Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and Its Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Morphological and Cytological Observations of Flower Buds
2.3. Water Transport of ‘Maya’ Jujube Shoots
2.4. Colchicine Treatment in Field
2.5. Ploidy Determination and Paternal Analysis
2.6. Phenotypic Trait Measurement of Triploid and Diploid Offspring
2.7. Statistical Analysis
3. Results
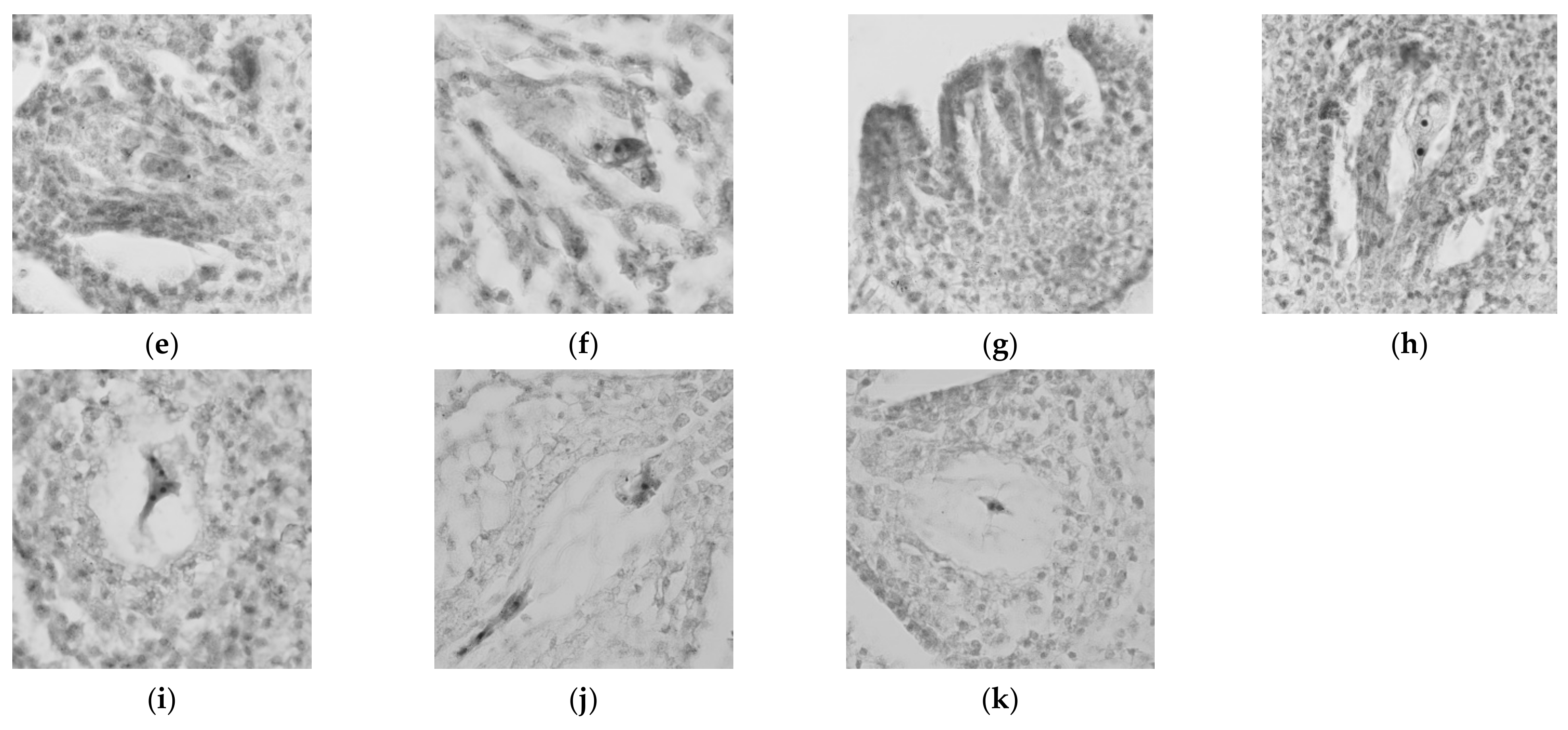
3.1. Development of the Flower Bud and Gametophytes of ‘Maya’ Jujube
3.2. Relationship between Bud Morphology and Gametophyte Development of ‘Maya’ Jujube
3.3. Water Transport of ‘Maya’ Jujube Shoots
3.4. Colchicine-Induced Chromosome Doubling of Female Gametes of ‘Maya’ Jujube
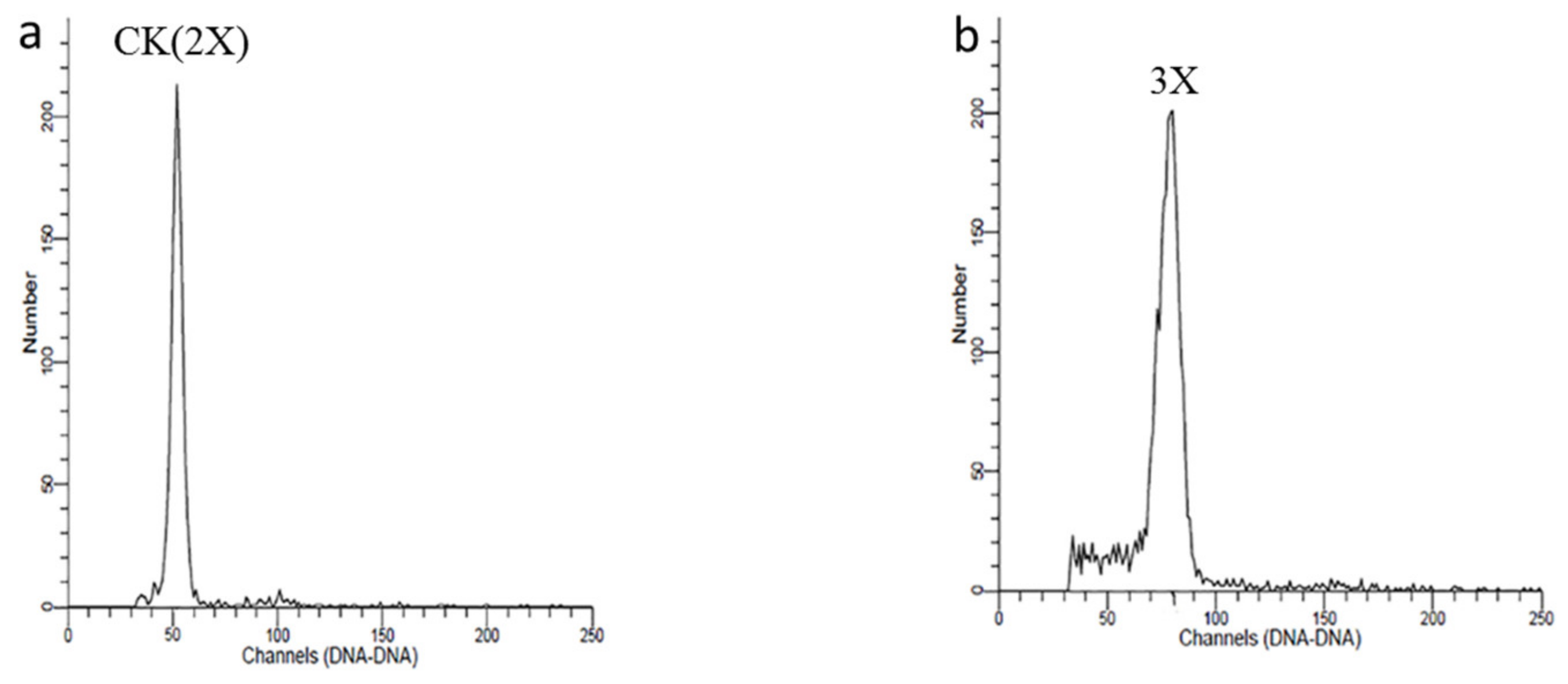
3.5. Ploidy Determination and Paternal Analysis
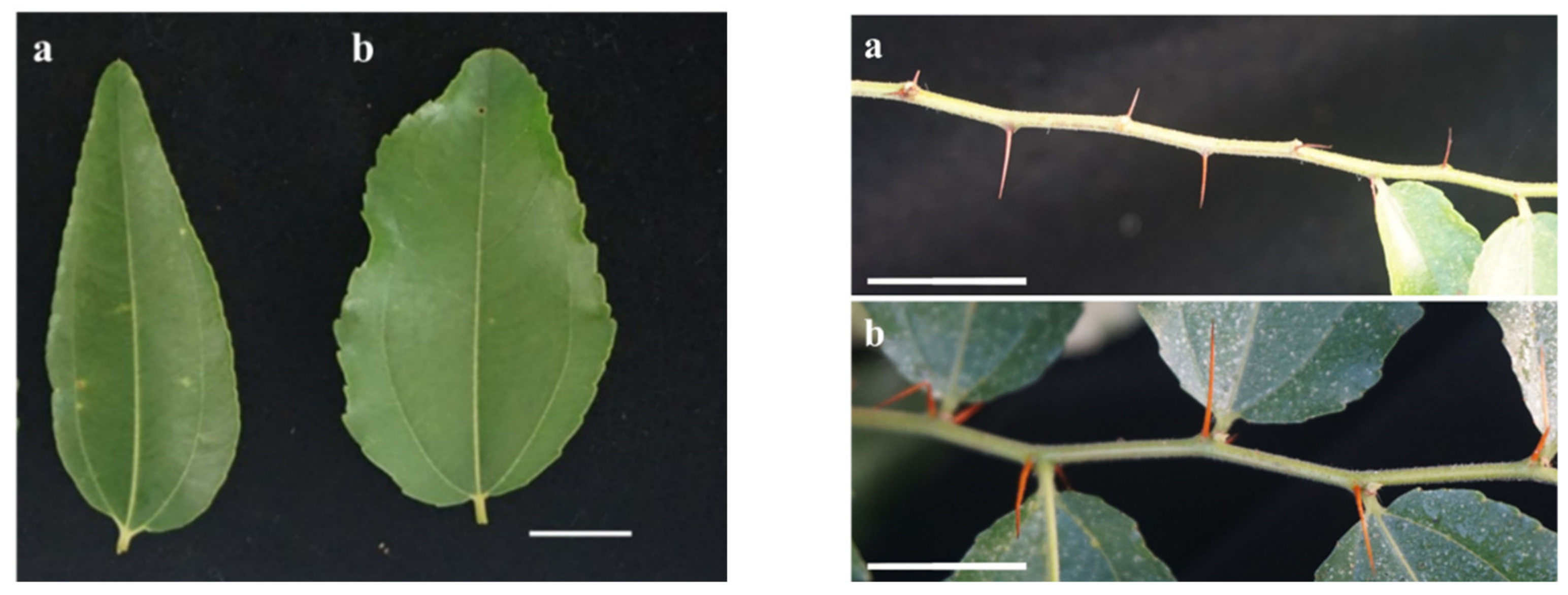
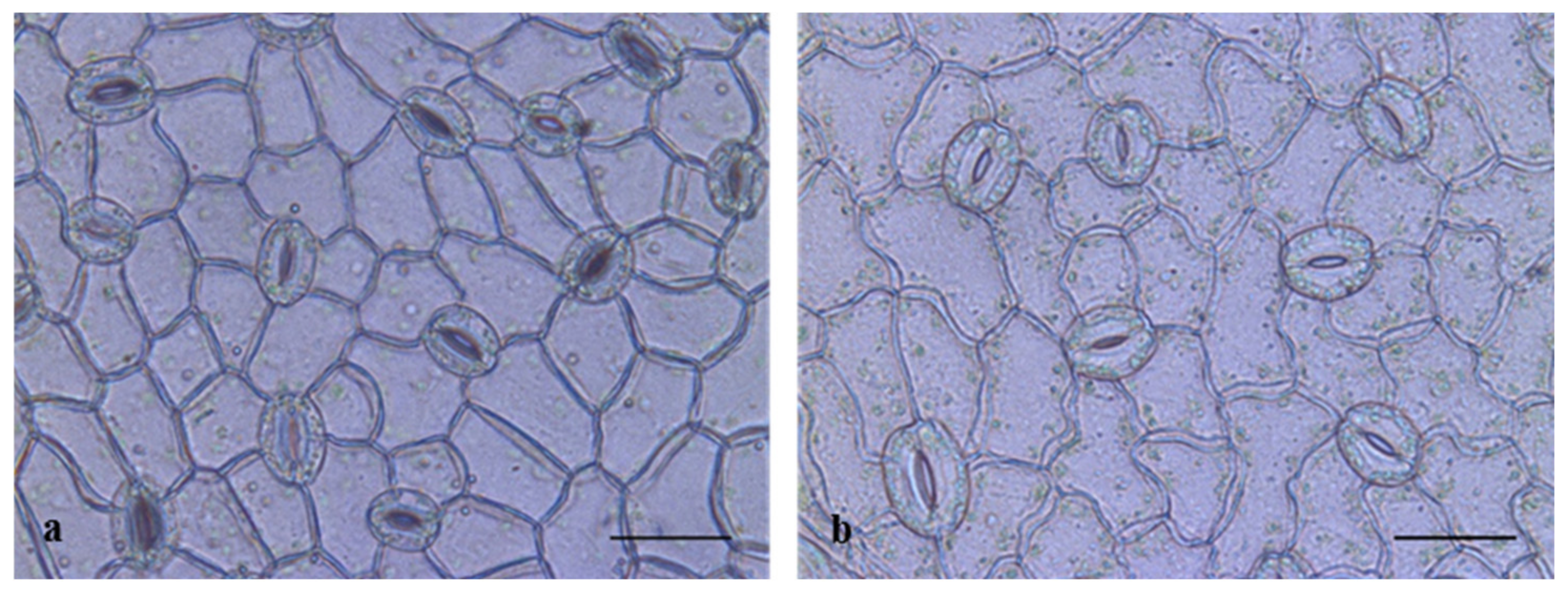
3.6. Phenotypic Trait Assessment of Triploid and Diploid Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Luo, Z.; Wang, L.; Deng, W.; Wei, H.; Liu, P.; Liu, M. Morphological, cytological and nutritional changes of autotetraploid compared to its diploid counterpart in Chinese jujube (Ziziphus jujuba Mill.). Sci. Hortic. 2019, 249, 263–270. [Google Scholar] [CrossRef]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Santini, J. Triploid citrus genotypes have a better tolerance to natural chilling conditions of photosynthetic capacities and specific leaf volatile organic compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Pomper, K.; Lowe, J.; Crabtree, S.; Vincent, J.; Raemakers, K. Ploidy level in american persimmon (Diospyros virginiana) cultivars. Hortscience 2020, 55, 4–7. [Google Scholar] [CrossRef]
- Hias, N.; Leus, L.; Davey, M.; Stijn, V. Effect of polyploidization on morphology in two apple (Malus × domestica) genotypes. Hortic. Sci. 2017, 44, 55–63. [Google Scholar]
- Nukaya, T.; Sudo, M.; Yahata, M.; Nakajo, Y.; Ohta, T.; Yasuda, K.; Tominaga, A.; Mukai, H.; Kunitake, H. Characteristics in autotetraploid kumquats (Fortunella spp.) induced by colchicine treatment to nucellar embryos and their utilization for triploid breeding. Sci. Hortic. 2019, 245, 210–217. [Google Scholar] [CrossRef]
- Rao, S.; Kang, X.; Li, J.; Chen, J. Induction, identification and characterization of tetraploidy in Lycium ruthenicum. Breed. Sci. 2019, 69, 160–168. [Google Scholar] [CrossRef]
