On the Below- and Aboveground Phenology in Deciduous Trees: Observing the Fine-Root Lifespan, Turnover Rate, and Phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula Roth for Two Growing Seasons
Abstract
:1. Introduction
1.1. The Functions of Fine-Roots
1.2. Contribution of Fine-Roots to Forest Biomass and Production
1.3. The Fine-Root Lifespan, Turnover Rate, and Phenology of Temperate Deciduous Trees
1.4. Research Questions and Hypotheses
2. Methods
2.1. Description of the Sites
2.1.1. Field Sites and Experimental Lay-Out
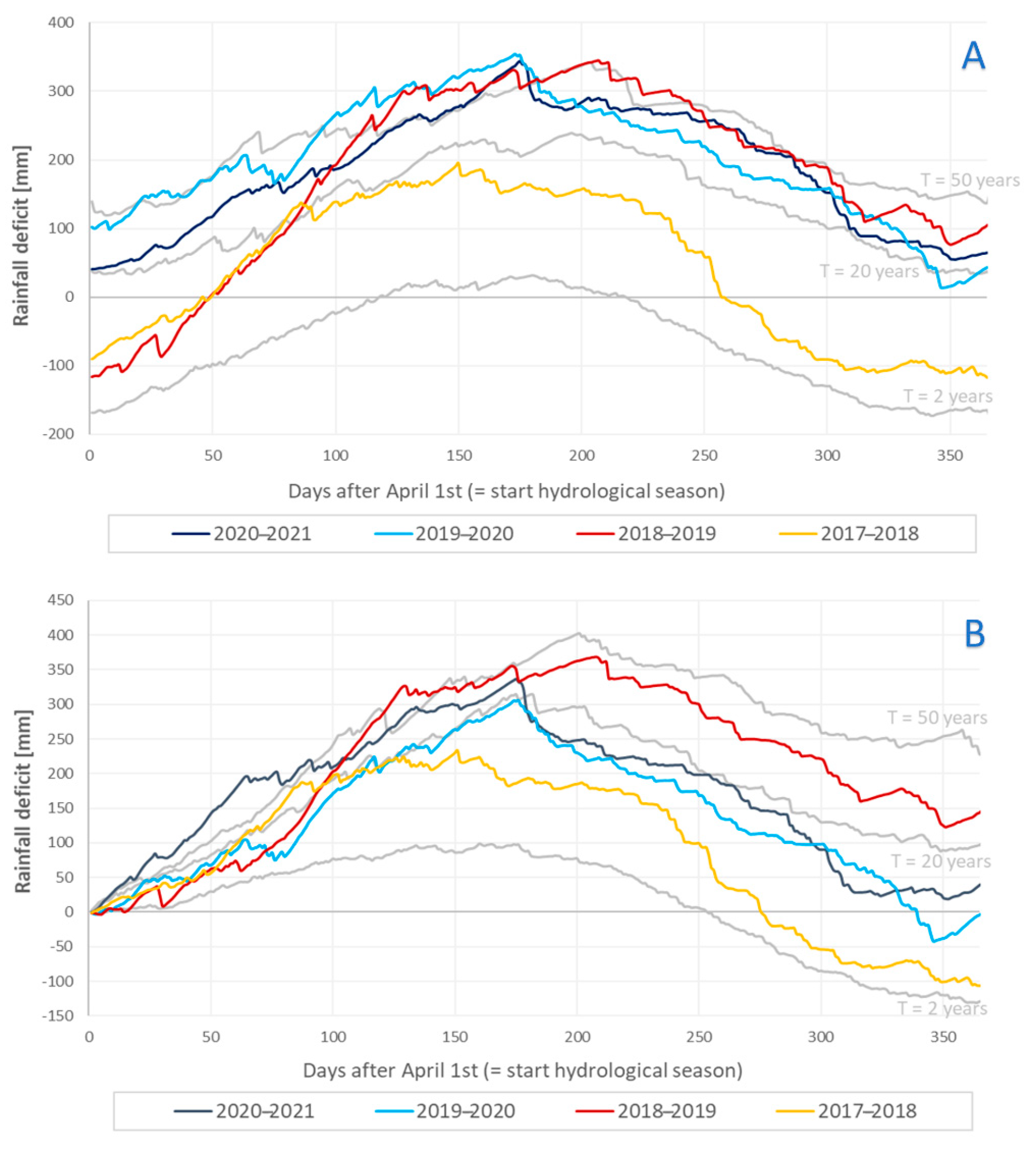
2.1.2. Meteorological Conditions
| Normal (1981–2010) | 2019 | 2020 | Normal (1991–2020) | 2021 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Winter | |
| Average temperature (°C) | 3.6 | 10.1 | 17.6 | 10.9 | 5.2 | 10.5 | 19.1 (++) | 11.3 | 6.3 (++) | 11.3 | 18.8 | 12.3 (+) | 4.1 | 4.7 |
| Total precipitation (mm) | 220.5 | 187.8 | 224.6 | 219.9 | 235.8 | 176.5 | 198.6 | 209.3 | 230.3 | 105.7 (-) | 168.2 | 219.2 | 228.6 | 264.1 |
| Average number of rainy days | 54.8 | 49 | 43.9 | 51 | 48 | 44 | 33 | 53 | 58 | 23 (---) | 46 | 43 | 55.2 | 54.8 |
| Relative humidity (%) | 84 | 74 | 73 | 82 | 84 (--) | 72 | 70 | 83 | 85 | 61 (--) | 66 (--) | 79 (--) | 84 | 84 |
| Sunshine duration (h:m) | 180:18 | 463:58 | 578:20 | 322:00 | 226:13 (+) | 489:42 | 714:38 (++) | 322:23 | 169:58 | 740:46 (+++) | 602:50 | 346:35 | 180:17 | 182:22 |
| Global solar radiation (kWh/m²) | 73.9 | 325 | 429.6 | 168.2 | 87.6 | 345.6 | 487.9 (+) | 178.4 | 73.3 | 61 (---) | 454.8 | 177 | 75.5 | 83.1 |
| Vapor pressure (hPa) | 6.9 | 9.2 | 14.5 | 11 | 6.9 | 9 | 15 | 11.2 | 8.2 (++) | 8.1 (--) | 13.9 | 11.2 | 7.1 | 7.4 |
| Air pressure (hPa) | 1017.3 | 1015.2 | 1016.2 | 1015.6 | 1016.4 | 1015.6 | 1015.4 | 1011 (--) | 1015.2 | 1017.8 | 1014.2 (--) | 1016.2 | 1017.1 | 1011.3 (-) |

2.1.3. Soil Conditions
2.2. Observing and Measuring Fine-Roots
2.3. Aboveground Phenological Data
2.4. Statistical Analyses
2.4.1. Detecting Trends in the Fine-Root Phenology Using Generalized Additive Mixed Models
g(𝔼(Yij)) = g(μij)
g(μij) = Speciesij+ f(Dayij, Speciesij) + Individual treei
g(𝔼(Yij)) = g(μij)
g(μij) = Speciesij+ f(Dayij, Speciesij) + Individual treei
g(𝔼(Yij)) = g(μij)
g(μij) = f(Dayij) + Individual treei
g(𝔼(Yij)) = g(μij)
g(μij) = f(Dayij) + Individual treei
2.4.2. Estimating the Fine-Root Lifespan and Turnover Rate Using Kaplan–Meier Survival Analyses
3. Results
3.1. Trends in the Cumulative Root Surface and Root Count
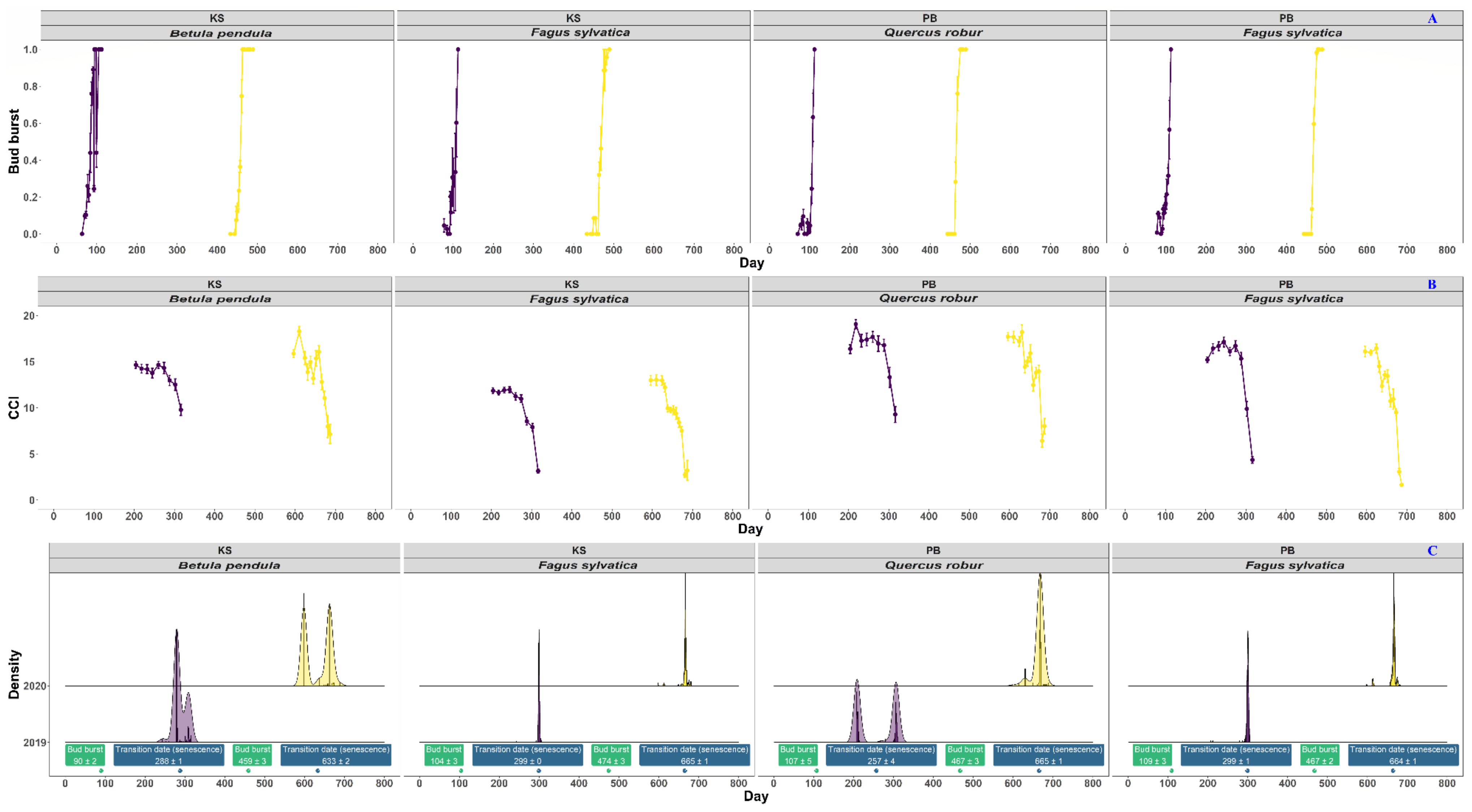
3.2. Linking the Above- and Belowground Phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula Roth
3.3. Fine-Root Lifespan and Turnover Rate
4. Discussion
4.1. The Trends (or Relative Lack Thereof) in the Fine-Root Phenology of Fagus Sylvatica, Quercus Robur, and Betula Pendula
4.2. Relationship between the Above- and Belowground Phenology of Deciduous Trees
4.3. Discussing the Fine-Root Lifespan and Turnover Rate
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogt, K.; Vogt, D.; Bloomfield, J. Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 1998, 200, 71–89. [Google Scholar] [CrossRef]
- Freschet, G.; Pagès, L.; Iversen, C.; Comas, L.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.; et al. A Starting Guide to Root Ecology: Strengthening Ecological Concepts and Standardizing Root Classification, Sampling, Processing and Trait Measurements. New Phytol. 2020, 232, 973–1122. [Google Scholar] [CrossRef]
- Lukac, M. Fine Root Turnover; Springer: Berlin/Heidelberg, Germany, 2012; pp. 363–373. [Google Scholar]
- Withington, J.M.; Goebel, M.; Bułaj, B.; Oleksyn, J.; Reich, P.B.; Eissenstat, D.M. Remarkable Similarity in Timing of Absorptive Fine-Root Production Across 11 Diverse Temperate Tree Species in a Common Garden. Front. Plant Sci. 2020, 11, 623722. [Google Scholar] [CrossRef]
- McCormack, M.; Dickie, I.; Eissenstat, D.; Fahey, T.; Fernandez, C.; Guo, D.; Helmisaari, H.-S.; Hobbie, E.; Iversen, C.; Jackson, R.; et al. Redefining fine roots improves understanding of belowground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Zadworny, M.; McCormack, M.L.; Mucha, J.; Reich, P.B.; Oleksyn, J. Scots pine fine roots adjust along a 2000-km latitudinal climatic gradient. New Phytol. 2016, 212, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagnoli, A.; Terzaghi, M.; Giussani, B.; Scippa, G.S.; Chiatante, D. An integrated method for high-resolution definition of new diameter-based fine root sub-classes of Fagus sylvatica L. Ann. For. Sci. 2018, 75, 76. [Google Scholar] [CrossRef] [Green Version]
- Majdi, H.; Pregitzer, K.; Morén, A.-S.; Nylund, J.-E.; Ågren, G.I. Measuring Fine Root Turnover in Forest Ecosystems. Plant Soil 2005, 276, 1–8. [Google Scholar] [CrossRef]
- Baddeley, J.A.; Watson, C.A. Influences of Root Diameter, Tree Age, Soil Depth and Season on Fine Root Survivorship in Prunus avium. Plant Soil 2005, 276, 15–22. [Google Scholar] [CrossRef]
- Brunner, I.; Godbold, D.L. Tree roots in a changing world. J. For. Res. 2007, 12, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [Green Version]
- Hamanishi, E.; Campbell, M. Genome-wide responses to drought in forest trees. Forestry 2011, 84, 273–283. [Google Scholar] [CrossRef]
- Holmes, R.; Likens, G. Chapter 09: Roots. In Hubbard Brook: The Story of a Forest Ecosystem; Fahey, T., Ed.; Yale University Press: New Haven, CT, USA, 2016; p. 288. [Google Scholar]
