Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Needle Sampling, Extraction and Gas Chromatography Analysis
2.3. Statistical Analysis
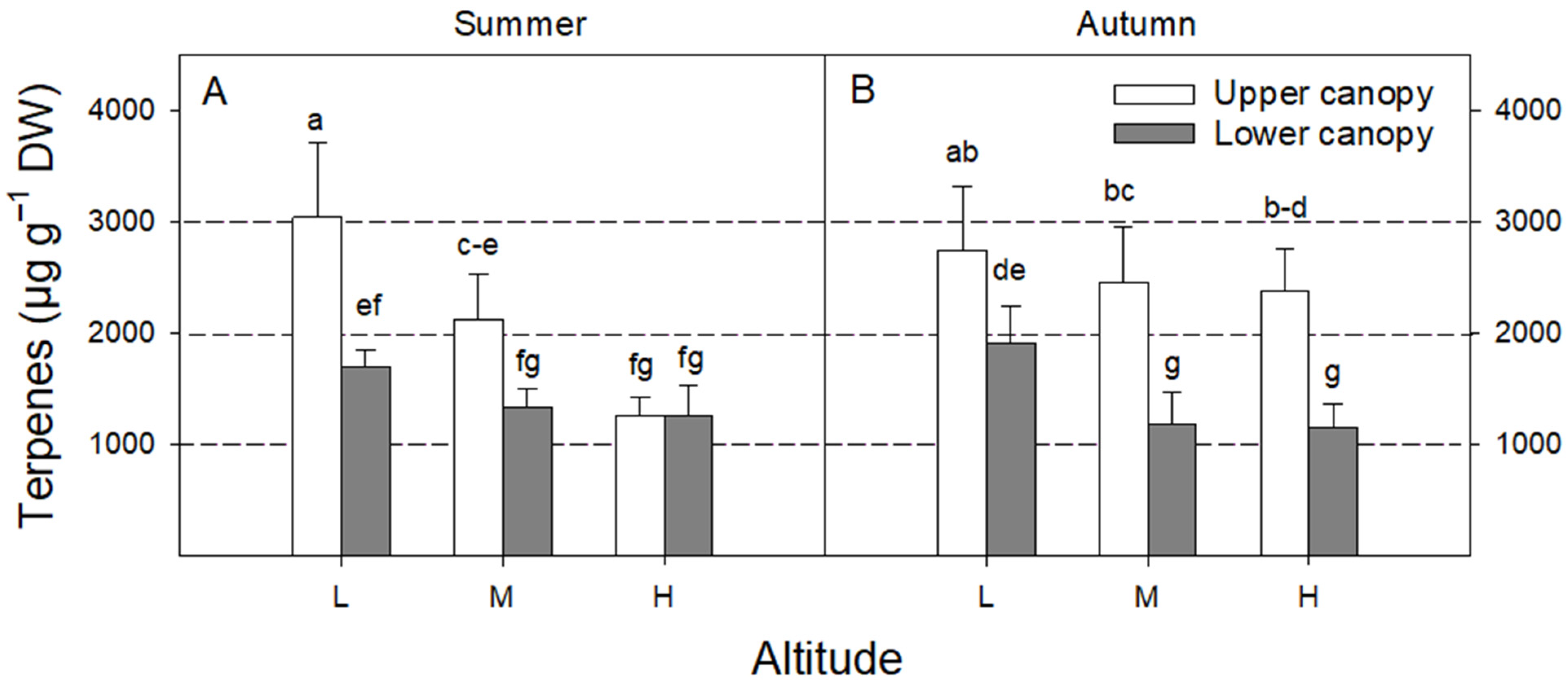
3. Results
4. Discussion
4.1. Terpenes under a Complex Environment
4.2. Effect of Season
4.3. Effect of Canopy Position
4.4. Effect of Altitude
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Summer | Autumn | ||||
|---|---|---|---|---|---|
| Altitude | Upper Canopy | Lower Canopy | Upper Canopy | Lower Canopy | |
| MT (µg g–1 DW) | |||||
| 3-carene | L | - | - | - | - |
| M | 2.17 ± 2.75 | - | 14.73 ± 2.54 | - | |
| H | 5.01 ± 1.53 | - | 20.37 ± 4.77 | - | |
| Borneol | L | 9.04 ± 3.22 | 0.42 ± 0.53 | 4.52 ± 4.54 | 10.06 ± 4.11 |
| M | 0.79 ± 1.10 | - | 3.77 ± 3.99 | - | |
| H | - | - | - | - | |
| Bornyl acetate | L | 77.02 ± 15.26 | 39.93 ± 10.86 | 62.53 ± 3.90 | 52.41 ± 3.18 |
| M | 49.93 ± 5.97 | 28.10 ± 5.01 | 66.71 ± 12.03 | 23.34 ± 4.03 | |
| H | 40.72 ± 6.33 | 31.69 ± 4.84 | 85.81 ± 9.49 | 38.70 ± 8.49 | |
| Camphene | L | 641.89 ± 98.85 | 503.86 ± 93.36 | 597.40 ± 78.90 | 572.50 ± 92.09 |
| M | 392.46 ± 57.35 | 386.79 ± 24.55 | 486.20 ± 73.07 | 314.92 ± 62.89 | |
| H | 237.91 ± 18.85 | 268.48 ± 51.85 | 489.60 ± 26.79 | 282.78 ± 36.18 | |
| Camphor | L | 17.43 ± 4.86 | 10.24 ± 3.57 | 17.70 ± 4.11 | 11.43 ± 1.99 |
| M | 4.84 ± 0.72 | 4.78 ± 1.14 | 8.67 ± 0.65 | 5.66 ± 1.49 | |
| H | 0.80 ± 0.21 | 1.42 ± 0.33 | 4.58 ± 0.83 | 1.98 ± 0.23 | |
| Eucalyptol | L | 203.29 ± 26.35 | 145.78 ± 23.15 | 211.03 ± 22.52 | 171.21 ± 21.03 |
| M | 153.89 ± 2.97 | 109.72 ± 8.58 | 201.69 ± 34.47 | 116.81 ± 14.07 | |
| H | 70.98 ± 5.45 | 94.06 ± 23.93 | 189.14 ± 36.38 | 120.90 ± 12.16 | |
| Fenchol | L | 19.17 ± 3.61 | 8.82 ± 1.77 | 15.59 ± 3.57 | 11.63 ± 2.51 |
| M | 10.73 ± 2.66 | 6.51 ± 1.42 | 16.01 ± 1.48 | 6.39 ± 1.35 | |
| H | 4.71 ± 0.80 | 2.13 ± 0.37 | 9.90 ± 2.83 | 4.63 ± 1.59 | |
| Limonene | L | 775.33 ± 204.19 | 399.76 ± 57.19 | 646.94 ± 82.76 | 433.21 ± 66.38 |
| M | 510.75 ± 130.79 | 311.57 ± 45.21 | 593.04 ± 137.33 | 278.41 ± 67.76 | |
| H | 381.83 ± 19.07 | 573.21 ± 115.44 | 630.51 ± 87.54 | 294.36 ± 66.69 | |
| Myrcene | L | 419.36 ± 105.44 | 84.31 ± 8.65 | 540.43 ± 231.41 | 149.09 ± 43.66 |
| M | 537.12 ± 117.06 | 147.06 ± 24.31 | 536.44 ± 134.78 | 153.13 ± 42.65 | |
| H | 236.00 ± 67.69 | 54.51 ± 6.03 | 421.96 ± 140.04 | 137.95 ± 27.32 | |
| Sabinene | L | 37.53 ± 4.92 | 25.44 ± 3.00 | 31.70 ± 6.14 | 28.37 ± 6.57 |