- Li, Y.; Kang, X.; Wang, S.; Zhang, Z.; Chen, H. Triploid induction in Populus alba × P. glandulosa by chromosome doubling of female gametes. Silvae Genet. 2008, 57, 37–40. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, Z.; Xiong, T.; Lan, H. Megaspore Chromosome Doubling in Eucalyptus urophylla S.T. Blake induced by colchicine treatment to produce triploids. Forests 2018, 11, 728. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, P.; Yang, J.; Kang, X. Induction of unreduced megaspores in Eucommia ulmoides by high temperature treatment during megasporogenesis. Euphytica 2016, 212, 515–524. [Google Scholar] [CrossRef]
- Ulrich, K.; Ewald, D. Methods for generation of triploid aspen and poplar. Landbauforschung 2018, 68, 1–10. [Google Scholar]
- Younis, A.; Hwang, Y.; Lim, K. Exploitation of induced 2n-gametes for plant breeding. Plant Cell Rep. 2014, 33, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Guo, L.; Xu, W.; Zhang, J.; Li, B. Megasporogenesis, megagametogenesis, and induction of 2n eggs with colchicine in poplar section Aigeiros. Scand. J. For. Res. 2014, 6, 527–536. [Google Scholar] [CrossRef]
- Lai, H.; Chen, X.; Chen, Z.; Ye, J.; Li, K.; Liu, J. Induction of female 2n gametes and creation of tetraploids through sexual hybridization in cassava (Manihot esculenta). Eupgytica 2015, 201, 265–273. [Google Scholar] [CrossRef]
- Yang, J.; Yao, P.; Li, Y.; Mo, J.; Wang, J. Induction of 2n pollen with colchicine during microsporogenesis in Eucalyptus. Euphytica 2016, 210, 69–78. [Google Scholar] [CrossRef]
- Zeng, R.; Zhu, J.; Xu, S.; Du, G.; Guo, H.; Chen, J.; Zhang, Z.; Xie, L. Unreduced male gamete formation in cymbidium and its use for developing sexual polyploid cultivars. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, S.; He, Y.; Yan, G.; Bi, Y.; Zhu, Y. Diploid female gametes induced by colchicine in Oriental lilies. Sci. Hortic. 2007, 114, 50–53. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, J.; Liao, T.; Zhu, X.; Suo, Y.; Kang, X. Transcriptomic changes following synthesis of a Populus full-sib diploid and allotriploid population with different heterozygosities driven by three types of 2n female gamete. Plant Mol. Biol. 2015, 89, 493–510. [Google Scholar] [CrossRef]