- Jackson, R.B.; Mooney, H.A.; Schulze, E.D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Leppälammi-Kujansuu, J.; Salemaa, M.; Schiestl-Aalto, P.; Kulmala, L.; Ukonmaanaho, L.; Nöjd, P.; Minkkinen, K.; Makita, N.; Železnik, P.; et al. Distinct patterns of below- and aboveground growth phenology and litter carbon inputs along a boreal site type gradient. For. Ecol. Manag. 2021, 489, 119081. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Aerts, R.; Bakker, C.; Caluwe, H. Root turnover as determinant of the cycling of C, N, and P in a dry heathland ecosystem. Biogeochemistry 1992, 15, 175–190. [Google Scholar] [CrossRef]
- Ruess, R.W.; Hendrick, R.L.; Bryant, J.P. Regulation of Fine Root Dynamics by Mammalian Browsers in Early Successional Alaskan Taiga Forests. Ecology 1998, 79, 2706–2720. [Google Scholar] [CrossRef]
- McCormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 2014, 95, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Addo-Danso, S.; Prescott, C.; Smith, A. Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: A review. For. Ecol. Manag. 2015, 359, 332–351. [Google Scholar] [CrossRef]
- Litton, C.M.; Raich, J.W.; Ryan, M.G. Carbon allocation in forest ecosystems. Glob. Chang. Biol. 2007, 13, 2089–2109. [Google Scholar] [CrossRef] [Green Version]
- Vogt, K.; Vogt, D.; Palmiotto, P.; Boon, P.; O’Hara, J.; Asbjornsen, H. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 1995, 187, 159–219. [Google Scholar] [CrossRef]
- Kubisch, P.; Hertel, D.; Leuschner, C. Fine Root Productivity and Turnover of Ectomycorrhizal and Arbuscular Mycorrhizal Tree Species in a Temperate Broad-Leaved Mixed Forest. Front. Plant Sci. 2016, 7, 1233. [Google Scholar] [CrossRef] [Green Version]
- Keyes, M.R.; Grier, C.C. Above- and below-ground net production in 40-year-old Douglas-fir stands on low and high productivity sites. Can. J. For. Res. 1981, 11, 599–605. [Google Scholar] [CrossRef]
- Müller-Haubold, H.; Hertel, D.; Seidel, D.; Knutzen, F.; Leuschner, C. Climate Responses of Aboveground Productivity and Allocation in Fagus sylvatica: A Transect Study in Mature Forests. Ecosystems 2013, 16, 1498–1516. [Google Scholar] [CrossRef]
- Timothy, J.F.; Hughes, J.W.; Mou, P.; Arthur, M.A. Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. For. Sci. 1988, 34, 744–768. [Google Scholar]
- Ostonen, I.; Lõhmus, K.; Pajuste, K. Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: Comparison of soil core and ingrowth core methods. For. Ecol. Manag. 2005, 212, 264–277. [Google Scholar] [CrossRef]
- Rosenvald, K.; Tullus, A.; Ostonen, I.; Uri, V.; Kupper, P.; Aosaar, J.; Varik, M.; Sõber, J.; Niglas, A.; Hansen, R. The effect of elevated air humidity on young silver birch and hybrid aspen biomass allocation and accumulation-acclimation mechanisms and capacity. For. Ecol. Manag. 2014, 330, 252–260. [Google Scholar] [CrossRef]
- Huang, Y.; Ciais, P.; Santoro, M.; Makowski, D.; Chave, J.; Schepaschenko, D.; Abramoff, R.; Goll, D.; Yang, H.; Chen, Y.; et al. A Global Map of Root Biomass across the World’s Forests. Earth Syst. Sci. Data 2021, 13, 4263–4274. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A. Critical analysis of root: Shoot ratios in terrestrial biomes. Glob. Chang. Biol. 2005, 12, 84–96. [Google Scholar] [CrossRef]
- Anderson, L.J.; Comas, L.H.; Lakso, A.N.; Eissenstat, D.M. Multiple risk factors in root survivorship: A 4-year study in Concord grape. New Phytol. 2003, 158, 489–501. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.; Raynal, D. Fine root growth phenology, production, and turnover in a northern hardwood forest ecosystem. Plant Soil 1994, 162, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Steinaker, D.F.; Wilson, S.D.; Peltzer, D.A. Asynchronicity in root and shoot phenology in grasses and woody plants. Glob. Chang. Biol. 2010, 16, 2241–2251. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T.; Yavitt, J.B. Environmental control of fine root dynamics in a northern hardwood forest. Glob. Chang. Biol. 2003, 9, 670–679. [Google Scholar] [CrossRef]
- Lyr, H.; Hoffmann, G. Growth Rates and Growth Periodicity of Tree Roots; Elsevier: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Mooney, H.A. The Carbon Balance of Plants. Annu. Rev. Ecol. Syst. 1972, 3, 315–346. [Google Scholar] [CrossRef]
- Waring, R.H.; Schlesinger, W.H. Forest Ecosystems: Concepts and Management; Academic Press: Cambridge, MA, USA, 1987; p. 340. [Google Scholar]
- Radville, L.; McCormack, M.L.; Post, E.; Eissenstat, D.M. Root phenology in a changing climate. J. Exp. Bot. 2016, 67, 3617–3628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a multidimensional root trait framework: A tree root review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weemstra, M.; Kiorapostolou, N.; van Ruijven, J.; Mommer, L.; de Vries, J.; Sterck, F. The role of fine-root mass, specific root length and life span in tree performance: A whole-tree exploration. Funct. Ecol. 2020, 34, 575–585. [Google Scholar] [CrossRef]
- Mohamed, A.; Stokes, A.; Mao, Z.; Jourdan, C.; Sabatier, S.; Pailler, F.; Fourtier, S.; Dufour, L.; Monnier, Y. Linking above- and belowground phenology of hybrid walnut growing along a climatic gradient in temperate agroforestry systems. Plant Soil 2018, 424, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Defrenne, C.E.; Childs, J.; Fernandez, C.W.; Taggart, M.; Nettles, W.R.; Allen, M.F.; Hanson, P.J.; Iversen, C.M. High-resolution minirhizotrons advance our understanding of root-fungal dynamics in an experimentally warmed peatland. Plants People Planet 2021, 3, 640–652. [Google Scholar] [CrossRef]