| M | 18.12 ± 3.17 | 22.08 ± 5.20 | 28.97 ± 6.47 | 14.16 ± 2.12 | |
| H | 8.49 ± 1.68 | 10.88 ± 2.98 | 32.18 ± 5.55 | 17.18 ± 4.96 | |
| Terpinolene | L | 3.64 ± 0.58 | 1.18 ± 0.29 | 2.20 ± 0.18 | 0.51 ± 0.12 |
| M | 2.16 ± 0.21 | 0.78 ± 0.12 | 1.79 ± 0.33 | - | |
| H | 0.83 ± 0.07 | 1.21 ± 0.28 | 2.38 ± 0.45 | 0.62 ± 0.15 | |
| Tricyclene | L | 65.96 ± 10.42 | 49.87 ± 6.64 | 59.53 ± 7.14 | 58.35 ± 10.40 |
| M | 40.13 ± 4.93 | 41.39 ± 3.40 | 46.07 ± 4.77 | 32.62 ± 6.27 | |
| H | 24.53 ± 2.06 | 20.10 ± 3.83 | 50.74 ± 1.91 | 29.62 ± 4.21 | |
| α-pinene | L | 469.85 ± 79.93 | 272.68 ± 44.58 | 341.18 ± 54.41 | 318.33 ± 48.96 |
| M | 232.07 ± 20.54 | 214.72 ± 20.54 | 275.57 ± 18.69 | 169.35 ± 34.20 | |
| H | 138.94 ± 5.32 | 144.17 ± 36.82 | 278.62 ± 20.35 | 152.24 ± 21.85 | |
| α-terpineol | L | 8.07 ± 0.56 | 3.66 ± 1.19 | 6.35 ± 1.03 | 2.20 ± 0.26 |
| M | 4.06 ± 0.81 | 0.98 ± 0.19 | 3.93 ± 1.03 | 1.83 ± 0.36 | |
| H | 0.66 ± 0.18 | 1.21 ± 0.17 | 4.02 ± 1.08 | 1.29 ± 0.13 | |
| β-pinene | L | 96.72 ± 15.40 | 34.66 ± 1.01 | 44.25 ± 5.80 | 34.24 ± 1.39 |
| M | 36.48 ± 7.21 | 21.92 ± 5.98 | 31.03 ± 3.09 | 16.67 ± 4.34 | |
| H | 20.26 ± 3.14 | 18.66 ± 3.74 | 42.29 ± 5.64 | 23.15 ± 3.00 | |
| SQT (µg g–1 DW) | |||||
| Germacrene D-4-ol | L | 177.45 ± 50.15 | 107.06 ± 27.79 | 152.33 ± 16.32 | 47.75 ± 5.94 |
| M | 115.24 ± 19.37 | 37.48 ± 3.73 | 131.63 ± 32.16 | 42.75 ± 12.43 | |
| H | 79.14 ± 17.43 | 35.01 ± 6.22 | 106.15 ± 22.00 | 41.26 ± 10.34 | |
| Humulene | L | 6.43 ± 2.38 | 6.79 ± 2.21 | 8.63 ± 2.17 | 10.06 ± 3.23 |
| M | 7.62 ± 1.18 | 5.99 ± 1.91 | 9.24 ± 1.92 | 7.22 ± 1.60 | |
| H | 6.36 ± 1.77 | 3.88 ± 0.54 | 6.75 ± 1.57 | 5.89 ± 1.39 | |
| Longifolene | L | 2.64 ± 0.35 | 1.81 ± 0.53 | 1.11 ± 0.07 | 0.97 ± 0.30 |
| M | 1.79 ± 0.35 | 0.89 ± 0.27 | 1.17 ± 0.13 | 0.85 ± 0.10 | |
| H | 0.88 ± 0.07 | 0.63 ± 0.14 | 1.04 ± 0.27 | 0.59 ± 0.09 | |
| β-cadinene | L | 2.73 ± 0.25 | 2.45 ± 0.60 | 1.21 ± 0.11 | 1.06 ± 0.17 |
| M | 1.95 ± 0.43 | 1.15 ± 0.18 | 1.40 ± 0.12 | 0.75 ± 0.09 | |
| H | 1.08 ± 0.10 | 0.68 ± 0.11 | 0.89 ± 0.25 | 0.56 ± 0.04 | |
| β-caryophyllene | L | 4.97 ± 0.77 | 3.93 ± 1.12 | 6.13 ± 1.34 | 5.67 ± 1.13 |
| M | 4.13 ± 0.52 | 2.87 ± 0.64 | 3.05 ± 0.45 | 3.95 ± 0.80 | |
| H | 4.04 ± 0.83 | 2.32 ± 0.53 | 4.89 ± 1.13 | 4.38 ± 1.10 | |
References