- Kang, X. Some understandings on polyploid breeding of poplars. J. Beijing For. Univ. 2010, 32, 149–153. [Google Scholar]
- Yao, P.; Li, G.; Qiu, Y.; Kang, X. Induction of 2n female gametes in rubber (Hevea brasiliensis) by high-temperature exposure during megasporogenesis as a basis for triploid breeding. Tree Genet. Genomes 2020, 16, 24. [Google Scholar] [CrossRef]
- Geng, X.; Han, Z.; Yang, J.; Kang, X. The different origins of artificially-induced unreduced female gametes and their effect on transmitted parental heterozygosity in Populus. Euphytica 2019, 215, 181. [Google Scholar] [CrossRef]
- Zhang, Y. Disciplines of Fruit-Set Success and Triploid Induction of Hevea brasiliensis. Master’s Thesis, Beijing Forest University, Beijing, China, 2013. [Google Scholar]
- Liu, M.; Wang, M. Chinese Jujube Germsplasm Resources, 1st ed.; China Forestry Publishing House: Beijing, China, 2009; pp. 39–44. [Google Scholar]
- Chen, J.; Chan, P.; Lam, C.; Li, Z.; Lam, K.; Yao, P.; Dong, T.; Lin, H.; Lam, H.; Tsim, K. Fruit of Ziziphus jujuba (Jujube) at two stages of maturity: Distinction by metabolic profiling and biological assessment. J. Agric. Food Chem. 2015, 63, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bian, Y.; Hou, S.; Li, X. Sugar transport played a more important role than sugar biosynthesis in fruit sugar accumulation during Chinese jujube domestication. Planta 2018, 248, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H. Promotion plant technology of Maya jujube. Tianjin Agric. Sci. 2008, 14, 23–24. [Google Scholar]
- Lu, C.; He, B.; Ma, S.; Zhang, M.; Song, J.; Wei, X.; Liu, W.; Peng, R.; Li, J. Fruits microstructure characteristics of different jujube varieties during maturity. Non-Wood For. Res. 2020, 38, 119–126. [Google Scholar]
- Wang, X.; Zhu, D.; Yang, R.; Li, X. Study on crack resistance of northern Shaanxi Jujube on mountain land. J. Fruit Sci. 2011, 28, 82–85. [Google Scholar]
- Bondada, B.; Matthews, M.; Shackel, K. Functional xylem in the post-veraison grape berry. J. Exp. Bot. 2005, 56, 2949–2957. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, M.; Li, Y.; Zhang, P. Slow-growing pollen-tube of colchicine-induced 2n pollen responsible for low triploid production rate in Populus. Euphtica 2017, 4, 94. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, L.; Li, X.; Huang, F.; Li, Y. In vitro induction of tetraploid Ziziphus jujuba Mill. var. spinose plants from leaf explants. Plant Cell Tiss. Org. 2017, 131, 175–182. [Google Scholar] [CrossRef]
