Allometric Models for Estimating Aboveground Biomass in Short Rotation Crops of Acacia Species in Two Different Sites in Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Trial Establishmet
2.3. Biomass Sampling According to Species, Location and Plant Density
2.4. Fitting of Linear and Non-Linear Functional Forms
2.4.1. Linear Forms
2.4.2. Non-Linear Forms
2.5. Selection of the Functional Forms
2.5.1. Preselection Based on Goodness of Fit
2.5.2. Selection Bases on Prediction Quality
2.6. Evaluation of the Additivity of the Selected Systems of Equations
3. Results
3.1. Classification and Predictive Quality of the Models
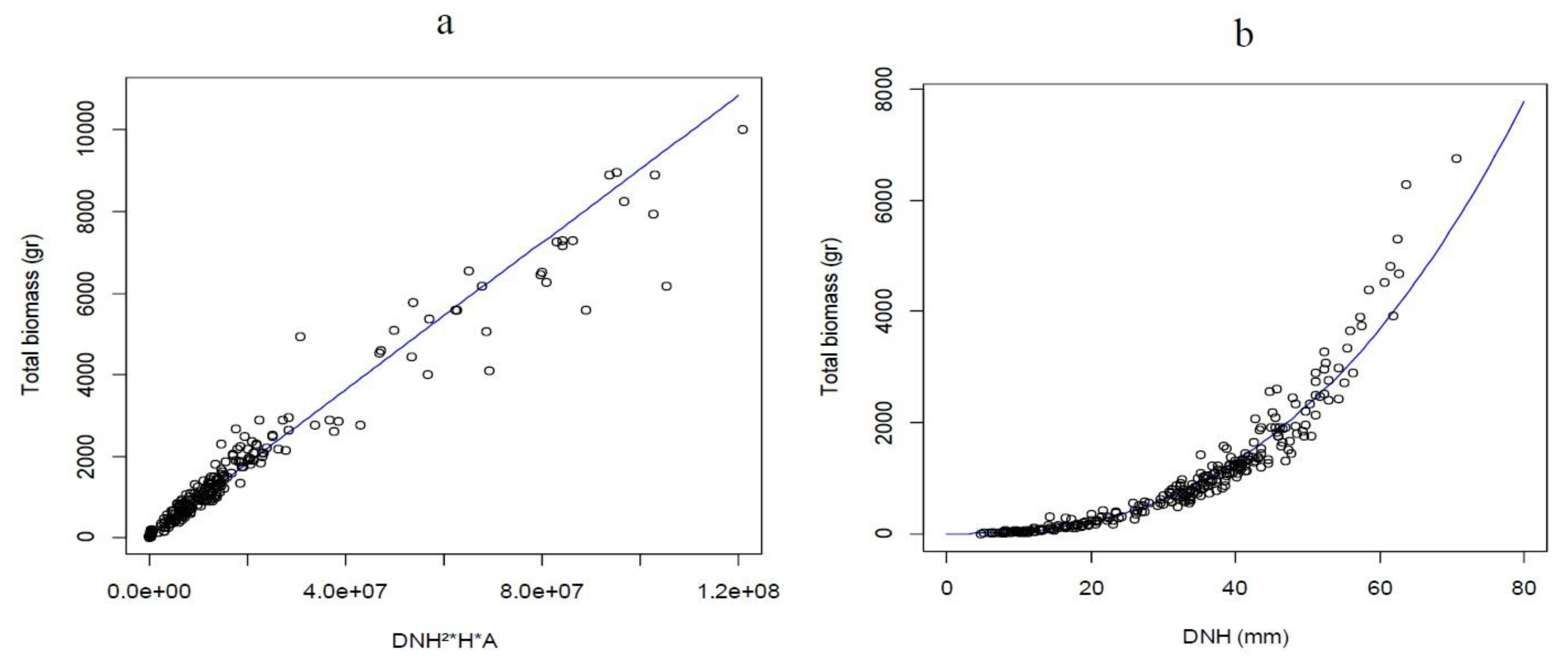
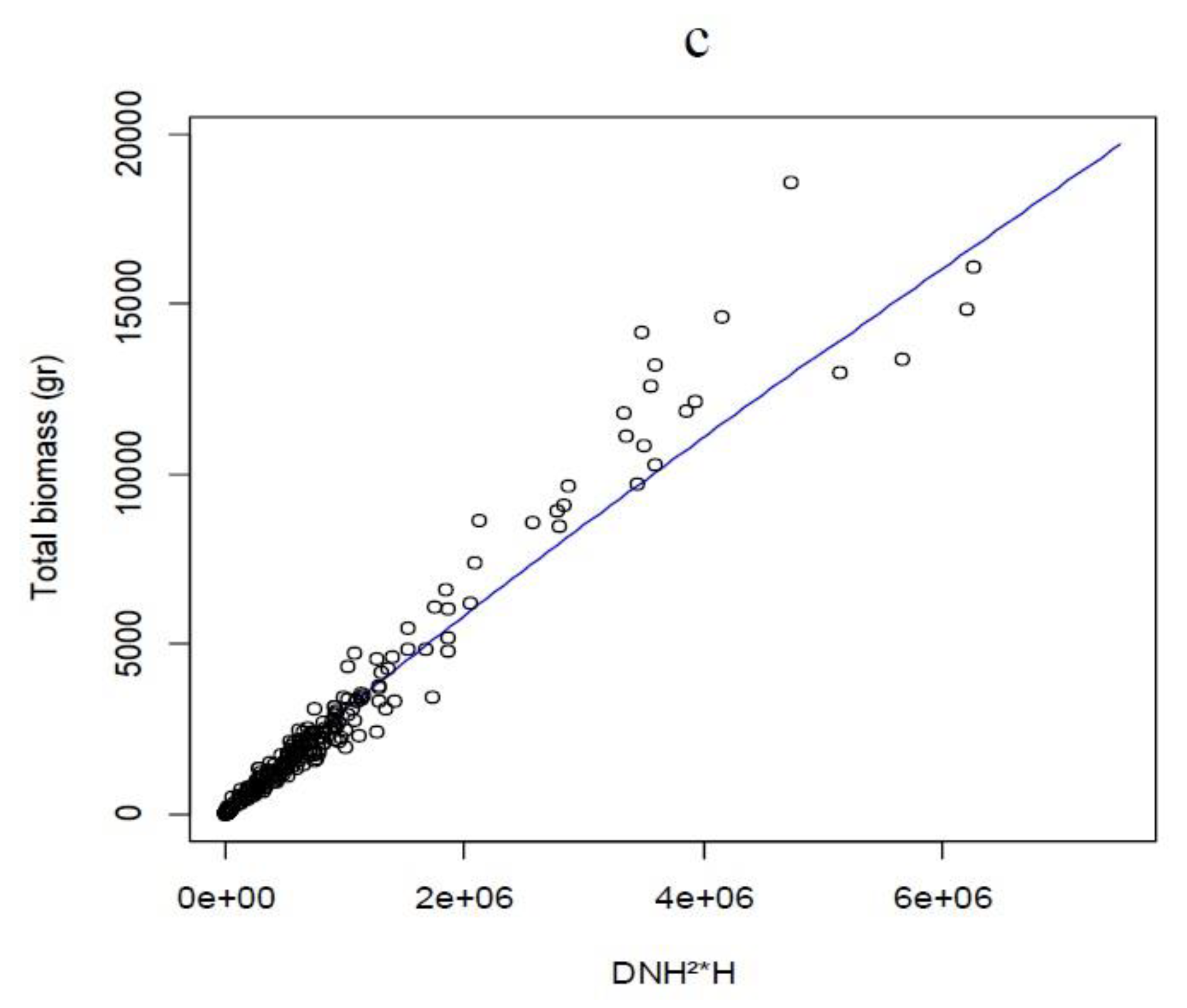
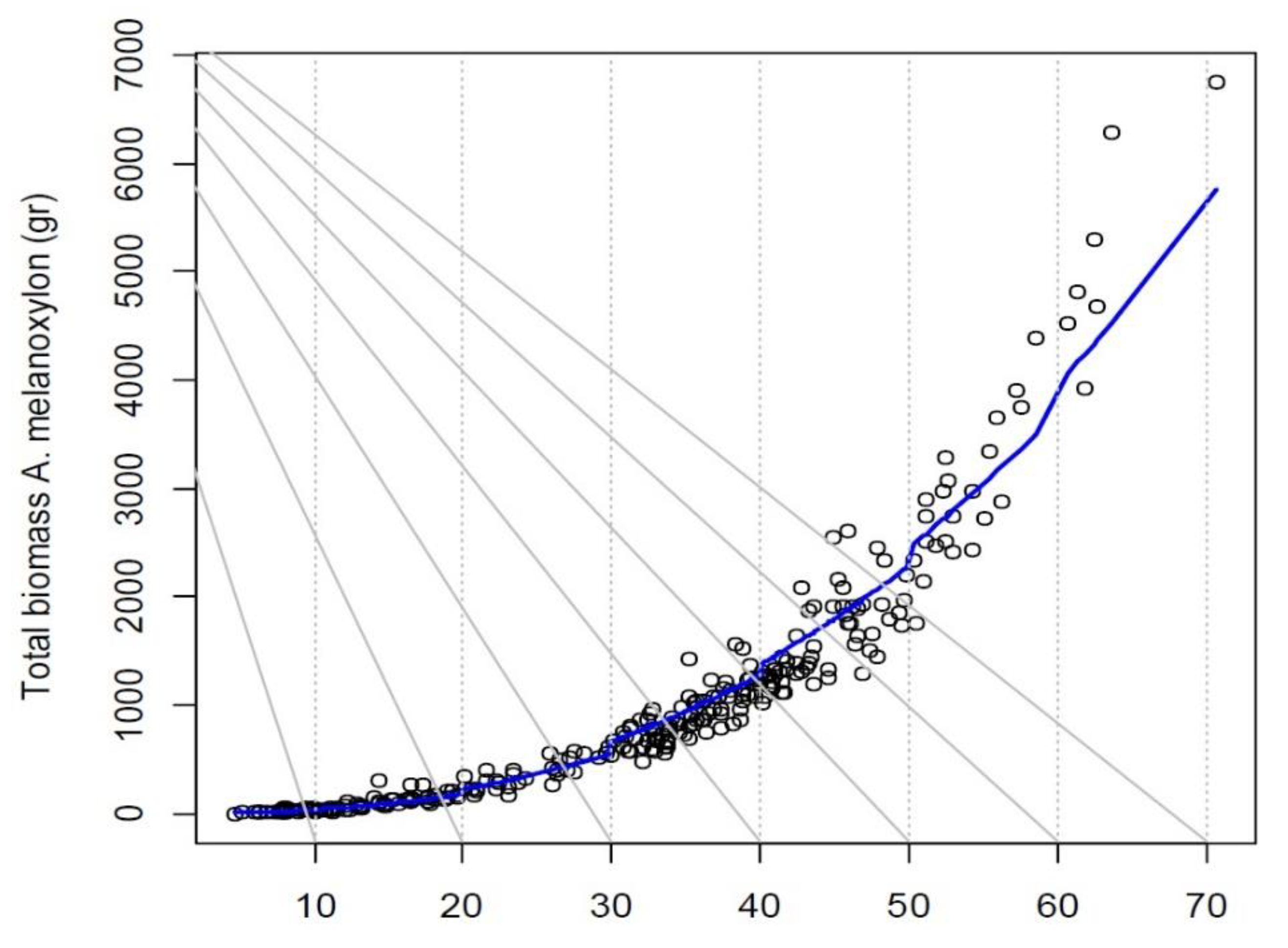
