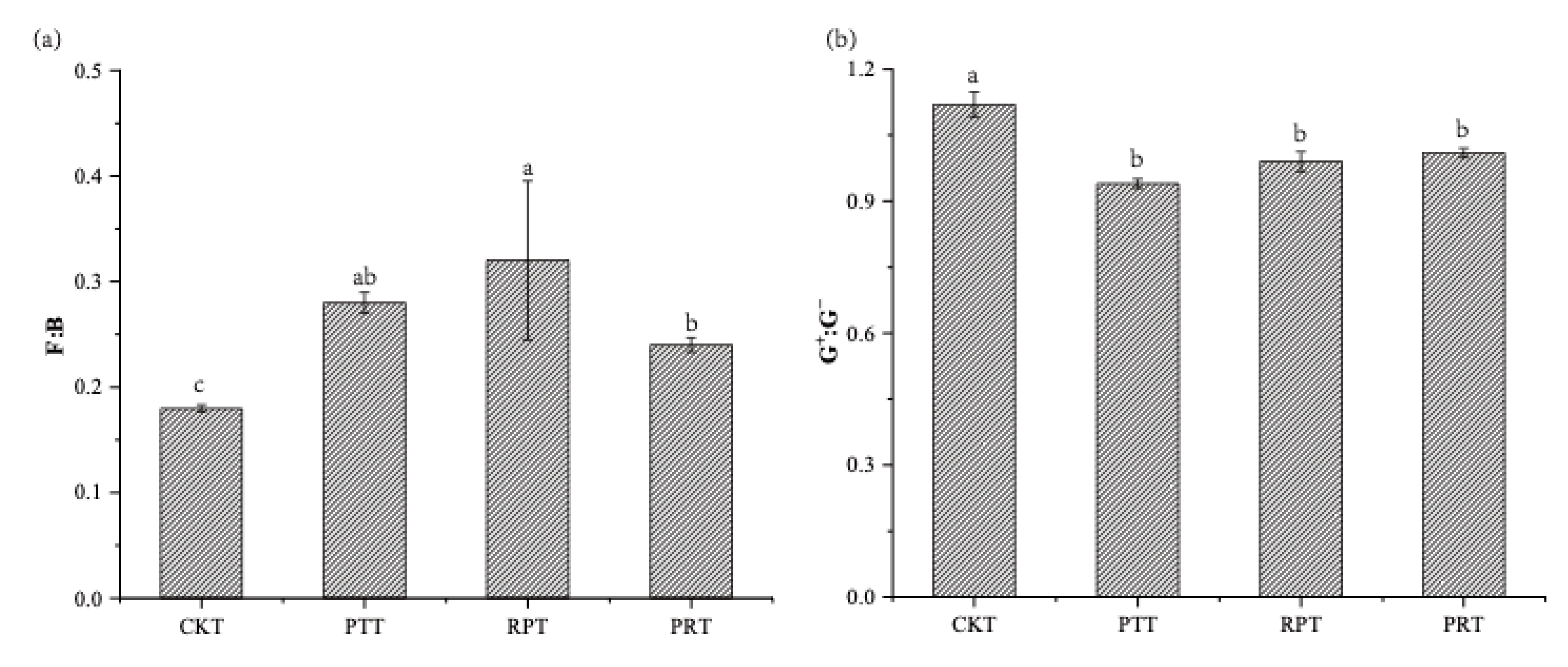
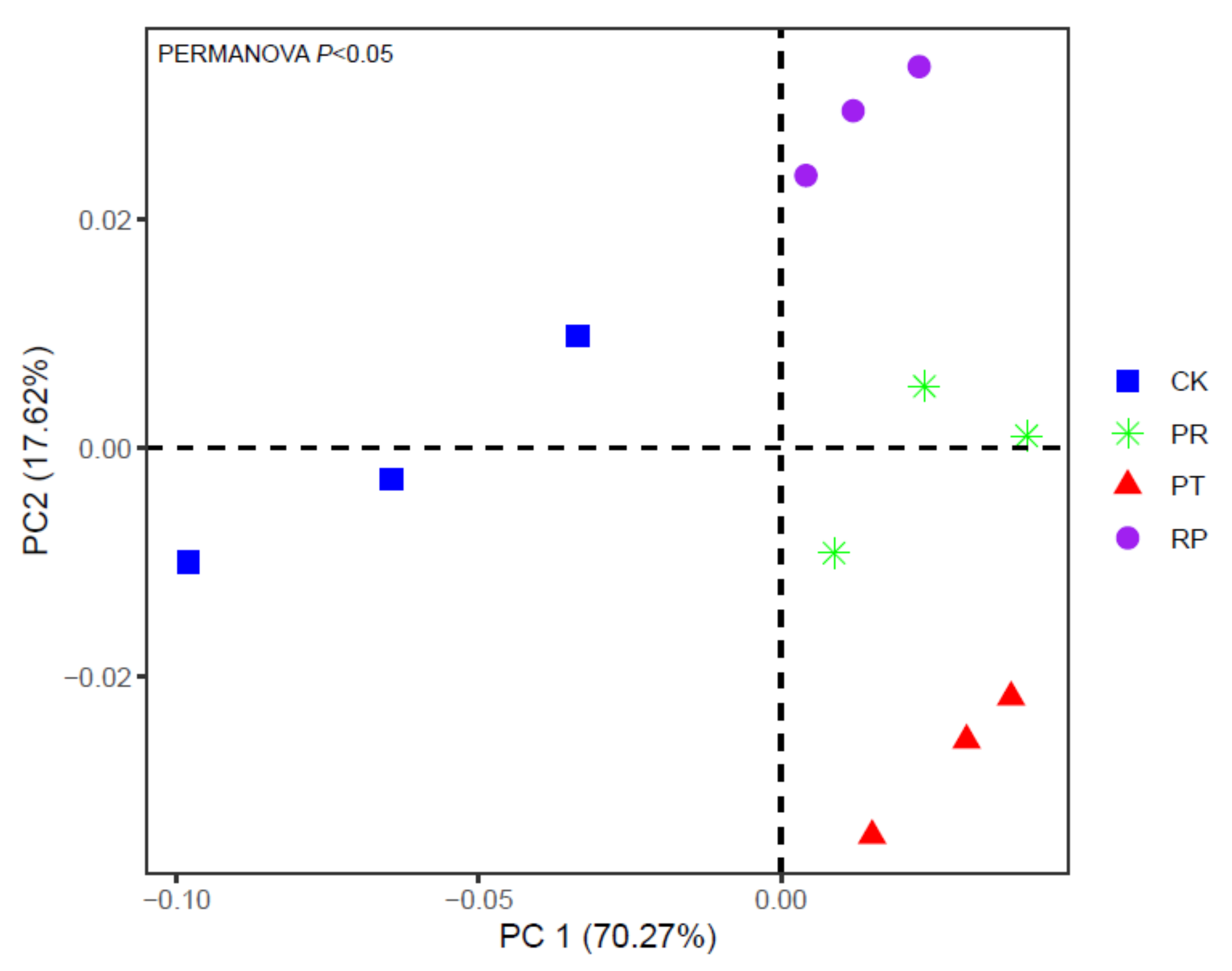
3.4. Soil Microbial Communities
3.5. Microbial Biomass Carbon
3.6. Soil Enzyme Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, A.A.; Exbrayat, J.F.; van Velde, I.R.; Feng, L.; Williams, M. The decadal state of the terrestrial carbon cycle: Global retrievals of terrestrial carbon allocation, pools, and residence times. Proc. Natl. Acad. Sci. USA 2016, 113, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.M.; Dijkstra, F.A.; Wang, P.; Zhu, B.; Cheng, W.X. Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytol. 2018, 218, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Liu, E.K.; Wang, J.B.; Zhang, Y.Q.; Angers, D.A.; Yan, C.R.; Oweis, T.; He, W.Q.; Liu, Q.; Chen, B.Q. Priming effect of 13C-labelled wheat straw in no-tillage soil under drying and wetting cycles in the Loess Plateau of China. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Liu, Y.Y.; Freschet, G.T.; Zhang, W.D.; Yu, X.; Zheng, W.H.; Guan, X.; Yang, Q.P.; Chen, L.C.; Dijkstra, F.A.; et al. Litter carbon and nutrient chemistry control the magnitude of soil priming effect. Funct. Ecol. 2019, 33, 876–888. [Google Scholar] [CrossRef]
- Shahzad, T.; Anwar, F.; Hussain, S.; Mahmood, F.; Arif, M.S.; Sahar, A.; Nawaz, M.F.; Perveen, N.; Sanaullah, M.; Rehman, K.; et al. Carbon dynamics in surface and deep soil in response to increasing litter addition rates in an agro-ecosystem. Geoderma 2019, 333, 1–9. [Google Scholar] [CrossRef]
- Hick, L.C.; Meri, P.; Nottingham, A.T.; Reay, D.S.; Stott, A.W.; Salinas, N.; Whitaker, J. Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol. Biochem. 2019, 129, 212–222. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.Y.; Song, Q.N.; Compson, Z.G.; LeRoy, C.J.; Luan, F.G.; Wang, H.; Hu, Y.L.; Yang, Q.P. Synergistic effects: A common theme in mixed-species litter decomposition. New Phytol. 2020, 227, 757–765. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.J.; Zou, J.W.; Siemann, E. Decomposition of Phragmitesaustralis litter retarded by invasive Solidagocanadensis in mixtures: An antagonistic non-additive effect. Sci. Rep. 2014, 4, 1–8. [Google Scholar]
