Closed-Canopy Tropical Forests of Hainan, (China) Are Resilient against Invasive Herbs and Shrubs
Abstract
:1. Introduction
2. Methods
2.1. Diversity and Distribution of Terrestrial Invasive Herbs and Shrubs in Hainan Island
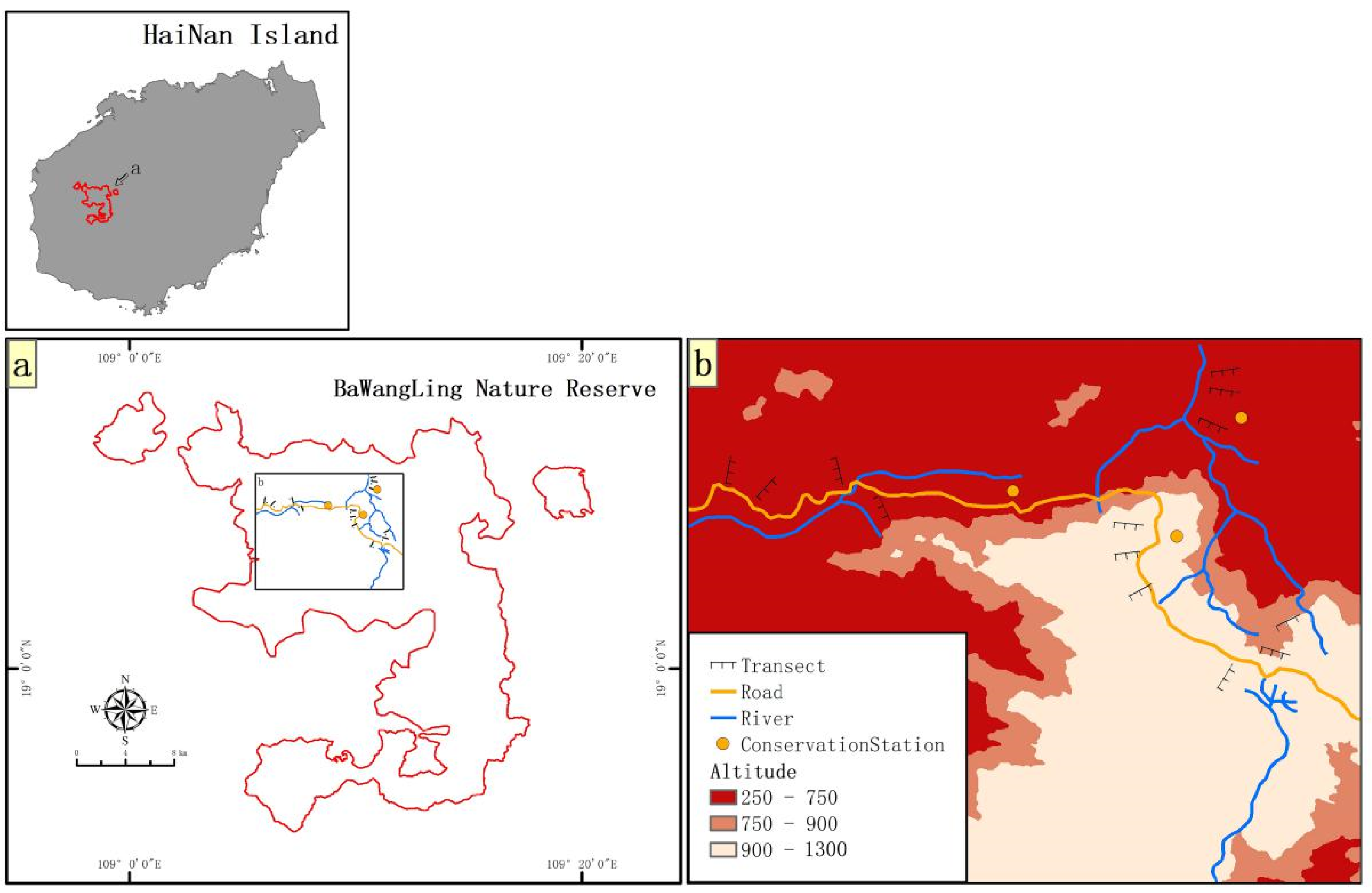
2.2. Forest Edge Effects on Distribution and Abundance of Terrestrial Invasive Herbs and Shrubs
2.3. Forest Edge Effects on Soil Seed Bank of Terrestrial Invasive Herbs and Shrubs
2.4. Seed Germination of Terrestrial Invasive Herbs and Shrubs in Forest Gaps
2.5. Statistical Analyses
3. Results
3.1. Species Diversity and Distribution of Terrestrial Invasive Herbs and Shrubs in Hainan Island
3.2. Forest Edge Effects on Terrestrial Invasive Herbs or Shrubs
3.3. Seed Germination and Seedling Survival of Terrestrial Invasive Herbs and Shrubs in Forest Gaps
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peerbhay, K.; Mutanga, O.; Ismail, R. The identification and remote detection of alien invasive plants in commercial forests: An Overview. S. Afr. J. Geomat. 2016, 5, 49–67. [Google Scholar]
- Walsh, J.R.; Carpenter, S.R.; Zanden, V.M.J. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proc. Natl. Acad. Sci. USA 2016, 113, 4081–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozlan, R.E.; Newton, A.C. Biological invasions: Benefits versus risks. Science 2009, 324, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; Boer, W.E.; Verhoeven, K.J.F.; Boschker, H.T.S.; Putten, W.H.V.D. Comparison of nutrient acquisition in exotic plant species and congeneric natives. J. Ecol. 2011, 99, 1308–1315. [Google Scholar] [CrossRef]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Chapter One—Impacts of Invasive Species on Food Webs: A Review of Empirical Data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar]
- Weidenhamer, J.D.; Callaway, R.M. Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. J. Chem. Ecol. 2010, 36, 59–69. [Google Scholar] [CrossRef]
- Prescott, C.E.; Zukswert, J.M. Invasive plant species and litter decomposition: Time to challenge assumptions. New Phytol. 2016, 209, 5–7. [Google Scholar] [CrossRef] [Green Version]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. Chapter Two—The Effects of Invasive Species on the Decline in Species Richness: A Global Meta-Analysis. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Webb, C.O.; Salamin, N. Exotic taxa less related to native species are more invasive. Proc. Natl. Acad. Sci. USA 2006, 103, 5841–5845. [Google Scholar] [CrossRef] [Green Version]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catford, J.A.; Baumgartner, J.B.; Vesk, P.A.; White, M.; Buckley, Y.M.; McCarthy, M.A. Disentangling the four demographic dimensions of species invasiveness. J. Ecol. 2016, 104, 1745–1758. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.W. Plant community diversity and native plant abundance decline with increasing abundance of an exotic annual grass. Oecologia 2011, 167, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Ström, L.; Jansson, R.; Nilsson, C. Invasibility of boreal wetland plant communities. J. Veg. Sci. 2014, 25, 1078–1089. [Google Scholar] [CrossRef]