- Körner, C. The use of altitude in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Kolář, T.; Čermák, P.; Trnka, M.; Žid, T.; Rybníček, M. Temporal changes in the climate sensitivity of Norway spruce and European beech along an elevation gradient in Central Europe. Agric. For. Meteorol. 2017, 239, 24–33. [Google Scholar] [CrossRef]
- Van de Weg, M.J.; Meir, P.; Grace, J.; Atkin, O.K. Altitudinal variation in leaf mass per unit area, leaf tissue density and foliar nitrogen and phosphorus content along an Amazon-Andes gradient in Peru. Plant Ecol. Divers. 2009, 2, 243–254. [Google Scholar] [CrossRef]
- Vats, S.K.; Kumar, N.; Kumar, S. Gas exchange response of barley and pea cultivars to altitude variation in Himalaya. Photosynthetica 2009, 47, 41–45. [Google Scholar] [CrossRef]
- Fan, Y.; Zhong, Z.; Zhang, X. Determination of photosynthetic parameters Vcmax and Jmax for a C3 plant (spring hulless barley) at two altitudes on the Tibetan Plateau. Agric. For. Meteorol. 2011, 151, 1481–1487. [Google Scholar] [CrossRef]
- Rajsnerová, P.; Klem, K.; Holub, P.; Novotná, K.; Večeřová, K.; Kozáčiková, M.; Rivas-Ubach, A.; Sardans, J.; Marek, M.V.; Peñuelas, J.; et al. Morphological, biochemical and physiological traits of upper and lower canopy leaves of European beech tend to converge with increasing altitude. Tree Physiol. 2015, 35, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Rana, P.S.; Saklani, P.; Chandel, C. Influence of altitude on secondary metabolites and antioxidant activity of Coleus forskohlii root extracts. Res. J. Med. Plant. 2020, 14, 43–52. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a better understanding of plant responses and acclimation to abiotic factors linked to global change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Holub, P.; Janssens, I.A.; Walker, T.W.N.; Pesqueda, A.; Peñuelas, J. Ecometabolomics of plant–herbivore and plant–fungi interactions: A synthesis study. Ecosphere 2021, 12, e03736. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defence response, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Hu, Z. Comparative anatomy of resin ducts of the Pinaceae. Trees 1997, 11, 135–143. [Google Scholar] [CrossRef]
- Manninen, A.M.; Pasanen, P.; Holopainen, J.K. Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmos. Environ. 2002, 36, 1763–1768. [Google Scholar] [CrossRef]
- Holzke, C.; Dinforf, T.; Kesselmeier, J.; Kuhn, U.; Koppmann, R. Terpene emissions from European beech (Fagus sylvatica L.): Pattern and emission behaviour over two vegetation periods. J. Atmos. Chem. 2006, 55, 81–102. [Google Scholar] [CrossRef]