- Hertel, D.; Strecker, T.; Müller-Haubold, H.; Leuschner, C. Fine root biomass and dynamics in beech forests across a precipitation gradient—Is optimal resource partitioning theory applicable to water-limited mature trees? J. Ecol. 2013, 101, 1183–1200. [Google Scholar] [CrossRef]
- Fogel, R. Root turnover and productivity of coniferous forests. Plant Soil 1983, 71, 75–85. [Google Scholar] [CrossRef]
- Chapin, F.S.; Woodwell, G.M.; Randerson, J.T.; Rastetter, E.B.; Lovett, G.M.; Baldocchi, D.D.; Clark, D.A.; Harmon, M.E.; Schimel, D.S.; Valentini, R.; et al. Reconciling Carbon-cycle Concepts, Terminology, and Methods. Ecosystems 2006, 9, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Iversen, C.M. Digging deeper: Fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol. 2010, 186, 346–357. [Google Scholar] [CrossRef]
- Ostle, N.J.; Smith, P.; Fisher, R.; Ian Woodward, F.; Fisher, J.B.; Smith, J.U.; Galbraith, D.; Levy, P.; Meir, P.; McNamara, N.P.; et al. Integrating plant-soil interactions into global carbon cycle models. J. Ecol. 2009, 97, 851–863. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root lifespan. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 27, pp. 1–60. [Google Scholar]
- Brunner, I.; Bakker, M.R.; Björk, R.G.; Hirano, Y.; Lukac, M.; Aranda, X.; Børja, I.; Eldhuset, T.D.; Helmisaari, H.S.; Jourdan, C.; et al. Fine-root turnover rates of European forests revisited: An analysis of data from sequential coring and ingrowth cores. Plant Soil 2013, 362, 357–372. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. Fuel Energy Abstr. 2011, 262, 2008–2023. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Fahey, T.J.; Horton, T.R.; Lovett, G.M. Belowground Ectomycorrhizal Fungal Community Change over a Nitrogen Deposition Gradient in Alaska. Ecology 2002, 83, 104–115. [Google Scholar] [CrossRef]
- Bakker, M.R. The effect of lime and gypsum applications on a sessile oak (Quercus petraea (M.) Liebl.) stand at La Croix-Scaille (French Ardennes) II. Fine root dynamics. Plant Soil 1999, 206, 109–121. [Google Scholar] [CrossRef]
- Børja, I.; de Wit, H.A.; Steffenrem, A.; Majdi, H. Stand age and fine root biomass, distribution and morphology in a Norway spruce chronosequence in southeast Norway. Tree Physiol. 2008, 28, 773–784. [Google Scholar] [CrossRef]
- Eldhuset, T.; Lange, H.; de Wit, H. Fine root biomass, necromass and chemistry during seven years of elevated aluminium concentrations in the soil solution of a middle-aged Picea abies stand. Sci. Total Environ. 2006, 369, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.H.W. Feinwurtel-Verteilung, -Vitalität, -Produktion und -umsatz voon Fichten (Picea abies (L.) Karst.) auf unterschiedlich versauerten Standorten. Ben. Forsch. Zent. Waldökosyst. 1999, 165, 1–11. [Google Scholar]
- Gaul, D.; Hertel, D.; Leuschner, C. Estimating fine root longevity in a temperate Norway spruce forest using three independent methods. Funct. Plant Biol. 2009, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Hertel, D. Das Feinwurzelsystem von Rein- und Mischbeständen der Rotbuche: Struktur, Dynamik und interspezifische Konkurrenz. Diss. Bot. 1999, 317, 1–185. [Google Scholar]
- Persson, H.; Stadenberg, I. Fine root dynamics in a Norway spruce forest (Picea abies (L.) Karst) in eastern Sweden. Plant Soil 2009, 330, 329–344. [Google Scholar] [CrossRef]
- Richter, A.K. Fine Root Growth and Vitality of European Beech in Acid Forest Soils with a Low Base Saturation; ETH: Zurich, Switzerland, 2007. [Google Scholar]
- Wu, K. Fine Root Production and Turnover and Its Contribution to Nutrient Cycling in Two Beech (Fagus sylvatica L.) Forest Ecosystems; Forschungszentrum Waldökosysteme: Göttingen, Germany, 2000. [Google Scholar]
- Hansson, K.; Helmisaari, H.-S.; Sah, S.; Lange, H. Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For. Ecol. Manag. 2013, 309, 58–65. [Google Scholar] [CrossRef]
- Peek, M.S. Explaining variation in fine root life span. In Progress in Botany; Esser, K., Löttge, U., Beyschlag, W., Murata, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 382–398. [Google Scholar]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Comparisons of Structure and Life Span in Roots and Leaves among Temperate Trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Cienciala, E.; Tatarinov, F. Application of BIOME-BGC model to managed forests: 2. Comparison with long-term observations of stand production for major tree species. For. Ecol. Manag. 2006, 237, 252–266. [Google Scholar] [CrossRef]
- Ponti, F.; Minotta, G.; Cantoni, L.; Bagnaresi, U. Fine root dynamics of pedunculate oak and narrow-leaved ash in a mixed-hardwood plantation in clay soils. Plant Soil 2004, 259, 39–49. [Google Scholar] [CrossRef]
- Van Praag, H.J.; Sougnez-Remy, S.; Weissen, F.; Carletti, G. Root turnover in a beech and a spruce stand of the Belgian Ardennes. Plant Soil 1988, 105, 87–103. [Google Scholar] [CrossRef]
- Dox, I.; Gricar, J.; Marchand, L.J.; Leys, S.; Zuccarini, P.; Geron, C.; Prislan, P.; Marien, B.; Fonti, P.; Lange, H.; et al. Timeline of autumn phenology in temperate deciduous trees. Tree Physiol. 2020, 40, 1001–1013. [Google Scholar] [CrossRef]