- Koerner, S.E.; Avolio, M.L.; Chang, C.C.; Gray, J.; Hoover, D.L.; Smith, M.D. Invasibility of a mesic grassland depends on the time-scale of fluctuating resources. J. Ecol. 2015, 103, 1538–1546. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol Env. 2009, 7, 142–149. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar]
- Sanderson, L.A.; Antunes, P.M. The exotic invasive plant Vincetoxicum rossicum is a strong competitor even outside its current realized climatic temperature range. Neobiota 2016, 16, 1–15. [Google Scholar]
- Syamsuardi, N.Y.; Yulianti, W.; Usman, S. Floristic analysis of alien invasive plant species at some conservation areas in tropical forest of West Sumatra. Der. Pharm. Lett. 2016, 8, 237–245. [Google Scholar]
- Fine, P.V.A. The invasibility of tropical forests by exotic plants. J. Trop. Ecol. 2002, 18, 687–705. [Google Scholar] [CrossRef] [Green Version]
- Denslow, J.S.; Dewalt, S.J. Exotic plant invasions in tropical forests: Patterns and hypotheses. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Miguel, J.M.D.; Martín-Forés, I.; Acosta-Gallo, B.; Pozo, A.D.; Ovalle, C.; Sánchez-Jardón, L.; Castro, L.; Casado, M.A. Non-random co-occurrence of native and exotic plant species in Mediterranean grasslands. Acta Oecologica 2016, 77, 18–26. [Google Scholar] [CrossRef]
- Ashbacher, A.C.; Cleland, E.E. Native and exotic plant species show differential growth but similar functional trait responses to experimental rainfall. Ecosphere 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Chen, B.M.; Li, S.; Liao, H.X.; Peng, S.L. Do forest soil microbes have the potential to resist plant invasion? A case study in Dinghushan Biosphere Reserve (South China). Acta Oecologica 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Mavimbela, L.Z.; Sieben, E.J.J.; Procheş, Ş. Invasive alien plant species, fragmentation and scale effects on urban forest community composition in Durban, South Africa. N. Z. J. For. Sci. 2018, 48, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ghulam, A.; Porton, I.; Freeman, K. Detecting subcanopy invasive plant species in tropical rainforest by integrating optical and microwave (InSAR/PolInSAR) remote sensing data, and a decision tree algorithm. ISPRS J. Photogramm. Remote. Sens. 2014, 88, 174–192. [Google Scholar] [CrossRef]
- Klinczar, A.G. The Effect of Treefall Gaps and Propagule Rain on the Spatial Distribution of Four Invasive Plants in a Mature Upland Forest in Maryland; Miami University: Oxford, OH, USA, 2014. [Google Scholar]
- Procheş, Ş.; Forest, F.; Jose, S.; Dominicis, M.D.; Ramdhani, S.; Wiggill, T. How do alien plants fit in the space-phylogeny matrix? PLoS ONE 2015, 10, e0123238. [Google Scholar] [CrossRef] [PubMed]