- Liang, T.; Sun, W.; Ren, H.; Ahmad, I.; Vu, N.; Marym; Huang, J. Genetic diversity of Ziziphus mauritiana germplasm based on SSR markers and ploidy level estimation. Planta 2019, 249, 1875–1887. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Song, L. Study of variation in the growth, photosynthesis, and content of secondary metabolites in Eucommia triploids. Trees 2019, 33, 817–826. [Google Scholar] [CrossRef]
- Yang, J. Reproductive Biology and Techniques of Chromosome Doubling in Eucalyptus. Ph.D. Thesis, Beijing Forest University Beijing, China, 2015. [Google Scholar]
- Wang, J.; Li, D.; Kang, X. Induction of unreduced megaspores with high temperature during megasporogenesis in Populus. Ann. For. Sci. 2012, 69, 59–67. [Google Scholar] [CrossRef]
- Li, D.; Tian, J.; Xue, Y.; Chen, H.; Wang, J. Triploid production via heat-induced diploidisation of megaspores in Populus pseudo-simonii. Euphytica 2019, 215, 10. [Google Scholar] [CrossRef]
- Huang, F.; Dou, S.; Zhang, Y.; Pang, X.; Li, Y. Megasporogenesis, microsporogenesis and development of female and male gametophytes of Ziziphus jujuba Mill. Cv. Dongzao. J. Nucl. Agric. Sci. 2017, 31, 1913–1920. [Google Scholar]
- Kang, X.; Zhu, Z.; Lin, H. Study on the effective treating period for pollen chromosome doubling of Populus tomentosa × P. bolleana. Sci. Silvae Sin. 1999, 35, 21–24. [Google Scholar]
- Li, Y.; Guo, Q.; Wang, J.; Tian, J.; Kang, X. Colchicine-induced pollen chromosome doubling and its cytological effects in Populus alba L. J. Nucl. Agric. Sci. 2014, 28, 749–756. [Google Scholar]
- Xi, X.; Jiang, X.; Li, D.; Guo, L.; Zhang, J.; Wei, Z.; Li, B. Induction of 2n pollen by colchicine in Populus × popularis and its triploids breeding. Silvae Genet. 2011, 60, 155–160. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Guo, L.; Zhao, J.; Zhang, J. High frequency 2n pollen formation in black poplar (Populus nigra L.) induced by colchicine. Not. Bot. Horti Agrobo. 2019, 47, 939–946. [Google Scholar] [CrossRef]
- Wang, J.; Kang, X.; Li, D.; Chen, H.; Zhang, P. Induction of diploid eggs with colchicine during embryo sac development in Populus. Silvae Genet. 2009, 59, 40–48. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, C.; Jia, M.; Zheng, M.; Cao, D.; Kang, L.; Wang, Y. Study on colchicine-induced tetraploid in Petunia hybrida Vilm. J. Shanxi Agric. Sci. 2016, 44, 951–953. [Google Scholar]