- Pasqua, G.; Monacelli, B.; Manfredini, C.; Loreto, F.; Perez, G. The role of isoprenoid accumulation and oxidation in sealing wounded needles of Mediterranean pines. Plant. Sci. 2002, 163, 355–359. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant. Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Esposito, R.; Lusini, I.; Večeřová, K.; Holišová, P.; Pallozzi, E.; Guidolotti, G.; Urban, O.; Calfapietra, C. Shoot-level terpenoids emission in Norway spruce (Picea abies) under natural field and manipulated laboratory conditions. Plant. Physiol. Biochem. 2016, 108, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Calfapietra, C.; Fares, S.; Loreto, F. Volatile organic compounds from Italian vegetation and their interaction with ozone. Environ. Pollut. 2009, 157, 1478–1486. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant. Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Lonati, M.; Cioni, P.L.; Pistelli, L.; Najar, B.; Scariot, V. Latitude and altitude influence secondary metabolite production in peripheral alpine populations of the Mediterranean species Lavandula angustifolia Mill. Front. Plant. Sci. 2018, 9, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, S.A.; Fernandez, C.; Greff, S.; Torre, F.; Derridj, A.; Gauquelin, T.; Mevy, J.P. Inter-population variability of terpenoid composition in leaves of Pistacia lentiscus L. from Algeria: A chemoecological approach. Molecules 2011, 16, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Martz, F.; Peltola, R.; Fontanay, S.; Duval, E.R.; Julkunen-Tiittol, R.; Stark, S. Effect of latitude and altitude on the terpenoid and soluble phenolic composition of juniper (Juniperus communis) needles and evaluation of their antibacterial activity in the boreal zone. J. Agric. Food Chem. 2009, 50, 9575–9584. [Google Scholar] [CrossRef]
- Schönwitz, R.; Lohwasser, K.; Kloos, M.; Ziegler, H. Seasonal variation in the monoterpenes in needles of Picea abies (L.) Karst. Trees 1990, 4, 34–40. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am. J. Bot. 2002, 87, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Gleizes, M.; Pauly, G.; Bernard-Dagan, C.; Jacques, R. Effect of light on terpene hydrocarbons synthesis in Pinus pinaster. Physiol. Plant. 1980, 50, 16–20. [Google Scholar] [CrossRef]