- Dox, I.; Prislan, P.; Gricar, J.; Mariën, B.; Delpierre, N.; Flores, O.; Leys, S.; Rathgeber, C.B.K.; Fonti, P.; Campioli, M. Drought elicits contrasting responses on the autumn dynamics of wood formation in late successional deciduous tree species. Tree Physiol. 2021, 41, 1171–1185. [Google Scholar] [CrossRef]
- Mariën, B.; Balzarolo, M.; Dox, I.; Leys, S.; Lorene, M.J.; Geron, C.; Portillo-Estrada, M.; AbdElgawad, H.; Asard, H.; Campioli, M. Detecting the onset of autumn leaf senescence in deciduous forest trees of the temperate zone. New Phytol. 2019, 224, 166–176. [Google Scholar] [CrossRef]
- Mariën, B.; Dox, I.; de Boeck, H.J.; Willems, P.; Leys, S.; Papadimitriou, D.; Campioli, M. Does drought advance the onset of autumn leaf senescence in temperate deciduous forest trees? Biogeosciences 2021, 18, 3309–3330. [Google Scholar] [CrossRef]
- Marchand, L.J.; Dox, I.; Gričar, J.; Prislan, P.; Leys, S.; van den Bulcke, J.; Fonti, P.; Lange, H.; Matthysen, E.; Peñuelas, J.; et al. Inter-individual variability in spring phenology of temperate deciduous trees depends on species, tree size and previous year autumn phenology. Agric. For. Meteorol. 2020, 290, 108031. [Google Scholar] [CrossRef] [PubMed]
- Mariën, B.; Papadimitriou, D.; Kotilainen, T.; Dox, I.; Verlinden, M.; Heinecke, T.; Mariën, J.; Campioli, M. Timing leaf senescence, a GAMLSS approach. 2021; In review. [Google Scholar]
- De vos, B. Capability of PlantCare Mini-Logger Technology for Monitoring of Soil Water Content and Temperature in Forest Soils: Test Results of 2015; Instituut voor Natuur-en Bosonderzoek: Brussels, Belgium, 2016; p. 85. [Google Scholar]
- Carrara, A.; Kowalski, A.S.; Neirynck, J.; Janssens, I.A.; Yuste, J.C.; Ceulemans, R. Net ecosystem CO2 exchange of mixed forest in Belgium over 5 years. Agric. For. Meteorol. 2003, 119, 209–227. [Google Scholar] [CrossRef]
- KMI. Klimatologisch Seizoenoverzicht, Herfst 2019; KMI: Brussels, Belgium, 2019. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Zomer 2019; KMI: Brussels, Belgium, 2019. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Winter 2019; KMI: Brussels, Belgium, 2019. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Lente 2019; KMI: Brussels, Belgium, 2019. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Winter 2020; KMI: Brussels, Belgium, 2020. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Lente 2020; KMI: Brussels, Belgium, 2020. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Zomer 2020; KMI: Brussels, Belgium, 2020. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Herfst 2020; KMI: Brussels, Belgium, 2020. [Google Scholar]
- KMI. Klimatologisch Seizoenoverzicht, Winter 2021; KMI: Brussels, Belgium, 2021. [Google Scholar]
- Buck, A.L. New Equations for Computing Vapor Pressure and Enhancement Factor. J. Appl. Meteorol. 1981, 20, 1527–1532. [Google Scholar] [CrossRef] [Green Version]
- Bultot, F.; Coppens, A.; Dupriez, G.L. Estimation de L’évapotranspiration Potentielle en Belgique: (Procédure Révisée); Institut Royal Météorologique de Belgique: Brussels, Belgium, 1983. [Google Scholar]
- Penman, H.L. Natural evaporation from open water, hare soil and grass. Proc. R. Soc. Lond. A Math. Phys. Sci. 1948, 193, 120–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguis, P.; Roulin, E.; Willems, P.; Ntegeka, V. Climate change scenarios for precipitation and potential evapotranspiration over central Belgium. Theor. Appl. Climatol. 2010, 99, 273–286. [Google Scholar] [CrossRef]
- Willems, P. Compound intensity/duration/frequency-relationships of extreme precipitation for two seasons and two storm types. J. Hydrol. 2000, 233, 189–205. [Google Scholar] [CrossRef]
- Willems, P. Multidecadal oscillatory behaviour of rainfall extremes in Europe. Clim. Chang. 2013, 120, 931–944. [Google Scholar] [CrossRef] [Green Version]
- Dondeyne, S.; van Ranst, E.; Deckers, J.; Bouhon, A.; Chapelle, J.; Vancampenhout, K.; Baert, G. Converting the Legend of the Soil Map of Belgium to World Reference Base for Soil Resources: Case Studies of the Flemish Region; Vlaamse Overheid, Departement Leefmilieu, Natuurlijke Rijkdommen: Bruseels, Belgium, 2012; p. 121. [Google Scholar]
- Van Ranst, E.; Sys, C. Eenduidige Legende voor de Digitale Bodemkaart van Vlaanderen (Schaal 1:20.000); Laboratorium Voor Bodemkunde (Universiteit Gent): Ghent, Belgium, 2000; p. 361. [Google Scholar]
- VPO. Databank Ondergrond Vlaanderen; Flanders: Antwerp, Belgium, 2017. [Google Scholar]
- FAO. Soil Map of the World. Revised Legend; Soils Bulletin; FAO: Rome, Italy, 1988. [Google Scholar]
- FAO-UNESCO. Soil Map of the World; FAO: Rome, Italy, 1974. [Google Scholar]
- Chesworth, W.; Spaargaren, O.; Hadas, A. Thermal regime. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Encyclopedia of Earth Sciences Series; Springer: Berlin/Heidelberg, Germany, 2008; pp. 767–772. [Google Scholar]
- Chesworth, W. Moisture regimes. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Encyclopedia of Earth Sciences Series; Springer: Berlin/Heidelberg, Germany, 2008; p. 485. [Google Scholar]
- Joslin, J.D.; Wolfe, M.H. Disturbances During Minirhizotron Installation Can Affect Root Observation Data. Soil Sci. Soc. Am. J. 1999, 63, 218–221. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.-Y.; Louvet, J.-M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Rigby, R.A.; Stasinopoulos, D.M. Generalized additive models for location, scale and shape. J. R. Stat. Soc. Ser. C-Appl. Stat. 2005, 54, 507–544. [Google Scholar] [CrossRef] [Green Version]