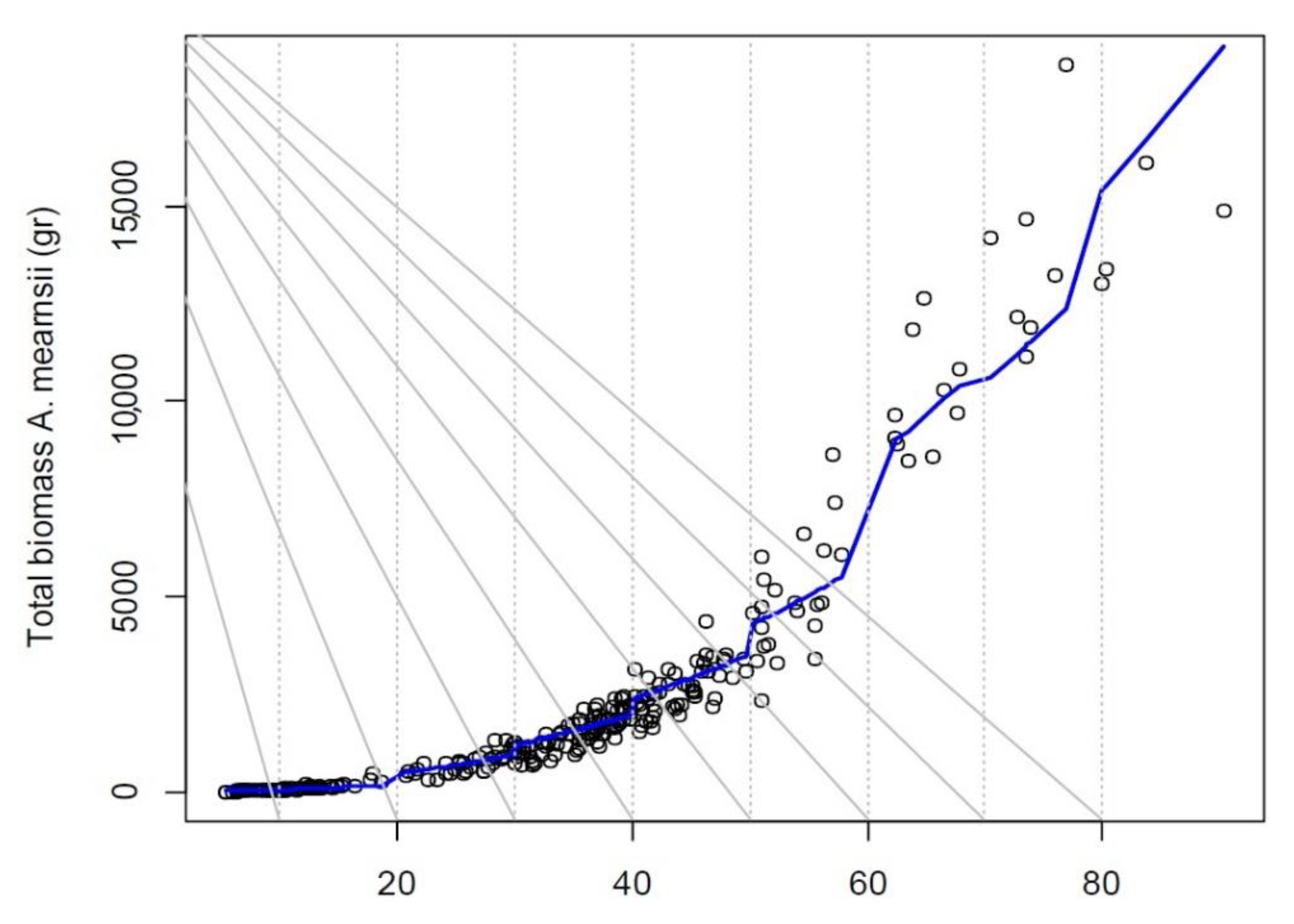
3.2. Model Selection by Linear and Non-Linear Regressions
3.3. Heteroscedasticity of the Residuals’ Variance
3.4. Additivity in Systems of Equations to Predict Acacia Species Biomass
Prediction Intervals for Total Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goel, V.L.; Behl, H.M. Productivity assessment of three leguminous species under high-density plantations on degraded soil sites. Biomass Bioenergy 2004, 27, 403–409. [Google Scholar] [CrossRef]
- Beadle, C.L.; Brown, A.G. Acacia Utilisation and Management-Adding Value: Proceedings of a Blackwood Industry Group (BIG) Workshop, Victoria, Australia, 26–29 April 2006; Rural Industries Research and Development Corporation: Kingston, Australia, 2007.