- Scheibe, A.; Gleixner, G. Influence of litter diversity on dissolved organic matter release and soil carbon formation in a mixed beech forest. PLoS ONE 2014, 9, 1–21. [Google Scholar] [CrossRef][Green Version]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.K.; Wang, Y.P.; Wang, S.L.; He, T.X.; Liu, L. Fresh carbon and nitrogen inputs alter organic carbon mineralization and microbial community in forest deep soil layers. Soil Biol. Biochem. 2014, 72, 145–151. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S. Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol. Lett. 2005, 8, 1075–1087. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Hu, G.; Dai, W.; Jiang, P.; Bai, E. The priming effect of soluble carbon inputs in organic and mineral soils from a temperate forest. Oecologia 2015, 178, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Hartley, I.P.; Hopkins, D.W.; Sommerkorn, M.; Wookey, P.A. The response of organic matter mineralisation to nutrient and substrate additions in sub-arctic soils. Soil Biol. Biochem. 2010, 42, 92–100. [Google Scholar] [CrossRef]
- Norris, C.E.; Quideau, S.A.; Oh, S.W. Microbial utilization of double-labeled aspen litter in boreal aspen and spruce soils. Soil Biol. Biochem. 2016, 100, 9–20. [Google Scholar] [CrossRef]
- Jílková, V.; Jandová, K.; Cajthaml, T.; Devetter, M.; Kukla, J.; Starý, J.; Vacířová, A. Organic matter decomposition and carbon content in soil fractions as affected by a gradient of labile carbon input to a temperate forest soil. Biol. Fertil. Soils 2020, 56, 411–421. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Stott, A.W.; Tanner, E.V.J. Nitrogen and phosphorus constrain stable and labile carbon turnover in lowland tropical forest soils. Soil Biol. Biochem. 2015, 80, 26–33. [Google Scholar] [CrossRef]
- Yu, G.C.; Zhao, H.B.; Chen, J.; Zhang, T.L.; Cai, Z.L.; Zhou, G.Y.; Li, Z.J.; Qiu, Z.J.; Wu, Z.M. Soil microbial community dynamics mediate the priming effects caused by in situ decomposition of fresh plant residues. Sci. Total Environ. 2020, 737, 1–13. [Google Scholar] [CrossRef]
- Whitaker, J.; Ostle, N.; McNamara, N.P.; Nottingham, A.T.; Scott, A.W.; Bardgett, R.D.; Salinas, N.; Ccahuana, A.J.Q.; Meri, P. Microbial carbon mineralization in tropical lowland and montane forest soils of Peru. Front. Microbiol. 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscularmycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Liang, C.; Bodè, S.; Huygens, D.; Boeckx, P. Phospholipid 13C stable isotopic probing during decomposition of wheat residues. Appl. Soil Ecol. 2016, 98, 65–74. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- DeVries, D.A.; Peter, J. Women on display: The effect of portraying the self online on women’s self-objectification. Comput. Hum. Behav. 