- Akanztiliotou, K.; Rigby, R.; Stasinopoulos, D. The R implementation of Generalized Additive Models for Location, Scale and Shape. Stat. Model. Soc. 2002, 54, 83. [Google Scholar]
- Rigby, R.; Stasinopoulos, D. The GAMLSS project: A flexible approach to statistical modelling. In Proceedings of the 16th International Workshop on Statistical Modelling, Odense, Denmark, 2–6 July 2001. [Google Scholar]
- Rasool, S.; Mir, B.A.; Rehman, M.U.; Amin, I.; Mir, M.U.R.; Ahmad, S.B. Abiotic stress and plant senescence. In Senescence Signalling and Control in Plants; Sarwat, M., Tuteja, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 15–27. [Google Scholar]
- Munne-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant. Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Garnier, S. Viridis: Default Color Maps from ‘Matplotlib’; R Package Version 0.5.1; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation; R Package Version 0.7.4; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’; R Package Version 1.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Simpson, G.L. Gratia: Graceful ‘ggplot’-Based Graphics and Other Functions for GAMs Fitted Using ‘mgcv’; R Package Version 0.3.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef] [Green Version]
- Simpson, G.L. Modelling Palaeoecological Time Series Using Generalised Additive Models. Front. Ecol. Evol. 2018, 6, 149. [Google Scholar] [CrossRef] [Green Version]
- Stasinopoulos, D.; Rigby, R.; Heller, G.; Voudouris, V.; de Bastiani, F. Flexible Regression and Smoothing: Using GAMLSS in R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Eilers, P.H.C.; Marx, B.D. Flexible smoothing with B-splines and penalties. Statist. Sci. 1996, 11, 89–121. [Google Scholar] [CrossRef]
- Eilers, P.; Marx, B.; Durbán, M. Twenty years of P-splines. Stat. Oper. Res. Trans. 2015, 39, 149–186. [Google Scholar]
- Reiss, P.; Ogden, R. Smoothing parameter selection for a class of semiparametric linear models. J. R. Stat. Soc. Ser. B 2009, 71, 505–523. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R; R Package Version 3.2-7; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Therneau, T.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Kassambra, A.; Kosinki, M.; Biecek, P. Survminer: Drawing Survival Curves Using ‘ggplot2’; R Package Version 0.4.9; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Harrington, D.P.; Fleming, T.R. A Class of Rank Test Procedures for Censored Survival Data. Biometrika 1982, 69, 553–566. [Google Scholar] [CrossRef]
- Bobinac, M.; Batos, B.; Miljković, D.; Radulovic, S. Polycyclism and Phenological Variability in the Common Oak (Quercus robur L.). Arch. Biol. Sci. 2012, 64, 97–105. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.J.; Benham, D.G.; Marland, L.A.; Fitter, A.H. Root production is determined by radiation flux in a temperate grassland community. Glob. Chang. Biol. 2004, 10, 209–227. [Google Scholar] [CrossRef] [Green Version]
- King, J.S.; Albaugh, T.J.; Allen, H.L.; Buford, M.; Strain, B.R.; Dougherty, P. Below-ground carbon input to soil is controlled by nutrient availability and fine root dynamics in loblolly pine. New Phytol. 2002, 154, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought responses at leaf, stem and fine root levels of competitive Fagus sylvatica L. and Quercus petraea (Matt.) Liebl. trees in dry and wet years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Newman, E.I. Relationship between Root Growth of Flax (Linum Usitatissimum) and Soil Water Potential. New Phytol. 1966, 65, 273–283. [Google Scholar] [CrossRef]
- Tryon, P.R.; Chapin, F.S., III. Temperature control over root growth and root biomass in taiga forest trees. Can. J. For. Res. 1983, 13, 827–833. [Google Scholar] [CrossRef]
- Comas, L.H.; Anderson, L.J.; Dunst, R.M.; Lakso, A.N.; Eissenstat, D.M. Canopy and environmental control of root dynamics in a long-term study of Concord grape. New Phytol. 2005, 167, 829–840. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Stasinopoulos, M.D.; Rigby, R.A.; Bastiani, F.D. GAMLSS: A distributional regression approach. Stat. Model. 2018, 18, 248–273. [Google Scholar] [CrossRef]
- Stasinopoulos, D.M.; Rigby, B. gamlss.dist: Distributions for Generalized Additive Models for Location Scale and Shape; Version 5.1-6; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- McDonald, J. Some Generalized Functions for the Size Distribution of Income. Econometrica 1984, 52, 647–663. [Google Scholar] [CrossRef]
- McDonald, J.B.; Xu, Y.J. A generalization of the beta distribution with applications. J. Econom. 1995, 66, 133–152. [Google Scholar] [CrossRef]
- McDonald, J.B. 14 Probability distributions for financial models. In Handbook of Statistics; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 427–461. [Google Scholar]
- López, B.; Sabaté, S.; Gracia, C.A. Annual and seasonal changes in fine root biomass of a Quercus ilex L. forest. Plant Soil 2001, 230, 125–134. [Google Scholar] [CrossRef]
- Kuhns, M.; Garrett, H.; Teskey, R.; Hinckley, T. Root Growth of Black Walnut Trees Related to Soil Temperature, Soil Water Potential, and Leaf Water Potential. For. Sci. 1985, 31, 617–629. [Google Scholar]