- Snow, M.D.; Bard, R.R.; Olszyk, D.M.; Minster, L.M.; Hager, A.N.; Tingey, D.T. Monoterpene levels in needles of Douglas fir exposed to elevated CO2 and temperature. Physiol. Plant. 2003, 117, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, A.; Koch, K.; Taipale, R.; Zimmer, I.; Schnitzler, J.-P.; Rinne, J. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant. Cell Environ. 2010, 33, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Bäck, J.; Hari, P.; Hakola, H.; Juurola, E.; Kulmala, M. Dynamics of monoterpene emissions in Pinus sylvestris during early spring. Boreal Environ. Res. 2005, 10, 409–424. [Google Scholar]
- Niinemets, U.; Reichstein, M.; Staudt, M.; Seufert, G.; Tenhunen, J.D. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant. Physiol. 2002, 130, 1371–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönwitz, R.; Merk, L.; Yiegler, H. Naturally occurring monoterpenoids in needles of Picea abies (L.) Karst. Trees 1987, 1, 88–93. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Wargula, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusia, J. Effects of carbon dioxide, water supply and seasonality on terpene content and emission by Rosmarinus officinalis. J. Chem. Ecol. 1996, 23, 979–993. [Google Scholar] [CrossRef] [Green Version]
- Pokorska, O.; Dewulf, J.; Amelynck, C.; Schoon, N.; Šimpraga, M.; Steppe, K.; Van Langenhove, H. Isoprene and terpenoid emissions from Abies alba: Identification and emission rates under ambient conditions. Atmos. Environ. 2012, 59, 501–508. [Google Scholar] [CrossRef]
- Kainulainen, P.; Oksanen, J.; Palomaki, V.; Holopainen, J.K.; Holopainen, T. Effect of drought and waterlogging stress on needle monoterpenes of Picea abies. Can. J. Bot. 1992, 70, 1613–1616. [Google Scholar] [CrossRef]
- Heller, W.; Rosemann, D.; Asswald, W.F.; Benz, B.; Schonwitz, R.; Lohwasser, K.; Kloos, M.; Sandermann, H., Jr. Biochemical response of Norway Spruce (Pice abies (L.) Karst.) towards 14-month exposure to ozone and acid mist: Part I—effect on polyphenol and monoterpene metabolism. Environ. Pollut. 1990, 64, 353–366. [Google Scholar] [CrossRef]
- Seufert, G.; Kotzias, D.; Sparta, C.; Versino, B. Volatile organics in Mediterranean shrubs and their potential role in a changing environment. In Anticipated Effects of a Changing Global Environment on Mediterranean Type Ecosystems; Oechel, W.C., Moreno, J.M., Eds.; Springer: Berlin, Germany, 1995; pp. 343–370. [Google Scholar] [CrossRef]
- Rudloff von, E. Seasonal variation of the terpenes of the leaves, buds and twigs of the blue spruce (Picea pungens). Can. J. Bot. 1975, 53, 2975–2982. [Google Scholar] [CrossRef]