- Liu, Y.; Zhang, Y.; Zhou, Q.; Wu, J.; Zhang, P. Colchicine did not affect the viability of induced 2n pollen in Populus tomentosa. Silva Fenn. 2019, 53, 1–3. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, J.; Sang, Y.; Zhao, Z.; Zhang, P.; Liu, M. Effects of colchicine on Populus canescens ectexine structure and 2n pollen production. Front. Plant Sci. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.; Wendel, J. Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Jing, J.; Li, H.; Liu, G. Seed vigor, photosynthesis and early growth of saplings of different triploid Betula families. Dendrobiology 2012, 68, 11–20. [Google Scholar]
- Zhang, Y.; Wang, B.; Qi, S.; Dong, M.; Wang, Z.; Li, Y.; Chen, S.; Li, B.; Zhang, J. Ploidy and hybridity effects on leaf size, cell size and related genes expression in triploids, diploids and their parents in Populus. Planta 2019, 249, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S. Polyploidy and cellular mechanisms changing leaf size: Comparison of diploid and autotetraploid populations in two species of Lolium. Ann. Bot. Lond. 2005, 96, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Cheng, S.; Zhu, X.; Min, Y.; Kang, X. Effects of triploid status on growth, photosynthesis, and leaf area in Populus. Trees 2016, 30, 1137–1147. [Google Scholar] [CrossRef]
- Warner, D.; Gerald, E. Effects of polyploidy on photosynthesis. Photosynth Res. 1993, 35, 135–147. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Mi, Z.; Bian, X.; Liu, M.; Sun, Y.; Jing, J.; Wang, F.; Li, S.; Cui, Y. Comparative analysis of growth and photosynthetic characteristics of (Populus simonii × P. nigra) × (P. nigra × P. simonii) hybrid clones of different ploidides. PLoS ONE 2015, 10, e0119259. [Google Scholar] [CrossRef]
- Beck, S.; Dunlop, R.; Fossey, A. Stomatal length and frequency as a measure of ploidy level in black wattle, Acacia mearnsii (de Wild). Bot. J. Linn. Soc. 2003, 141, 177–181. [Google Scholar] [CrossRef]
- Diego, P.; Amr, M.; Benedeeta, C.; Antonietta, M.G.; Valli, P.S.S.K. Ploidy levels in Citrus clementine affects leaf morphology, stomatal density and water content. Theor. Exp. Plant Phys. 2013, 25, 283–290. [Google Scholar]