- Webb, D.P. Root Growth in Acer saccharum Marsh. Seedlings: Effects of Light Intensity and Photoperiod on Root Elongation Rates. Bot. Gaz. 1976, 137, 211–217. [Google Scholar] [CrossRef]
- Teskey, R.O.; Hinckley, T.M. Influence of temperature and water potential on root growth of white oak. Physiologia Plantarum 1981, 52, 363–369. [Google Scholar] [CrossRef]
- Liang, E.; Balducci, L.; Ren, P.; Rossi, S. Chapter 3—Xylogenesis and moisture stress. In Secondary Xylem Biology; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 45–58. [Google Scholar]
- Sass-Klaassen, U.; Sabajo, C.R.; den Ouden, J. Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia 2011, 29, 171–175. [Google Scholar] [CrossRef]
- Puchałka, R.; Koprowski, M.; Gričar, J.; Przybylak, R. Does tree-ring formation follow leaf phenology in Pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 2017, 136, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Rathgeber, C.B.K.; Cuny, H.E.; Fonti, P. Biological Basis of Tree-Ring Formation: A Crash Course. Front. Plant Sci. 2016, 7, 734. [Google Scholar] [CrossRef] [Green Version]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the intra-annual wood formation of three European species (Fagus sylvatica, Quercus petraea and Pinus sylvestris) as related to leaf phenology and non-structural carbohydrate dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, E.; Baas, P.; Gasson, P. IAWA List of Microcopie Features for Hardwood Identification. IAWA J. 1989, 10, 219–332. [Google Scholar] [CrossRef]
- Marchand, L.J.; Dox, I.; Gričar, J.; Prislan, P.; van den Bulcke, J.; Fonti, P.; Campioli, M. Timing of spring xylogenesis in temperate deciduous tree species relates to tree growth characteristics and previous autumn phenology. Tree Physiol. 2021, 41, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Côté, B.; Bélanger, N.; Courchesne, F.; Fyles, J.; Hendershot, W. A cyclical but asynchronous pattern of fine root and woody biomass production in a hardwood forest of southern Quebec and its relationships with annual variation of temperature and nutrient availability. Plant Soil 2003, 250, 49–57. [Google Scholar] [CrossRef]
- Dougherty, P.M.; Teskey, R.O.; Phelps, J.E.; Hinckley, T.M. Net Photosynthesis and Early Growth Trends of a Dominant White Oak (Quercus alba L.). Plant Physiol. 1979, 64, 930. [Google Scholar] [CrossRef] [Green Version]
- Farrar, J.F.; Jones, D.L. The control of carbon acquisition by roots. New Phytol. 2000, 147, 43–53. [Google Scholar] [CrossRef]
- Konôpka, B.; Curiel Yuste, J.; Janssens, I.; Ceulemans, R. Comparison of Fine Root Dynamics in Scots Pine and Pedunculate Oak in Sandy Soil. Plant Soil 2005, 276, 33–45. [Google Scholar] [CrossRef]
- Brundrett, M.; Kendrick, B. The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can. J. Bot. 1988, 66, 1153–1173. [Google Scholar] [CrossRef]
- Engler, A. Untersuchungen über das Wurzelwachstum der Holzarten Mittelungen des Forstliches Versuchswezen. Fäsi Beer 1903, 7, 243–317. [Google Scholar]
- Leibundgut, H.; Dafis, S.; Richard, E. Untersuchungen über das Wurzelwachstum verschiedener Baumarten. Schweiz. Z. Forstwes. 1963, 114, 621–646. [Google Scholar]
- Noguchi, K.; Sakata, T.; Mizoguchi, T.; Takahashi, M. Estimating the production and mortality of fine roots in a Japanese cedar (Cryptomeria japonica D. Don) plantation using a minirhizotron technique. J. For. Res. 2005, 10, 435–441. [Google Scholar] [CrossRef]
- Norby, R.J.; Ledford, J.; Reilly, C.D.; Miller, N.E.; O’Neill, E.G. Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc. Natl. Acad. Sci. USA 2004, 101, 9689–9693. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Montagnoli, A.; di Iorio, A.; Terzaghi, M.; Trupiano, D.; Scippa, G.; Chiatante, D. Influence of soil temperature and water content on fine-root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 2014, 133, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Joslin, J.D.; Wolfe, M.H.; Hanson, P.J. Effects of altered water regimes on forest root systems. New Phytol. 2000, 147, 117–129. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Häberle, K.-H.; Matyssek, R.; Matzner, E.; Borken, W. Effects of drought stress on photosynthesis, rhizosphere respiration, and fine-root characteristics of beech saplings: A rhizotron field study. J. Plant Nutr. Soil Sci. 2014, 177, 168–177. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Offermann, C.A.; Gessler, A.; Winkler, J.B.; Ferrio, J.P.; Buchmann, N.; Barnard, R.L. Drought effects on allocation of recent carbon: From beech leaves to soil CO2 efflux. New Phytol. 2009, 184, 950–961. [Google Scholar] [CrossRef]
- Herzog, C.; Steffen, J.; Pannatier, E.; Hajdas, I.; Brunner, I. Nine Years of Irrigation Cause Vegetation and Fine Root Shifts in a Water-Limited Pine Forest. PLoS ONE 2014, 9, e96321. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z. Fine Root Biomass, Production, Turnover Rates, and Nutrient Contents in Boreal Forest Ecosystems in Relation to Species, Climate, Fertility, and Stand Age: Literature Review and Meta-Analyses. Crit. Rev. Plant Sci. 2010, 29, 204. [Google Scholar] [CrossRef]