- Parendes, L.A. Spatial Patterns of Invasion by Exotic Plants in a Forested Landscape; Oregon State University: Corvallis, OR, USA, 1997. [Google Scholar]
- Wijayabandara, S.; Jayasuriya, K.; Jayasinghe, J. Seed Dormancy, Storage Behavior and Germination of an Exotic Invasive Species, Lantana camara L. (Verbenaceae). Int. Res. J. Biol. Sci. 2013, 2, 7–14. [Google Scholar]
- Marques, A.R.; Costa, C.F.; Atman, A.P.F.; Garcia, Q.S. Germination characteristics and seedbank of the alien species Leucaena leucocephala (Fabaceae) in Brazilian forest: Ecological implications. Weed Res. 2015, 54, 576–583. [Google Scholar] [CrossRef]
- Dawson, W.; Burslem, D.F.R.P.; Hulme, P.E. Consistent effects of disturbance and forest edges on the invasion of a continental rain forest by alien plants. Biotropica 2015, 47, 27–37. [Google Scholar] [CrossRef]
- González-Moreno, P.; Pino, J.; Gassó, N.; Vilà, M. Landscape context modulates alien plant invasion in mediterranean forest edges. Biol. Invasions 2013, 15, 547–557. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.Q.; Fu, S.H.; Yang, X.B.; Chen, Y.K.; Zhou, W.; Yang, Q.; Tao, C.; Zhou, W.S. Distribution patterns of alien invasive plants and their influences on native plants of Hainan Island. Chin. J. Plant Ecol. 2015, 39, 486–500. [Google Scholar]
- Cook, J.G.; Stutzman, T.W.; Bowers, C.W.; Brenner, K.A.; Irwin, L.L. Spherical densiometers produce biased estimates of forest canopy cover. Wildl. Soc. Bulletin. 1995, 23, 711–717. [Google Scholar]
- Winkler, E.; Marcante, S.; Erschbamer, B. Demography of the alpine pioneer species Saxifraga aizoides in different successional stages at the glacier foreland of the Rotmoosferner (Obergurgl, Ötztal, Austria). Tuexenia 2015, 35, 267–283. [Google Scholar]
- Liénard, J.F.; Gravel, D.; Strigul, N.S. Data-intensive modeling of forest dynamics. Environ. Model. Softw. 2015, 67, 138–148. [Google Scholar] [CrossRef]
- Oduor, A.M.O.; Leimu, R.; Kleunen, M.V. Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. J. Ecol. 2016, 104, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. USA 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.U.; Dukes, J.S. Dukes J.S. Functional composition controls invasion success in a California serpentine grassland. J. Ecol. 2010, 98, 764–777. [Google Scholar] [CrossRef]





| Species | Code | Family | Growth Form | Dispersal Mechanism | Native to | Geographical Distribution |
|---|---|---|---|---|---|---|
| Chromolaena odorata (L.) R.M.King & H.Rob. | Co | Asteraceae | Herb | Wind | Americas | South America, Africa and tropical Asia |
| Praxelis clematidea (G.) R. M. King et H. Rob. | Pc | Asteraceae | Herb | Wind | South America | South America, tropical regions of the Eastern Hemisphere |
| Mimosa pudica Linn | Mp | Leguminous | Herb or subshrub | Wind | Americas | Tropical regions |
| Parthenium hysterophorus L. | Ph | Asteraceae | Herb | Wind | Americas | America, Northern Vietnam and Southern China |