- He, P.; Li, L.; Cheng, L.; Wang, H.; Chang, Y. Variation in ploidy level and morphological traits in the progeny of the triploid apple variety Jonagold. Czech J. Genet. Plant 2018, 54, 135–142. [Google Scholar]
- Warner, D. Effects of Polyploidy on Photosynthesis in Three C4 Plant Species. Ph.D. Thesis, Washington State University, Washington, DC, USA, 1988. [Google Scholar]
- Wang, X.; Cheng, Z.; Zhi, S.; Xu, F. Breeding triploid plants: A review. Czech J. Genet. Plant 2016, 52, 41–54. [Google Scholar] [CrossRef]







| Treatment Number | Colchicine Concentration (%) | Treatment Method |
|---|---|---|
| 1 | 0.3 | injection |
| 2 | 0.4 | injection |
| 3 | 0.5 | injection |
| 4 | 0.3 | improved cotton leaching |
| 5 | 0.4 | improved cotton leaching |
| 6 | 0.5 | improved cotton leaching |
| Name | Repeat Motifs | Forward Primer (5′—3′) | Reverse Primer (3′—5′) | Expected Length (bp) |
|---|---|---|---|---|
| BFU0308 | (TC)11 | TTTCCACCCCAAAATACCAA | AGACGCTGGATGAGGATGAT | 176 |
| BFU0574 | (CA)7 | GAAGGTTGAAGATGCTCTCTCTC | CCTGACATCCATTTGAAGGAA | 114 |
| BFU0546 | (CT)8 | CGTGACAGCACAATGTTTGA | AAACCATGAAATATCCAAGCAA | 156 |
| BFU0277 | (GA)11 | GCACTACCCTGTGGAACTCAA | AGTGTTGACCTGGCAAGAAGA | 236 |
| BFU1205 | (CA)8 | TGTTGCTGGTTCAATTCCAG | CTTATGGCTTTTTCATTTTGTGA | 151 |
| BFU0377 | (CT)10 | CCAGCTGGTATCCAATTGCT | ACGACGATGCCATGAAAGAT | 283 |
| BFU0467 | (TC)9 | CCGGACCGAGTGGAGTTATTA | AGAATATGGCATCAACCTATACCA | 222 |
| BFU1157 | (GA)9 | TCCCTAAATTACCCTTCCCAAT | AAAGCGACAGCGAAAACTGT | 234 |
| External Morphology of Bud | Bud Diameter (mm) | Bud Form | Microspore Mother Cell Development | Megaspore Mother Cell Development |
|---|---|---|---|---|
| a | 0.80–1.12 | Slightly raised, green | Archespore, microspore mother cell | Undifferentiated |
| b | 1.13–1.20 | Slightly enlarged, green | Leptotene–diakinesis | Undifferentiated |
| c | 1.21–1.33 | Continue to swell, green | Metaphase I–telophase I | Undifferentiated |
| d | 1.34–1.38 | Continue to swell, light green | Prophase II–telophase II | Archespore |
| e | 1.39–1.56 | Continue to swell, light green | tetrad stage—slope nucleus centered | Archespore-sporogenous |
| f | 1.57–1.75 | Sepals distinct, pale green | slope nucleus centered—mononuclear fringe phase | Sporogenous |