- Leuschner, C.; Hertel, D.; Schmid, I.; Koch, O.; Muhs, A.; Hölscher, D. Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility. Plant Soil 2004, 258, 43–56. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Sharp, R.; Lenoble, M.; Else, M.; Thorne, E.; Gherardi, F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: Evidence for an interaction with ethylene. J. Exp. Bot. 2000, 51, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smet, I. Abscisic Acid in Root Growth and Development. Front. Plant Sci. 2013, 32, 11–16. [Google Scholar]
- Stober, C.; George, E.; Persson, H. Root growth and response to nitrogen. In Carbon and Nitrogen Cycling in European Forest Ecosystems; Schulze, E.-D., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 99–121. [Google Scholar]
- Hickler, T.; Smith, B.; Prentice, I.C.; MjÖFors, K.; Miller, P.; Arneth, A.; Sykes, M.T. CO2 fertilization in temperate FACE experiments not representative of boreal and tropical forests. Glob. Chang. Biol. 2008, 14, 1531–1542. [Google Scholar] [CrossRef]
- Pietsch, S.; Hasenauer, H.; Thornton, P. BGC-model parameters for tree species growing in central European forests. For. Ecol. Manag. 2005, 211, 264–295. [Google Scholar] [CrossRef]
- White, M.; Thornton, P.; Running, S.; Nemani, R. Parameterization and Sensitivity Analysis of the BIOME–BGC Terrestrial Ecosystem Model: Net Primary Production Controls. Earth Interact. 2000, 4, 1–85. [Google Scholar] [CrossRef]
- Rasse, D.P.; Rumpel, C.; Dignac, M.-F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 2005, 269, 341–356. [Google Scholar] [CrossRef]
- McCormack, M.L.; Guo, D. Impacts of environmental factors on fine root lifespan. Front. Plant Sci. 2014, 5, 205. [Google Scholar] [CrossRef] [Green Version]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Withington, J.M.; Elkin, A.D.; Bułaj, B.; Olesiński, J.; Tracy, K.N.; Bouma, T.J.; Oleksyn, J.; Anderson, L.J.; Modrzyński, J.; Reich, P.B.; et al. The impact of material used for minirhizotron tubes for root research. New Phytol. 2003, 160, 533–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Yi | Model Equation | Family Distribution | Link Function | Adjusted R² | Site | Smooth Term | Species | Edf | F or Chi.sq | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| New root count | g(𝔼(yi)) = f1 (Dayi) + ζID + εi | Negative binomial | Logarithmic | 0.28 | PB | Day | Fagus sylvatica L. | 6 | 18 | <0.05 |
| New root count | g(𝔼(yi)) = β1Speciesi + f1Speciesi(Dayi) + ζID + εi | Negative binomial | Logarithmic | 0.11 | KS | Day | Fagus sylvatica L. | 1 | 30 | <0.001 |
| PB | Quercus robur L. | 2.6 | 59 | <0.001 | ||||||
| KS | Betula pendula Roth | 7.5 | 89 | <0.001 | ||||||
| Cumulative root surface | g(𝔼(yi)) = f1(Dayi) + ζID + εi | Gamma | Inverse | 0.2 | PB | Day | Fagus sylvatica L. | 5.3 | 3.6 | <0.01 |
| Cumulative root surface | g(𝔼(yi)) = β1Speciesi + f1Speciesi(Dayi) + ζID + εi | Gamma | Inverse | 0.25 | KS | Day | Fagus sylvatica L. | 4 | 4.2 | <0.001 |
| PB | Quercus robur L. | 2.8 | 6.5 | <0.001 | ||||||
| KS | Betula pendula Roth | 1 | 0.6 | ns |
| Survival Curve Per Species (with Root Diameter < 2 mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | n | Events | Mean Lifespan (Days) | Median Lifespan (Days) | Root Turnover Rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Fagus sylvatica | 4272 | 4165 | 412 | 3 | 470 | 463 | 470 | 0.78 | 0.79 | 0.78 |
| Quercus robur | 2511 | 2434 | 442 | 4 | 460 | 447 | 468 | 0.79 | 0.82 | 0.78 |
| Betula pendula | 6529 | 6228 | 426 | 3 | 440 | 408 | 470 | 0.83 | 0.89 | 0.78 |
| Survival Curve for Fagus sylvatica Per Root Diameter Class | ||||||||||
| Root Diameter Class | n | Events | Mean lifespan (Days) | Median lifespan (Days) | Root turnover rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Root Diameter > 2 mm | 2321 | 2260 | 418 | 4 | 444 | 435 | 447 | 0.82 | 0.84 | 0.82 |
| 1 mm < Root Diameter > 2 mm | 3470 | 3371 | 409 | 3 | 470 | 447 | 470 | 0.78 | 0.82 | 0.78 |
| 0.5 mm < Root Diameter > 1 mm | 516 | 513 | 438 | 8 | 470 | 435 | 470 | 0.78 | 0.84 | 0.78 |
| Root Diameter < 0.5 mm | 286 | 281 | 399 | 12 | 511 | 310 | 511 | 0.71 | 1.18 | 0.71 |
| Survival Curve for Fagus sylvatica Per Season of Root Birth (with Root Diameter < 2 mm) | ||||||||||
| Season of Root Birth | n | Events | Mean Lifespan (Days) | Median Lifespan (Days) | Root Turnover Rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Autumn | 1497 | 1497 | 399 | 5 | 470 | 447 | 470 | 0.78 | 0.82 | 0.78 |
| Spring | 884 | 884 | 406 | 6 | 441 | 379 | 470 | 0.83 | 0.96 | 0.78 |
| Summer | 1271 | 1271 | 428 | 5 | 470 | 470 | 495 | 0.78 | 0.78 | 0.74 |
| Winter | 620 | 513 | 417 | 7 | 447 | 406 | 470 | 0.82 | 0.90 | 0.78 |
| Survival Curve for Quercus robur Per Root Diameter Class | ||||||||||
| Root Diameter Class | n | Events | Mean Lifespan (Days) | Median Lifespan (Days) | Root Turnover Rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Root diameter > 2 mm | 1468 | 1408 | 471 | 5 | 526 | 506 | 555 | 0.69 | 0.72 | 0.66 |
| 1 mm < root diameter > 2 mm | 2001 | 1935 | 446 | 4 | 460 | 460 | 468 | 0.79 | 0.79 | 0.78 |
| 0.5 mm < root diameter > 1 mm | 299 | 290 | 502 | 8 | 499 | 460 | 534 | 0.73 | 0.79 | 0.68 |
| Root diameter < 0.5 mm | 211 | 209 | 308 | 14 | 241 | 220 | 376 | 1.51 | 1.66 | 0.97 |
| Survival Curve for Quercus robur Per Season of Root Birth (with Root Diameter < 2 mm) | ||||||||||
| Season of Root Birth | n | Events | Mean Lifespan (Days) | Median Lifespan (Days) | Root Turnover Rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Autumn | 912 | 912 | 446 | 6 | 460 | 447 | 473 | 0.79 | 0.82 | 0.77 |