- Albaugh, T.J.; Rubilar, R.A.; Maier, C.A.; Acuña, E.A.; Cook, R.L. Biomass and nutrients mass of Acacia dealbata and Eucalyptus globulus bioenergy plantations. Biomass Bioenergy 2017, 97, 162–171. [Google Scholar] [CrossRef] [Green Version]
- Dutcă, I. The Variation Driven by Differences between Species and between Sites in Al-lometric Biomass Models. Forests 2019, 10, 976. [Google Scholar] [CrossRef] [Green Version]
- Roxburgh, S.H.; Paul, K.I.; Clifford, D.; England, J.R.; Raison, R.J. Guidelines for constructing allometric models for the prediction of woody biomass: How many individuals to harvest? Ecosphere 2016, 6, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Li, Z.; Zhou, G.; Qiu, Z.; Wu, Z. Site-Specific Allometric Models for Prediction of Above-and Belowground Biomass of Subtropical Forests in Guangzhou, Southern China. Forests 2019, 10, 862. [Google Scholar] [CrossRef] [Green Version]
- Miah, M.D.; Islam, K.N.; Kabir, M.H.; Koike, M. Allometric models for estimating aboveground biomass of selected homestead tree species in the plain land Narsingdi district of Bangladesh. Trees For. People 2020, 2, 100035. [Google Scholar] [CrossRef]
- Rahman, M.D.; Donoghue, D.M.; Bracken, L.J.; Mahmood, H. Biomass estimation in mangrove forests: A comparision of allometric models incorporating species and structural information. Environ. Res. Lett. 2021, 16, 124002. [Google Scholar] [CrossRef]
- Parresol, B. Assessing tree and stand biomass: A review with examples and critical comparisons. For. Sci. 1999, 45, 573–593. [Google Scholar] [CrossRef]
- Parresol, B. Additivity of nonlinear biomass equations. Can. J. For. Res. 2001, 31, 865–878. [Google Scholar] [CrossRef]
- Böhm, C.; Quinkenstein, A.; Freese, D. Yield prediction of young black locust (Robinia pseudoacacia L.) plantations for woody biomass production using allometric relations. Ann. For. Res. 2011, 54, 215–227. [Google Scholar] [CrossRef]
- Sandoval, S.; Cancino, J.; Rubilar, R.; Esquivel, E.; Acuña, E.; Muñoz, F. Probability distributions in high-density dendroenergy plantations. For. Sci. 2012, 58, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Jiban, C.D.; Abdul, M.; Enam, A. An allometric equation for estimating stem biomass of Acacia auriculiformis in the north-eastern region of Bangladesh. South For. 2012, 74, 103–113. [Google Scholar] [CrossRef]
- Ríos-Saucedo, J.C.; Acuña-Carmona, E.; Cancino-Cancino, J. Allometric equations commonly used for estimating shoot biomass in short-rotation wood energy species: A review. Rev. Chapingo Ser. Cienc. For. Ambiente 2016, 22, 193–202. [Google Scholar] [CrossRef]
- Adam, N.S.; Jusoh, I. Allometric Model for Predicting Aboveground Biomass and Carbon Stock of Acacia Plantations in Sarawak, Malaysia. Bioresources 2018, 13, 7381–7394. [Google Scholar] [CrossRef]
- CIREN. Descripciones de Suelos, Materiales y Símbolos. Estudio Agrológico VIII Región; Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 1999. [Google Scholar]
- Morales, L.; Acevedo, E.; Castellaro, G.; Román-Osorio, L.; Morales-Inostroza, J.; Alonso, M.F. A simple method for estimating suitable territory for bioenergy species in Chile. Cienc. Investig. Agrar. 2015, 42, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Probosque: Plan de Manejo Forestal. Available online: http://www.probosque.cl/Docs/20151103Plan_de_manejo.pdf (accessed on 14 April 2021).