2013, 29, 1483–1489. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Turner, B.L.; Chamberlain, P.M.; Stott, A.W.; Tanner, E.V.J. Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility. Biogeochemistry 2012, 111, 219–237. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, R.D., Eds.; American Society of Agronomy and Soil Science Society of American: Madison, WI, USA, 1982; pp. 101–129. [Google Scholar]
- Gallaher, R.N.; Weldon, C.O.; Boswell, F.C. A semiautomated procedure for total nitrogen in plant and soil samples. Soil Sci. Soc. Am. J. 1976, 40, 887–889. [Google Scholar] [CrossRef]
- Dranski, J.A.L.; Malavasi, U.C.; Malavasi, M.D. Relationship between lignin content and quality of Pinustaeda seedlings. Rev. Arvore 2015, 39, 905–913. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Lyu, M.K.; Xie, J.S.; Vadeboncoeur, M.A.; Wang, M.H.; Qiu, X.; Ren, Y.B.; Jiang, M.H.; Yang, Y.S.; Kuzyakov, Y. Simulated leaf litter addition causes opposite priming effects on natural forest and plantation soils. Biol. Fertil. Soils 2018, 54, 925–934. [Google Scholar] [CrossRef]
- White, D.; Stair, J.; Ringelberg, D. Quantitative comparisons of in situ microbial biodiversity by signature biomarker analysis. J. Ind. Microbiol. Biotechnol. 1996, 17, 185–196. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.J.; Wei, W.J.; He, Z.L.; Kuzyakov, Y.; Bol, R.; Hu, R.G. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Qiao, N.; Wang, J.; Xu, X.L.; Shen, Y.X.; Long, X.E.; Hu, Y.H.; Schaefer, D.; Li, S.G.; Wang, H.M.; Kuzyakov, Y. Priming alters soil carbon dynamics during forest succession. Biol. Fertil. Soils 2019, 55, 339–350. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Olsson, P.A.; Thingstrup, I.; Jakobsen, I.; Bååtha, E. Estimation of the biomass of arbuscularmycorrhizal fungi in a linseed field. Soil Biol. Biochem. 1999, 31, 1879–1887. [Google Scholar] [CrossRef]
- Williams, M.A.; Myrold, D.D.; Bottomley, P.J. Carbon flow from 13C-labeled straw and root residues into the phospholipid fatty acids of a soil microbial community under field conditions. Soil Biol. Biochem. 2006, 38, 759–768. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis: Part III, Chemical Methods, 2nd ed.; Sparks, D.L., Ed.; Soil Science Society America: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- DuPont, S.T.; Culman, S.W.; Ferris, H.; Buckley, D.H.; Glover, J.D. No-tillage conversion of harvested perennial grassland to annual cropland reduces root biomass, decreases active carbon stocks, and impacts soil biota. Agric. Ecosyst. Environ. 2010, 137, 25–32. [Google Scholar] [CrossRef]