- Riedlmeier, M.; Ghirardo, A.; Wenig, M.; Knappe, C.; Koch, K.; Georgii, E.; Dey, S.; Parker, J.E.; Schnitzler, J.-P.; Vlot, A.C. Monoterpenes support systemic acquired resistance within and between plants. Plant. Cell 2017, 29, 1440–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef]
- Urban, O.; Klem, K.; Ač, A.; Havránková, K.; Holišová, P.; Navrátil, M.; Zitová, M.; Kozlová, K.; Pokorný, R.; Šprtová, M.; et al. Impact of clear and cloudy sky conditions on the vertical distribution of photosynthetic CO2 uptake within a spruce canopy. Func. Ecol. 2012, 26, 46–55. [Google Scholar] [CrossRef]
- Loreto, F.; Nascetti, P.; Graverini, A.; Mannozzi, M. Emission and content of monoterpenes in intact and wounded needles of Mediterranean pine, Pinus pinea. Funct. Ecol. 2020, 14, 589–595. [Google Scholar] [CrossRef]
- Kawoosa, T.; Singh, H.; Kumar, A.; Sharma, S.K.; Devi, K.; Dutt, S.; Vats, S.K.; Sharma, M.; Ahuja, P.S.; Kumar, S. Light and temperature regulated terpene biosynthesis: Hepatoprotective monoterpene picroside accumulation in Picrorhiza kurrooa. Funct. Integr. Genomics 2010, 10, 393–404. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Pavlovic, R.; Giorgi, A. Influence of altitude on phytochemical composition of hemp inflorescence: A metabolomic approach. Molecules 2020, 25, 1381. [Google Scholar] [CrossRef] [Green Version]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Geographic-variation of Greek oregano (Origanum vulgare ssp Hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; Maddalena, G.; Passera, A.; Casati, P.; Bianco, P.A.; Quaglino, F. Role of terpenes in plant defense to biotic stress. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 401–417. [Google Scholar] [CrossRef]
- Williams, K.K.; McMillin, J.D.; DeGomez, T.E.; Clancy, K.M.; Miller, A. Influence of elevation on bark beetle (Coleoptera: Curculionidae, Scolytinae) community structure and flight periodicity in ponderosa pine forests of Arizona. Environ. Entomol. 2014, 37, 94–109. [Google Scholar] [CrossRef] [Green Version]