- Universidad Austral de Chile. Análisis Químico de Suelo Predio Santa Luisa; Laboratorio de Nutrición Y Suelos Forestales, Facultad de Ciencias Forestales: Valdivia, Chile, 2004. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Baskerville, G.L. Use of logarithmic regression in the estimation of plant biomass. Can. J. For. Res. 1972, 2, 49–53. [Google Scholar] [CrossRef]
- Picard, N.; Saint-André, L.; Henry, M. Manual de Construcción de Ecuaciones Alométricas Para Estimar el Volumen y la Biomasa de los Árboles. Del Trabajo de Campo a la Predicción; CIRAD and FAO: Roma, Italy, 2012. [Google Scholar]
- Furnival, G. An index for comparing equations used in constructing volume tables. For. Sci. 1961, 7, 337–341. [Google Scholar]
- Burnham, K.; Anderson, D. Model Selection and Multimodel Inference: Practical Information–Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- SAS Institute Inc. SAS/IML® 9.22 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Carvalho, J.; Parresol, B. Additivity in tree biomass components of Pyrenean oak (Quercus pyrenaica Willd.). For. Ecol. Manag. 2003, 179, 269–276. [Google Scholar] [CrossRef]
- Pardé, J.D. Forest Biomass. For. Abstr. 1980, 41, 343–362. [Google Scholar]
- Mäkelä, A.; Vanninen, P. Impacts of size and competition on tree form and distribution of aboveground biomass in Scots pine. Can. J. For. Res. 1998, 28, 216–227. [Google Scholar] [CrossRef]
- Meadows, J.; Hodges, J. Sapwood area as an estimator of leaf area and foliar weight in cherrybark oak and Green ash. For. Sci. 2002, 48, 69–76. [Google Scholar] [CrossRef]
- Hakkila, P. Utilization of Residual Forest Biomass, 1st ed.; Springer Series in Wood Sciences: Berlin, Germany, 1989. [Google Scholar]
- Socha, J.; Wezyk, P. Allometric equations for estimating the foliage biomass of Scots pine. Eur. J. For. Res. 2007, 126, 263–270. [Google Scholar] [CrossRef]
- Campoe, O.; Stape, J.L.; Laclau, J.P.; Marsden, C.; Nouvellon, Y. Stand level patterns of carbon fluxes and partitioning in a Eucalyptus grandis plantation across a gradient of productivity in São Paulo State, Brazil. Tree Physiol. 2012, 32, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter-Mikaelian, M.; Korzukhin, M. Biomass equations for sixty-five North American tree species. For. Ecol. Manag. 1997, 97, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Snowdon, P. A ratio estimator for bias correction in logarithmic regressions. Can. J. For. Res. 1991, 21, 720–724. [Google Scholar] [CrossRef]
- Hayes, A.; Cai, L. Using heteroscedasticity-consistent standard error estimators in OLS regression: An introduction and software implementation. Behav. Res. Methods 2007, 39, 709–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judge, G.; Hill, R.; Griffiths, W.; Lutkepohl, H.; Lee, T. Introduction to the Theory and Practice of Econometrics, 2nd ed.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Zellner, A. An efficient method of estimating seemingly unrelated regressions and test for aggregation bias. J. Am. Stat. Assoc. 1962, 57, 348–368. [Google Scholar] [CrossRef]