- Leavitt, S.W.; Follett, R.F.; Paul, E.A. Estimation of slow-and fast-cycling soil organic carbon pools from 6 N HCl hydrolysis. Radiocarbon 1996, 38, 231–239. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.; Karuma, A. Assessment of soil organic fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation extraction-an autoclaved procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzyme and Research Methods; Agricultural Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Qi, R.M.; Li, J.; Lin, Z.A.; Li, Z.J.; Li, Y.T.; Yang, X.D.; Zhang, J.J.; Zhao, B.Q. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Wang, H.; Boutton, T.W.; Xu, W.H.; Hu, G.Q.; Jiang, P.; Bai, E. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Keiser, A.D.; Keiser, D.A.; Strickland, M.S.; Bradford, M.A. Disentangling the mechanisms underlying functional differences among decomposer communities. J. Ecol. 2014, 102, 603–609. [Google Scholar] [CrossRef]
- Cuchietti, A.; Marcotti, E.; Gurvich, D.E.; Cingolani, A.M.; Harguindeguy, N.P. Leaf litter mixtures and neighbor effects: Low-nitrogen and high-lignin species increase decomposition rate of high-nitrogen and low-lignin neighbours. Appl. Soil Ecol. 2014, 82, 44–51. [Google Scholar] [CrossRef]
- Mason-Jones, K.; Schmucker, N.; Kuzyakov, Y. Contrasting effects of organic and mineral nitrogen challenge the N-mining hypothesis for soil organic matter priming. Soil Biol. Biochem. 2018, 124, 38–46. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, M.; Jiang, C.Y.; Liu, M.; Wu, M.; Liu, P.; Li, Z.P.; Uchimiya, M.; Yuan, X.Y. Soil microbiome-induced changes in the priming effects of 13C-labelled substrates from rice residues. Sci. Total Environ. 2020, 726, 1–11. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, X.J.; He, X.B.; Song, F.Q.; Ren, L.L.; Jiang, P. Effect of litter quality on its decomposition in broad leaf and coniferous forest. Eur. J. Soil Biol. 2008, 44, 392–399. [Google Scholar] [CrossRef]
- Li, Z.Q.; Song, M.; Li, D.D.; Ma, L.; Zhao, B.Z.; Zhang, J.B. Effect of long-term fertilization on decomposition of crop residues and their incorporation into microbial communities of 6-year stored soils. Biol. Fertil. Soils 2020, 56, 25–37. [Google Scholar] [CrossRef]