| Spring | 401 | 432 | 432 | 10 | 447 | 437 | 468 | 0.82 | 0.84 | 0.78 |
| Summer | 757 | 757 | 444 | 7 | 460 | 460 | 468 | 0.79 | 0.79 | 0.78 |
| Winter | 441 | 364 | 437 | 10 | 447 | 437 | 468 | 0.82 | 0.84 | 0.78 |
| Survival Curve for Betula pendula Per Root Diameter Class | ||||||||||
| Root Diameter Class | n | Events | Mean Lifespan (Days) | Median Lifespan (Days) | Root Turnover Rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Root diameter > 2 mm | 3765 | 3620 | 425 | 4 | 407 | 394 | 433 | 0.90 | 0.93 | 0.84 |
| 1 mm < root diameter > 2 mm | 5509 | 5260 | 422 | 3 | 440 | 394 | 470 | 0.83 | 0.93 | 0.78 |
| 0.5 mm < root diameter > 1 mm | 776 | 736 | 465 | 8 | 562 | 435 | 595 | 0.65 | 0.84 | 0.61 |
| Root diameter < 0.5 mm | 244 | 232 | 386 | 15 | 394 | 379 | 470 | 0.93 | 0.96 | 0.78 |
| Survival Curve for Betula pendula Per Season of Root Birth (with Root Diameter < 2 mm) | ||||||||||
| Season of Root Birth | n | Events | Mean Lifespan (Days) | Median Lifespan (Days) | Root Turnover Rate (Year−1) | |||||
| Mean | Mean SE | Median | Lower 95% CI | Upper 95% CI | Rate | Lower 95% CI | Upper 95% CI | |||
| Autumn | 2240 | 2240 | 376 | 5 | 379 | 358 | 379 | 0.96 | 1.02 | 0.96 |
| Spring | 1642 | 1642 | 479 | 5 | 511 | 471 | 559 | 0.71 | 0.77 | 0.65 |
| Summer | 1832 | 1832 | 387 | 5 | 387 | 371 | 394 | 0.94 | 0.98 | 0.93 |
| Winter | 815 | 514 | 544 | 6 | 626 | 595 | 653 | 0.58 | 0.61 | 0.56 |
| Survival Curve Per Species (with Root Diameter < 2 mm) | ||||
|---|---|---|---|---|
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Species | Fagus sylvatica | Quercus robur | ||
| Fagus sylvatica | - | 0.001 | <0.001 | |
| Betula pendula | 0.001 | 0.001 | ||
| Survival Curve for Fagus sylvatica Per Root Diameter Class | ||||
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Root Diameter Class | Root Diameter < 0.5 mm | Root Diameter > 2 mm | 0.5 mm < Root Diameter > 1 mm | |
| Root Diameter > 2 mm | ns | - | - | <0.001 |
| 0.5 mm < Root Diameter > 1 mm | ns | ns | - | |
| 1 mm < Root Diameter > 2 mm | <0.05 | <0.001 | <0.001 | |
| Survival Curve for Fagus sylvatica Per Season of Root Birth (with Root Diameter < 2 mm) | ||||
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Season of root birth | Autumn | Spring | Summer | |
| Spring | ns | - | - | <0.001 |
| Summer | <0.001 | ns | - | |
| Winter | <0.001 | <0.01 | ns | |
| Survival Curve for Quercus robur Per Root Diameter Class | ||||
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Root Diameter Class | Root Diameter < 0.5 mm | Root Diameter > 2 mm | 0.5 mm < Root Diameter > 1 mm | |
| Root Diameter > 2 mm | <0.001 | - | - | <0.001 |
| 0.5 mm < Root Diameter > 1 mm | <0.001 | ns | - | |
| 1 mm < Root Diameter > 2 mm | <0.001 | <0.001 | <0.05 | |
| Survival Curve for Quercus robur Per Season of Root Birth (with Root Diameter < 2 mm) | ||||
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Season of Root Birth | Autumn | Spring | Summer | |
| Spring | ns | - | - | <0.001 |
| Summer | <0.001 | ns | - | |
| Winter | <0.001 | <0.01 | ns | |
| Survival Curve for Betula pendula Per Root Diameter Class | ||||
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Root Diameter Class | Root Diameter < 0.5 mm | Root Diameter > 2 mm | 0.5 mm < Root Diameter > 1 mm | |
| Root Diameter > 2 mm | ns | - | - | <0.001 |
| 0.5 mm < Root Diameter > 1 mm | <0.001 | <0.001 | - | |
| 1 mm < Root Diameter > 2 mm | ns | <0.01 | <0.001 | |
| Survival Curve for Betula pendula Per Season of Root Birth (with Root Diameter < 2 mm) | ||||
| Post-Hoc Analysis | Log-Rank Test | |||
| p Value | p Value | |||
| Season of Root Birth | Autumn | Spring | Summer | |
| Spring | <0.001 | - | - | <0.001 |
| Summer | ns | <0.001 | - | |
| Winter | <0.001 | <0.001 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariën, B.; Ostonen, I.; Penanhoat, A.; Fang, C.; Xuan Nguyen, H.; Ghisi, T.; Sigurðsson, P.; Willems, P.; Campioli, M. On the Below- and Aboveground Phenology in Deciduous Trees: Observing the Fine-Root Lifespan, Turnover Rate, and Phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula Roth for Two Growing Seasons. Forests 2021, 12, 1680. https://doi.org/10.3390/f12121680
Mariën B, Ostonen I, Penanhoat A, Fang C, Xuan Nguyen H, Ghisi T, Sigurðsson P, Willems P, Campioli M. On the Below- and Aboveground Phenology in Deciduous Trees: Observing the Fine-Root Lifespan, Turnover Rate, and Phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula Roth for Two Growing Seasons. Forests. 2021; 12(12):1680. https://doi.org/10.3390/f12121680
Chicago/Turabian StyleMariën, Bertold, Ivika Ostonen, Alice Penanhoat, Chao Fang, Hòa Xuan Nguyen, Tomáš Ghisi, Páll Sigurðsson, Patrick Willems, and Matteo Campioli. 2021. "On the Below- and Aboveground Phenology in Deciduous Trees: Observing the Fine-Root Lifespan, Turnover Rate, and Phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula Roth for Two Growing Seasons" Forests 12, no. 12: 1680. https://doi.org/10.3390/f12121680
APA StyleMariën, B., Ostonen, I., Penanhoat, A., Fang, C., Xuan Nguyen, H., Ghisi, T., Sigurðsson, P., Willems, P., & Campioli, M. (2021). On the Below- and Aboveground Phenology in Deciduous Trees: Observing the Fine-Root Lifespan, Turnover Rate, and Phenology of Fagus sylvatica L., Quercus robur L., and Betula pendula Roth for Two Growing Seasons. Forests, 12(12), 1680. https://doi.org/10.3390/f12121680