| Species | Parameter | Average | S.D. | Min | Max |
|---|---|---|---|---|---|
| A. dealbata | DNH 1 (mm) | 32 | 15.38 | 6.3 | 80 |
| H total (cm) | 337.98 | 178.02 | 50 | 807 | |
| Age (months) | 19.51 | 7.79 | 8.5 | 33.2 | |
| Wstem (g) | 837.6 | 1194.26 | 3.41 | 6822.87 | |
| Wbranches (g) | 240.28 | 342.21 | 0.69 | 1796.16 | |
| Wleaves (g) | 401.76 | 473.18 | 3.2 | 2598.36 | |
| A. melanoxylon | DNH 1 (mm) | 31.17 | 15.05 | 4.69 | 70.7 |
| H total (cm) | 265.05 | 127.7 | 12 | 605 | |
| Age (months) | 19.83 | 7.98 | 8.5 | 33.2 | |
| Wstem (g) | 572.93 | 635.85 | 0.73 | 3985.9 | |
| Wbranches (g) | 165.52 | 207.7 | 0.23 | 1411.14 | |
| Wleaves (g) | 262.84 | 269.21 | 0.09 | 1572.07 | |
| A. mearnsii | DNH 1 (mm) | 33 | 17.91 | 5.4 | 90.4 |
| H total (cm) | 353.6 | 193.71 | 43 | 895 | |
| Age (months) | 19.94 | 7.77 | 8.5 | 33.2 | |
| Wstem (g) | 1265.55 | 2008.9 | 0.91 | 11,084.01 | |
| Wbranches (g) | 395.7 | 529.48 | 0.1 | 2905.15 | |
| Wleaves (g) | 597.64 | 728.66 | 1.34 | 4742.78 |
| Hierarchy | Functional Form | Variance Model | FI | AIC | Bias% |
|---|---|---|---|---|---|
| Total Biomass | |||||
| A. melanoxylon | Btotal = 0.09416 × (DNH)².⁵⁸³⁶ | (DNH¹.⁷⁸¹⁷)² | 2480.58 | −1.01 | |
| A. dealbata | Btotal = 46.2256 + 0.00009 × (DNH² × H × A) | (DNH¹.⁹⁵²)² | 184.49 | 0.11 | |
| A. mearnsii | Btotal = 0.009484 × (DNH² × H)⁰.⁹¹⁸⁹ | (DNH².³¹⁴⁹)² | −1.50 | ||
| Stem Biomass | |||||
| A. melanoxylon-Santa Luisa | Bstem = 2.3284 + 0.001276 × (DNH² × H) | 9.30 | −0.81 | ||
| A. melanoxylon-Luanco | Bstem = 0.00027 × (DNH)¹.⁹⁹⁶⁴ × (H)⁰.⁸⁹¹³ × (A)⁰.⁶⁹⁹³ | 93.05 | −0.10 | ||
| A. dealbata-SantaLuisa | Bstem = 6.8092 + 0.001381 × (DNH² × H) | (DNH¹.⁸⁰⁶⁶)² | 11.48 | 0.06 | |
| A. dealbata-Luanco | Bstem = 0.0001065 × (DNH² × H × A)⁰.⁹⁵⁸² | (DNH².⁴⁹³⁸)² | 1667.52 | −0.68 | |
| A. mearnsii | Bstem = 0.0004323 × (DNH)¹.⁷⁴⁸⁴ × (H)¹.⁰⁶⁰² × (A)⁰.⁵⁹⁹⁷ | (DNH².³⁶⁵⁶)² | 2391.77 | −0.80 | |
| Branches Biomass | |||||
| A. melanoxylon | Bbranches = 0.009182 × (DNH)².¹²⁹⁶ × (A)⁰.⁶⁹⁵⁴ | (DNH².⁰⁵⁰⁴⁴)² | 1997.17 | −8.30 | |
| A. dealbata | Bbranches = 4.9792 + 0.000014 × (DNH² × H × A) | (DNH².³¹⁸⁵)² | 44.50 | 6.82 | |
| A. mearnsii | Bbranches = 0.002183 × (DNH² × H)⁰.⁸⁹⁹³ | (DNH².²⁹¹⁰⁶)² | 2135.16 | 0.08 | |
| Leaves Biomass | |||||
| A. melanoxylon | Bleaves = 12.168 + 0.00059 × (DNH² × H) | (DNH¹.³⁸⁸²)² | 71.60 | −5.56 | |
| A. dealbata | Bleaves = 20.0042 + 0.000613 × (DNH² × H) | (DNH¹.⁸⁰¹⁸)² | 72.58 | 10.35 | |