- Magan, N.; Fragoeiro, S.; Bastos, C. Environmental factors and bioremediation of xenobiotics using white rot fungi. Mycobiology 2010, 38, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Xiao, C.; Guenet, B.; Zhou, Y.; Su, J.; Janssens, I.A. Priming of soil organic matter decomposition scales linearly with microbial biomass response to litter input in steppe vegetation. Oikos 2015, 124, 649–657. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Khomyakov, N.; Myachina, O.; Bogomolova, I.; Blagodatsky, S.; Kuzyako, Y. Microbial interactions affect sources of priming induced by cellulose. Soil Biol. Biochem. 2014, 74, 39–49. [Google Scholar] [CrossRef]
- Ferreira, V.; Faustino, H.; Raposeiro, P.M.; Gonçalves, V. Replacement of native forests by conifer plantations affects fungal decomposer community structure but not litter decomposition in Atlantic island streams. For. Ecol. Manag. 2017, 389, 323–330. [Google Scholar] [CrossRef]
- Finley, B.K.; Dijkstra, P.; Rasmussen, C.; Schwartz, E.; Mau, R.L.; Liu, X.J.A.; Getel, N.; Hungate, B.A. Soil mineral assemblage and substrate quality effects on microbial priming. Geoderma 2018, 322, 38–47. [Google Scholar] [CrossRef]




| Topsoil (0–20cm) | Deep Soil (60–80 cm) | PT | RP | PR | |
|---|---|---|---|---|---|
| pH | 7.63 | 8.03 | - | - | - |
| TOC (g·kg−1) | 11.60 | 3.32 | 648.96 | 484.59 | 533.73 |
| TN (g·kg−1) | 0.88 | 0.41 | 14.84 | 59.18 | 38.45 |
| C:N | 13.26 | 8.19 | 44.68 | 8.24 | 13.91 |
| -N (mg·kg−1) | 4.43 | 1.72 | - | - | - |
| -N (mg·kg−1) | 7.26 | 3.67 | - | - | - |
| Celulose (%) | - | - | 15.31 | 18.52 | 16.83 |
| Lignin (%) | - | - | 36.28 | 26.28 | 32.03 |
| Celulose: Lignin | - | - | 0.42 | 0.69 | 0.53 |
| δ13C(‰) | −21.64 | −21.18 | 652.75 | 1464.79 | 1254.85 |
| Treatments | TOC (g·kg−1) | LOC (g·kg−1) | RC (g·kg−1) | Carbon Lability |
|---|---|---|---|---|
| CK-T | 10.86 ± 0.20b | 5.21 ± 0.28a | 5.65 ± 0.43b | 0.93 ± 0.12b |
| PTT | 12.01 ± 0.20a | 5.52 ± 0.16a | 6.49 ± 0.31a | 0.85 ± 0.06b |
| RPT | 12.19 ± 0.40a | 5.35 ± 0.33a | 6.84 ± 0.74a | 0.79 ± 0.13b |
| PRT | 12.15 ± 0.33a | 5.63 ± 0.18a | 6.51 ± 0.49a | 0.87 ± 0.09b |
| CK-D | 3.54 ± 0.10e | 2.34 ± 0.05d | 1.20 ± 0.07d | 1.96 ± 0.11b |
| PTD | 4.53 ± 0.17d | 3.74 ± 0.26b | 0.79 ± 0.43d | 6.18 ± 3.90a |
| RPD | 5.35 ± 0.04c | 3.15 ± 0.37c | 2.20 ± 0.40c | 1.48 ± 0.42b |
| PRD | 5.10 ± 0.07c | 3.15 ± 0.13c | 1.95 ± 0.17c | 1.63 ± 0.20b |