| Pseudosorghum fasciculare (S.) A. Camus | Pf | Gramineae | Herb | Animals, farm tools, running water | Mediterranean | Tropical and subtropical regions from 55° N to 45° S |
| Sphagneticola trilobata | St | Asteraceae | Herb | Stolon | South America | South America, Southeast Asia and southern China |
| Alternanthera philoxeroides (M.) Griseb. | Ap | Amaranthaceae | Herb | Stolon | South America | South America, North America, Oceania, Southeast Asia, southern Europe and Africa |
| Conyza sumatrensis | Cs | Asteraceae | Herb | Wind | South America | Widely distributed in tropical and subtropical regions |
| Lantana camara L. | Lc | Verbenaceae | shrub | Animals | Americas | Widely distributed in tropical and subtropical regions |
| Forest Gap | Coordinate Point | Altitude | |
|---|---|---|---|
| I | 19°05′46.6″ E, 109°10′35″ N | 17.5 × 16.0 m | 1002 m |
| II | 19°05′50.6″ E, 109°10′25″ N | 14.5 × 15.0 m | 1092 m |
| III | 19°05′27.3″ E, 109°10′56″ N | 12.6 × 13.3 m | 1014 m |
| Response Variable | Model Type | Forest Type | Dispersed Distance | Altitude | Canopy | Seed Mass | Seed Production |
|---|---|---|---|---|---|---|---|
| Canopy cover | Linear | + | + | + | |||
| Pooled species richness | Poisson | + | + | + | + | ||
| Pooled abundance | NegBin. | + | + | + | + | + | + |
| Dispersed distance | Linear | + | + | + | + | + | |
| Presence in the soil seed bank | NegBin. | + | + | + | + |
| Habitat (N) | Ap | Co | Cs | Lc | Mp | Pc | Pf | Ph | St | Species Per Site (Mean ± Stdev) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abandoned field (17) | + | + | + | + | + | + | + | + | + | 4.6 ± 1.3 |
| Plantation (20) | 0 | + | + | + | + | + | 0 | 0 | + | 4.2 ± 1.0 |
| Rural (14) | + | + | + | + | + | + | + | + | + | 3.9 ±0.8 |
| Grassland (11) | + | + | + | + | + | + | 0 | 0 | + | 3.4 ± 0.9 |
| Field (14) | + | + | + | + | + | + | + | + | + | 3.6 ± 0.8 |
| Forest edge (11) | 0 | + | + | + | + | + | 0 | 0 | + | 2.7 ± 1.3 |
| Rain forest (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 ± 0.0 |
| TIHS | Frequency of Terrestrial Invasive Herbs and Shrubs | |||||
|---|---|---|---|---|---|---|
| AF (17) | PL (20) | RV (14) | SE (11) | FI (14) | GR (11) | |
| Pc | 0.700 ± 0.500 a | 0.946 ± 0.472 a | 0.485 ± 0.298 b | 0.556 ± 0.529 b,c | 0.790 ± 0.451 b,c | 0.685 ± 0.218 b |
| Mp | 0.502 ± 0.407 a | 0.331 ± 0.357 a | 0.274 ± 0.351 a,b | 0.204 ± 0.430 a | 0.592 ± 0.455 c | 0.474 ± 0.250 a,b |
| Co | 0.749 ± 0.493 a | 0.701 ± 0.465 a | 0.628 ± 0.326 a | 0.556 ± 0.529 a | 0.733 ± 0.459 a | 0.771 ± 0.226 a |
| Cs | 0.273 ± 0.431 a | 0.339 ± 0.443 a | 0.291 ± 0.394 a | 0.186 ± 0.347 a | 0.355 ± 0.527 a | 0.191 ± 0.094 a |
| PF | 0.118 ± 0.223 a | - | 0.112 ± 0.185 a | - | 0.051 ± 0.125 a | - |
| Lc | 0.118 ± 0.311 a | 0.025 ± 0.092 a | 0.112 ± 0.185 a | 0.099 ± 0.188 a | 0.051 ± 0.125 a | 0.092 ± 0.137 a |
| Ap | 0.126 ± 0.211 a | 0.022 ± 0.115 a | 0.032 ± 0.105 a | - | 0.091 ± 0.114 a | 0.052 ± 0.137 a |
| St | 0.126 ± 0.391 a | 0.226 ± 0.173 b | 0.029 ± 0.140 a | 0.050 ± 0.169 a | 0.179 ± 0.397 a | 0.129 ± 0.140 a |