- Dostálek, T.; Rokaya, M.B.; Maršík, P.; Rezek, J.; Skuhrovec, J.; Pavela, R.; Münzbergová, Z. Trade-off among different anti-herbivore defence strategies along an altitudinal gradient. AoB Plants 2016, 8, plw026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terhonen, E.; Langer, G.J.; Bußkamp, J.; Rӑscuţoi, D.R.; Blumenstein, K. Low water availability increases necrosis in Picea abies after artificial inoculation with fungal root rot pathogens Heterobasidion parviporum and Heterobasidion annosum. Forests 2019, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Llusià, J.; Peñuelas, J. Changes in terpene content and emission in potted Mediterranean woody plants under severe drought. Can. J. Bot. 1998, 76, 1366–1373. [Google Scholar] [CrossRef]
- Turtola, S.; Manninen, A.M.; Rikala, R.; Kainulainen, P. Drought stress alters the concentration of wood terpenoids in Scots pine and Norway spruce seedlings. J. Chem. Ecol. 2003, 29, 1981–1995. [Google Scholar] [CrossRef]
- Copolovici, L.; Niinemets, U. Environmental impacts on plant volatile emissions. In Deciphering Chemical Language of Plant Communication; Signaling and Communication in Plants; Blande, J.D., Glinwood, R., Eds.; Springer: Cham, Switzerland, 2016; pp. 175–210. [Google Scholar] [CrossRef]
- Nandini, Y.; Samir, S. Reactive oxygen species, oxidative stress and ROS scavenging system in plants. J. Chem. Pharm. Res. 2016, 8, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.R.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant. J. 2016, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Tiiva, P.; Rinnan, R.; Faubert, P.; Ranasen, J.; Holopainen, T.; Kyro, E.; Holopainen, J.K. Isoprene emission from a subarctic peatland under enhanced UV-B radiation. New Phytol. 2007, 176, 346–355. [Google Scholar] [CrossRef]
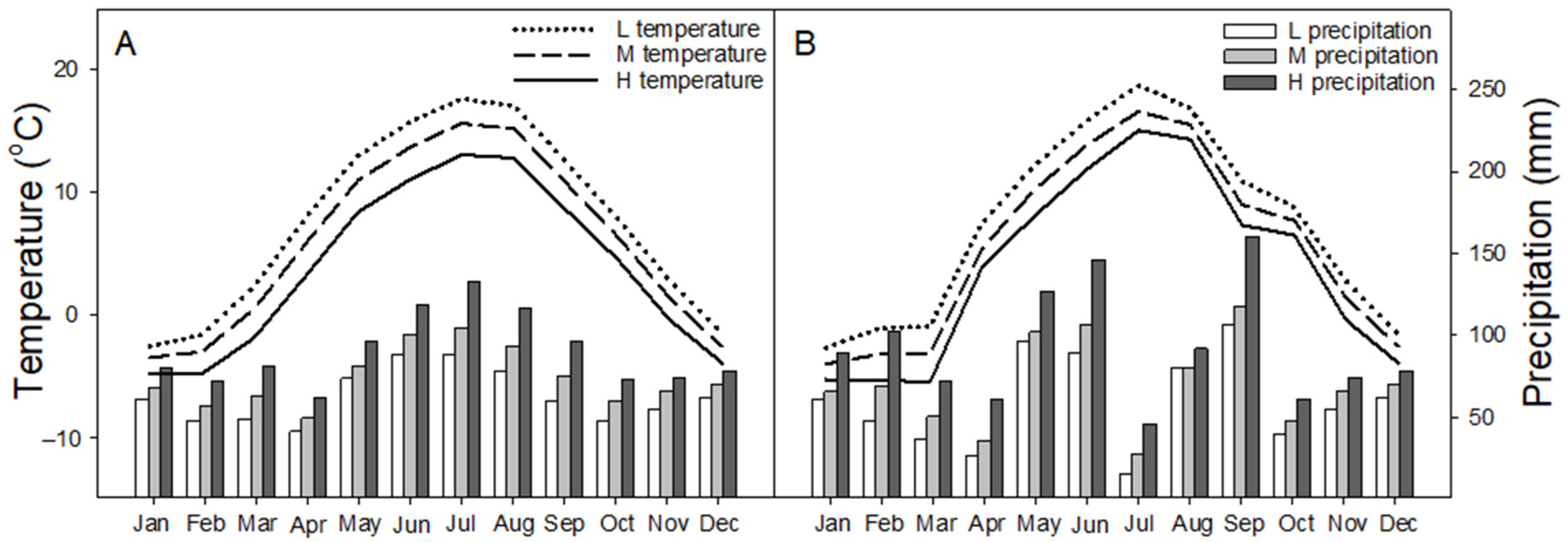


| L | M | H | |||||
|---|---|---|---|---|---|---|---|
| Summer | Autumn | Summer | Autumn | Summer | Autumn | ||
| Tree characteristics | |||||||
| H [m] | 36 ± 0.6 a | 32 ± 0.6 b | 28 ± 0.8 c | ||||
| DBH [cm] | 47 ± 1.8 a | 45 ± 1.7 a | 43 ± 1.5 a | ||||
| Needle characteristics | |||||||
| LMA [g m–2] | Upper | 284 ± 9.4 c | 422 ± 28.1 a | 254 ± 4.6 cd | 338 ± 10.9 b | 225 ± 7.8 d–f | 351 ± 10.3 b |
| Lower | 219 ± 14.1 d–f | 235 ± 10.1 d–f | 198 ± 10.9 f | 237 ± 14.4 de | 214 ± 11.2 e | 235 ± 18.1 d–f | |
| LWC [%] | Upper | 41 ± 0.7 d | 42 ± 0.4 cd | 37 ± 0.7 e | 43 ± 0.3 cd | 34 ± 1.5 f | 43 ± 0.5 cd |
| Lower | 46 ± 0.7 a | 44 ± 0.7 bc | 46 ± 1.1 ab | 43 ± 0.6 c | 47 ± 0.9 a | 43 ± 0.6 c | |
| Effect | A | C | S | A × C | A × S | C × S | A × C × S |
|---|---|---|---|---|---|---|---|
| Tricyclene | ≤0.001 | ≤0.001 | 0.002 | 0.306 | ≤0.001 | 0.126 | ≤0.001 |