| Treatments | Total PLFAs (nmol·g−1) | Bacteria (nmol·g−1) | Fungi (nmol·g−1) | G+ (nmol·g−1) | G− (nmol·g−1) | AMF (nmol·g−1) |
|---|---|---|---|---|---|---|
| CK-T | 64.75 ± 16.30b | 26.02 ± 6.95b | 5.47 ± 1.45b | 16.20 ± 4.22a | 10.56 ± 3.13b | 1.58 ± 0.45a |
| PTT | 92.86 ± 13.00ab | 36.84 ± 5.60ab | 12.68 ± 1.28a | 20.09 ± 3.29a | 16.44 ± 2.79ab | 1.72 ± 0.27a |
| RPT | 95.94 ± 21.50ab | 40.50 ± 8.93a | 15.09 ± 3.76a | 23.26 ± 5.98a | 18.11 ± 3.66a | 2.13 ± 0.49a |
| PRT | 105.64 ± 22.17a | 43.82 ± 9.52a | 13.39 ± 3.41a | 24.78 ± 5.58a | 19.12 ± 4.49a | 2.14 ± 0.51a |
| Treatments | β-Glucosidase (mg·g−1·h−1) | Cellulase (mg·g−1·d−1) | N-Acetylglucosaminidase (μmol·g−1·d−1) | Alkaline Phosphatase (mg·g−1·h−1) |
|---|---|---|---|---|
| CK-T | 1.06 ± 0.14c | 0.10 ± 0.01d | 243.36 ± 20.50d | 14.42 ± 1.26cd |
| PTT | 1.17 ± 0.06bc | 0.16 ± 0.02c | 270.96 ± 18.25c | 17.63 ± 0.88c |
| RPT | 1.57 ± 0.24a | 0.23 ± 0.00a | 291.64 ± 11.81c | 26.25 ± 3.06ab |
| PRT | 1.63 ± 0.03a | 0.16 ± 0.01c | 524.03 ± 8.58a | 22.67 ± 2.09b |
| CK-D | 0.11 ± 0.02d | 0.11 ± 0.02d | 16.38 ± 1.18g | 3.47 ± 0.28e |
| PTD | 0.19 ± 0.02d | 0.10 ± 0.01d | 117.30 ± 6.44f | 3.71 ± 0.19e |
| RPD | 1.39 ± 0.25ab | 0.20 ± 0.01b | 326.61 ± 6.04b | 26.62 ± 4.55a |
| PRD | 1.12 ± 0.18bc | 0.17 ± 0.01c | 152.30 ± 8.54e | 12.75 ± 0.53d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Dong, L.-G.; Fei, S.-X.; Zhang, J.-W.; Jiang, X.-M.; Wang, Y.; Yu, X. Responses of Soil Organic Carbon Mineralization and Microbial Communities to Leaf Litter Addition under Different Soil Layers. Forests 2021, 12, 170. https://doi.org/10.3390/f12020170
Zhang M, Dong L-G, Fei S-X, Zhang J-W, Jiang X-M, Wang Y, Yu X. Responses of Soil Organic Carbon Mineralization and Microbial Communities to Leaf Litter Addition under Different Soil Layers. Forests. 2021; 12(2):170. https://doi.org/10.3390/f12020170
Chicago/Turabian StyleZhang, Min, Li-Guo Dong, Shi-Xuan Fei, Jia-Wen Zhang, Xu-Meng Jiang, Ying Wang, and Xuan Yu. 2021. "Responses of Soil Organic Carbon Mineralization and Microbial Communities to Leaf Litter Addition under Different Soil Layers" Forests 12, no. 2: 170. https://doi.org/10.3390/f12020170
APA StyleZhang, M., Dong, L.-G., Fei, S.-X., Zhang, J.-W., Jiang, X.-M., Wang, Y., & Yu, X. (2021). Responses of Soil Organic Carbon Mineralization and Microbial Communities to Leaf Litter Addition under Different Soil Layers. Forests, 12(2), 170. https://doi.org/10.3390/f12020170