| Ph | 0.085 ± 0.121 a | 0.015 ± 0.002 a | 0.112 ± 0.105 a | - | 0.051 ± 0.125 a | 0.092 ± 0.037 a |
| AFTIHS ** | 2.806 | 2.605 | 2.075 | 1.651 | 2.893 | 2.486 |
| Species | Frequency (Presence/Soil Sample) | Furthest Distance Observed (m) | Range (Seedlings m−2) | Density (Seedlings m−2) | Total Germinated Seedlings of TIHS | Proportion of All Germinated Seedlings (57,138) (%) |
|---|---|---|---|---|---|---|
| A. conyzoides | 0.138 | 20 | 25–625 | 501.9 ± 697.0 | 542 | 0.95 |
| P. clematidea | 0.159 | 20 | 25–7000 | 192.5 ± 267.0 | 511 | 0.89 |
| B. latifolia | 0.041 | 20 | 25–1400 | 387.5 ± 483.8 | 143 | 0.25 |
| C. odorata | 0.149 | 20 | 25–2900 | 104.5 ± 139.0 | 117 | 0.2 |
| R. repen | 0.015 | 0 | 225–1375 | 783.3 ± 575.0 | 94 | 0.16 |
| C. crepidioide | 0.185 | 20 | 25–300 | 54.9 ± 59.7 | 79 | 0.14 |
| M. diplotricha | 0.015 | 10 | 25–275 | 166.5 ± 128.0 | 20 | 0.04 |
| M. pudica | 0.036 | 15 | 25–125 | 67.9 ± 37.4 | 19 | 0.03 |
| S. jamaicensis | 0.010 | 20 | 25–175 | 100.0 ± 106.1 | 8 | 0.01 |
| B. pilosa | 0.015 | 15 | 25–75 | 41.6 ± 28.8 | 5 | 0.01 |
| L. camara | 0.021 | 0 | 25 | 25.0 | 4 | 0.01 |
| T. procumbens | 0.015 | 10 | 25 | 25.0 | 3 | 0.01 |
| Total | 1545 | 2.7 |
| Effects | Parameter | Estimate (±1 SE) |
|---|---|---|
| (A) Pooled species richness of exotic species ~ Distance from edge + Canopy | Intercept | 1.114 (0.212) |
| Distance from the edge | −0.780 (0.383) | |
| Canopy cover | −0.796 (0.453) | |
| (B) Pooled abundance of exotic seeds ~Distance from edge + Canopy cover + seed mass | Intercept | 3.455 (0.302) |
| Distance from the edge | −4.291 (0.650) | |
| seed mass | −2.151 (0.715) | |
| Canopy cover | −0.526 (0.361) | |
| (C) Presence of exotic plants in the soil seed bank ~Distance from edge + Canopy cover | Intercept | 6.910 (2.867) |
| Distance from the edge | −2.627 (0.741) | |
| Canopy cover | 0.265 (0.820) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, L.; Lv, X.; Luo, W.; Li, D.; Liang, C.; Wee, A.K.S.; Long, W. Closed-Canopy Tropical Forests of Hainan, (China) Are Resilient against Invasive Herbs and Shrubs. Forests 2021, 12, 1596. https://doi.org/10.3390/f12111596
Yang X, Li L, Lv X, Luo W, Li D, Liang C, Wee AKS, Long W. Closed-Canopy Tropical Forests of Hainan, (China) Are Resilient against Invasive Herbs and Shrubs. Forests. 2021; 12(11):1596. https://doi.org/10.3390/f12111596
Chicago/Turabian StyleYang, Xiaobo, Long Li, Xiaobo Lv, Wenqi Luo, Donghai Li, Caiqun Liang, Alison K. S. Wee, and Wenxing Long. 2021. "Closed-Canopy Tropical Forests of Hainan, (China) Are Resilient against Invasive Herbs and Shrubs" Forests 12, no. 11: 1596. https://doi.org/10.3390/f12111596
APA StyleYang, X., Li, L., Lv, X., Luo, W., Li, D., Liang, C., Wee, A. K. S., & Long, W. (2021). Closed-Canopy Tropical Forests of Hainan, (China) Are Resilient against Invasive Herbs and Shrubs. Forests, 12(11), 1596. https://doi.org/10.3390/f12111596