| α-pinene | ≤0.001 | ≤0.001 | 0.358 | 0.136 | ≤0.001 | 0.499 | ≤0.001 |
| Camphene | ≤0.001 | ≤0.001 | 0.008 | 0.986 | 0.015 | 0.014 | ≤0.001 |
| β-pinene | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | 0.002 | ≤0.001 |
| Sabinene | ≤0.001 | ≤0.001 | ≤0.001 | 0.786 | ≤0.001 | ≤0.001 | ≤0.001 |
| Myrcene | 0.003 | ≤0.001 | 0.012 | 0.083 | 0.187 | 0.396 | 0.752 |
| Limonene | ≤0.001 | ≤0.001 | 0.669 | 0.007 | 0.609 | 0.009 | ≤0.001 |
| Eucalyptol | ≤0.001 | ≤0.001 | ≤0.001 | 0.030 | 0.002 | 0.004 | 0.004 |
| 3-caren | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
| Terpinolene | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
| Camphor | ≤0.001 | ≤0.001 | 0.011 | ≤0.001 | 0.550 | 0.190 | 0.363 |
| Fenchol | ≤0.001 | ≤0.001 | 0.003 | 0.084 | 0.030 | 0.662 | ≤0.001 |
| Borneol | ≤0.001 | 0.064 | 0.051 | 0.377 | 0.306 | 0.008 | ≤0.001 |
| α-terpineol | ≤0.001 | ≤0.001 | 0.422 | ≤0.001 | ≤0.001 | 0.102 | ≤0.001 |
| Bornyl acetate | ≤0.001 | ≤0.001 | ≤0.001 | 0.316 | ≤0.001 | 0.027 | ≤0.001 |
| β-caryophyllene | ≤0.001 | 0.012 | ≤0.001 | 0.348 | 0.041 | 0.015 | 0.465 |
| Humulene | 0.004 | 0.127 | 0.002 | 0.093 | 0.483 | 0.494 | 0.754 |
| Longifolene | ≤0.001 | ≤0.001 | ≤0.001 | 0.377 | ≤0.001 | 0.023 | 0.041 |
| β-cadinene | ≤0.001 | ≤0.001 | ≤0.001 | 0.023 | ≤0.001 | 0.455 | 0.977 |
| Germacrene D-4-ol | ≤0.001 | ≤0.001 | 0.454 | 0.090 | ≤0.001 | 0.097 | 0.769 |
| Total | ≤0.001 | ≤0.001 | 0.063 | 0.110 | 0.071 | 0.045 | 0.003 |
| Monoterpenes | ≤0.001 | ≤0.001 | 0.044 | 0.111 | 0.079 | 0.041 | ≤0.001 |
| Sesquiterpenes | ≤0.001 | ≤0.001 | 0.726 | 0.189 | 0.004 | 0.146 | 0.718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Večeřová, K.; Klem, K.; Veselá, B.; Holub, P.; Grace, J.; Urban, O. Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles. Forests 2021, 12, 1737. https://doi.org/10.3390/f12121737
Večeřová K, Klem K, Veselá B, Holub P, Grace J, Urban O. Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles. Forests. 2021; 12(12):1737. https://doi.org/10.3390/f12121737
Chicago/Turabian StyleVečeřová, Kristýna, Karel Klem, Barbora Veselá, Petr Holub, John Grace, and Otmar Urban. 2021. "Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles" Forests 12, no. 12: 1737. https://doi.org/10.3390/f12121737
APA StyleVečeřová, K., Klem, K., Veselá, B., Holub, P., Grace, J., & Urban, O. (2021). Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles. Forests, 12(12), 1737. https://doi.org/10.3390/f12121737