| g | 1.76–2.12 | Sepals distinct, yellowish green | mononuclear fringe phase—dikaryophase | Megaspore mother cell |
| h~i | 2.13–2.50 | Sepals distinct, yellowish green | Dikaryophase | Dyad-function macrospore |
| j | 2.51–2.90 | Sepals about to crack, yellow | Mature pollen grain | Mononuclear embryo Sac-tetruclear embryo sac |
| k~l | >2.90 | Sepals about to crack, yellow | Mature pollen grain | Ockaryotic embryo sac |
| Treatment | Number of Treated Shoots | Number of Treated Bearing Shoots | Number of Survived Bearing Shoots | External Morphology of Bearing Shoots | Number of Treated Seeds | Number of Germinated Seeds | Germination Rate (%) | Seedling Number | Seedling Survival Rate (%) | Triploid Number | Triploid Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 164 | 160 | Normal growth | 107 | 67 | 62.6 | 42 | 39.3 | 0 | 0 |
| 2 | 6 | 151 | 151 | Normal growth | 108 | 60 | 55.6 | 53 | 49.1 | 0 | 0 |
| 3 | 6 | 172 | 167 | Some appear wilted and yellow | 95 | 52 | 54.7 | 44 | 46.3 | 0 | 0 |
| 4 | 6 | 183 | 183 | Normal growth | 126 | 72 | 57.1 | 61 | 48.4 | 2 | 3.3 |
| 5 | 6 | 196 | 196 | Normal growth | 92 | 57 | 62.0 | 49 | 53.3 | 0 | 0 |
| 6 | 6 | 185 | 180 | Some appear wilted and yellow | 88 | 50 | 56.8 | 37 | 42.0 | 0 | 0 |
| Total | 36 | 1051 | 1037 | 616 | 358 | 58.1 | 286 | 46.4 | 2 | 3.3 |
| Offspring Name | Mother Name | Pair LOD Score | Candidate Father |
|---|---|---|---|
| TM | Maya jujube | 7.73 | Sour jujube |
| DM-6-10 | Maya jujube | 6.46 | Datian Sour jujube |
| DM-6-11 | Maya jujube | 6.34 | Dabai jujube |
| DM-6-12 | Maya jujube | 7.52 | Sour jujube |
| DM-6-14 | Maya jujube | 6.54 | Sour jujube |
| DM-6-15 | Maya jujube | 3.80 | Datian Sour jujube |
| DM-6-17 | Maya jujube | 4.75 | Sour jujube |
| DM-6-18 | Maya jujube | 7.84 | Xiaobai jujube |
| DM-6-19 | Maya jujube | 5.32 | Datian Sour jujube |
| DM-6-20 | Maya jujube | 7.84 | Xiaobai jujube |
| DM-6-21 | Maya jujube | 7.25 | Datian Sour jujube |
| DM-7-1 | Maya jujube | 7.84 | Dabai jujube |
| DM-7-2 | Maya jujube | 7.20 | Dabai jujube |
| DM-7-3 | Maya jujube | 7.84 | Dabai jujube |
| DM-7-4 | Maya jujube | 4.74 | Sour jujube |
| DM-7-5 | Maya jujube | 6.06 | Sour jujube |
| Ploidy | Height (cm) | Ground Diameter (mm) | Thorn Length (mm) | Leaf Length (cm) | Leaf Width (cm) | Leaf Area (cm2) | Chlorophyll Content |
|---|---|---|---|---|---|---|---|