| Species | Parameters | Adjusted Using OLS | Adjusted Using Variance Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated Value | S.E. | t Value | Prob (>|t|) | IF | Confidence Interval | Estimated Value | S.E. | t Value | Prob (>|t|) | FI | Confidence Interval | Difference in Se (%) | ||
| A. dealbata | a1 | 205.48 | 35.44 | 5.80 | 0 | 400.5 | (134.59 276.36) | 46.23 | 3.78 | 12.21 | 0 | 184.52 | (38.66 53.8) | −89.32 |
| b1 | 8 × 10−5 | 1.33 × 10−6 | 59.23 | 0 | (7.7 × 10−5 8.3 × 10−5) | 9 × 10−5 | 2 × 10−6 | 54.63 | 0 | (8.6 × 10−5 9.4 × 10−5) | 50.83 | |||
| A. melanoxylon | a1 | 0.02233 | 0.005439 | 4.1047 | 0 | 2719.72 | (0.01145 0.0332) | 0.0942 | 0.01503 | 6.2614 | 0 | 2480.58 | (0.06414 0.1243) | 176.34 |
| b1 | 2.9602 | 0.062 | 47.7444 | 0 | (2.8362 3.0842) | 2.5836 | 0.0432 | 59.8529 | 0 | (2.4972 2.67) | −30.39 | |||
| A. mearnsii | a1 | 0.00474 | 0.001308 | 3.6228 | 4 × 10−4 | 3150.61 | (2.1 × 10−3 7.4 × 10−3) | 0.009484 | 0.001334 | 7.1066 | 0 | 2629.13 | (0.0068 0.012) | 1.99 |
| b1 | 0.9707 | 0.01847 | 52.5417 | 0 | (0.9338 1.0076) | 0.9189 | 0.0104 | 87.6228 | 0 | (0.8981 0.9397) | −43.69 | |||
| Species | Average Values | Prediction Interval of the Individual Model | Prediction Interval: Method 1 | Prediction Intervals: SUR and NSUR Method | ||
|---|---|---|---|---|---|---|
| DNH (mm) | Height (cm) | Age (months) | ||||
| A. dealbata | 32 | 338 | 20 | 697.88 g ± 663.14 g | 719.32 g ± 459.13 g | 704.7 g ± 402.44 g |
| A. melanoxylon | 31 | 265 | 20 | 663.04 g ± 382.45 g | 627.91 g ± 334.28 g | 658.372 g ±279.13 g |
| A. mearnsii | 33 | 354 | 20 | 1284 g ± 549.84 g | 1201.84 g ± 514.53 g | 1252 g ± 557.517 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Ariza, A.; Valdés, S.; Gilabert, H.; Santelices-Moya, R.E.; Alonso-Valdés, M. Allometric Models for Estimating Aboveground Biomass in Short Rotation Crops of Acacia Species in Two Different Sites in Chile. Forests 2021, 12, 1767. https://doi.org/10.3390/f12121767
Cabrera-Ariza A, Valdés S, Gilabert H, Santelices-Moya RE, Alonso-Valdés M. Allometric Models for Estimating Aboveground Biomass in Short Rotation Crops of Acacia Species in Two Different Sites in Chile. Forests. 2021; 12(12):1767. https://doi.org/10.3390/f12121767
Chicago/Turabian StyleCabrera-Ariza, Antonio, Sara Valdés, Horacio Gilabert, Rómulo Eduardo Santelices-Moya, and Máximo Alonso-Valdés. 2021. "Allometric Models for Estimating Aboveground Biomass in Short Rotation Crops of Acacia Species in Two Different Sites in Chile" Forests 12, no. 12: 1767. https://doi.org/10.3390/f12121767
APA StyleCabrera-Ariza, A., Valdés, S., Gilabert, H., Santelices-Moya, R. E., & Alonso-Valdés, M. (2021). Allometric Models for Estimating Aboveground Biomass in Short Rotation Crops of Acacia Species in Two Different Sites in Chile. Forests, 12(12), 1767. https://doi.org/10.3390/f12121767