| DM-6-14 | 40.33 ± 1.15b | 4.37 ± 0.12b | 3.75 ± 0.27bc | 3.41 ± 0.35c | 1.29 ± 0.22c | 3.84 ± 0.53c | 34.58 ± 4.64b |
| DM-7-4 | 42.67 ± 1.52ab | 4.40 ± 0.10b | 8.23 ± 0.23b | 4.36 ± 0.56a | 1.81 ± 0.20b | 5.42 ± 1.74b | 32.77 ± 3.99b |
| DM-7-5 | 43.33 ± 1.15a | 4.43 ± 0.12b | 4.55 ± 0.13c | 3.84 ± 0.48b | 1.77 ± 0.24b | 3.99 ± 0.90c | 35.11 ± 2.89b |
| TM | 29.22 ± 1.53c | 5.06 ± 0.057a | 9.35 ± 0.14a | 4.19 ± 0.42ab | 2.26 ± 0.32a | 6.73 ± 1.18a | 39.46 ± 6.36a |
| Ploidy. | Pn (µmol·m −2·s−1) | Gs (mol·m−2·s−1) | Ci (µmol·m–1) | Tr (mmol·m−2·s−1) | WUEi | PEw (µmol·s−1) |
|---|---|---|---|---|---|---|
| DM-6-14 | 6.163 ± 0.24b | 0.161 ± 0.017b | 233.1 ± 46.26ab | 4.043 ± 0.11b | 1.526 ± 0.088ab | 0.00237 ± 0.0081b |
| DM-7-4 | 4.873 ± 0.48c | 0.093 ± 0.54b | 188.8 ± 7.80c | 2.967 ± 0.34c | 1.679 ± 0.45ab | 0.00264 ± 0.0082b |
| DM-7-5 | 5.236 ± 0.38c | 0.168 ± 0.069b | 228.4 ± 16.3ab | 4.123 ± 0.10b | 1.269 ± 0.082c | 0.00209 ± 0.0091b |
| TM | 9.583 ± 0.24a | 0.270 ± 0.62a | 279.7 ± 9.69a | 4.837 ± 0.17a | 1.982 ± 0.022a | 0.00645 ± 0.0062a |
| Ploidy | Stomata Height (µm) | Stomata Width (µm) | Stomata Density (No./mm2) |
|---|---|---|---|
| DM-6-14 | 18.78 ± 0.73b | 16.00 ± 0.52b | 465.88 ± 17.07a |
| DM-7-4 | 17.29 ± 0.20c | 16.37 ± 0.61b | 474.23 ± 22.05a |
| DM-7-5 | 17.84 ± 0.55bc | 15.83 ± 0.68b | 460.71 ± 26.79a |
| TM | 22.34 ± 0.52a | 17.61 ± 0.41a | 356.25 ± 23.51b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, C.; Yang, W.; Li, X.; Hou, L.; Li, M.; Pang, X.; Li, Y. Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and Its Characteristics. Forests 2021, 12, 112. https://doi.org/10.3390/f12020112
Liu S, Zhang C, Yang W, Li X, Hou L, Li M, Pang X, Li Y. Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and Its Characteristics. Forests. 2021; 12(2):112. https://doi.org/10.3390/f12020112
Chicago/Turabian StyleLiu, Songshan, Chenxing Zhang, Weicong Yang, Xiang Li, Lu Hou, Meng Li, Xiaoming Pang, and Yingyue Li. 2021. "Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and Its Characteristics" Forests 12, no. 2: 112. https://doi.org/10.3390/f12020112
APA StyleLiu, S., Zhang, C., Yang, W., Li, X., Hou, L., Li, M., Pang, X., & Li, Y. (2021). Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and Its Characteristics. Forests, 12(2), 112. https://doi.org/10.3390/f12020112






