The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems
Abstract
1. Introduction
An Overview of Forest Tree Pests and Pathogens
2. Insect Pests
3. Pathogens
3.1. Phytopathogenic Fungi
3.2. Root Rot
3.3. Rusts
3.4. Wilt Fungi
4. Oomycetes
5. Phytopathogenic Bacteria
6. Phytoplasmas
7. Viruses
8. Nematodes
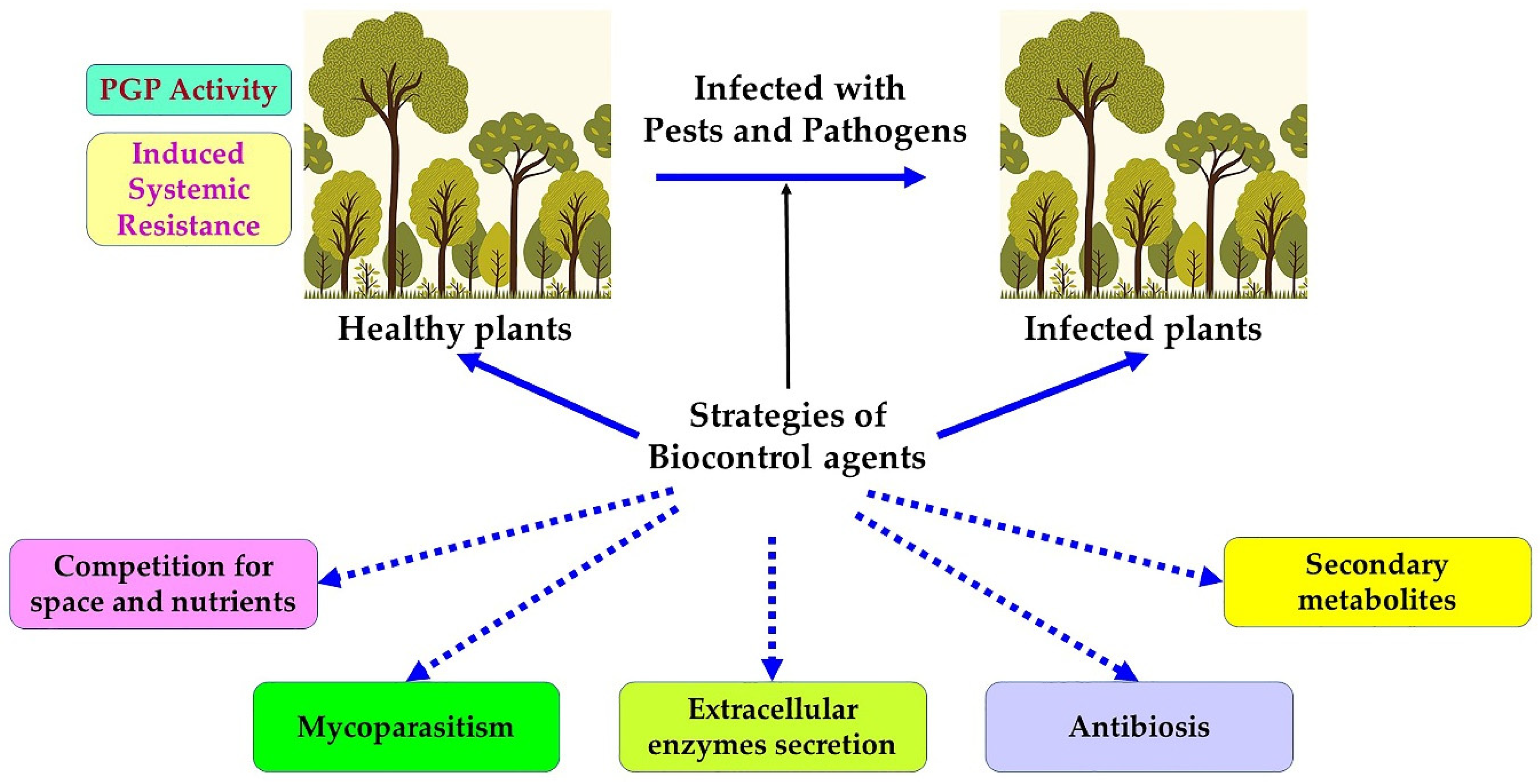
9. Biological Control Strategies against Phytopathogenic Agents
10. Entomopathogenic Fungi
11. Biological Control Using Bacteria
11.1. Bacillus and Pseudomonas
11.2. Streptomyces
11.3. Myxobacteria
12. Biological Control Using Viruses
13. Biological Control Using Insects and Nematodes
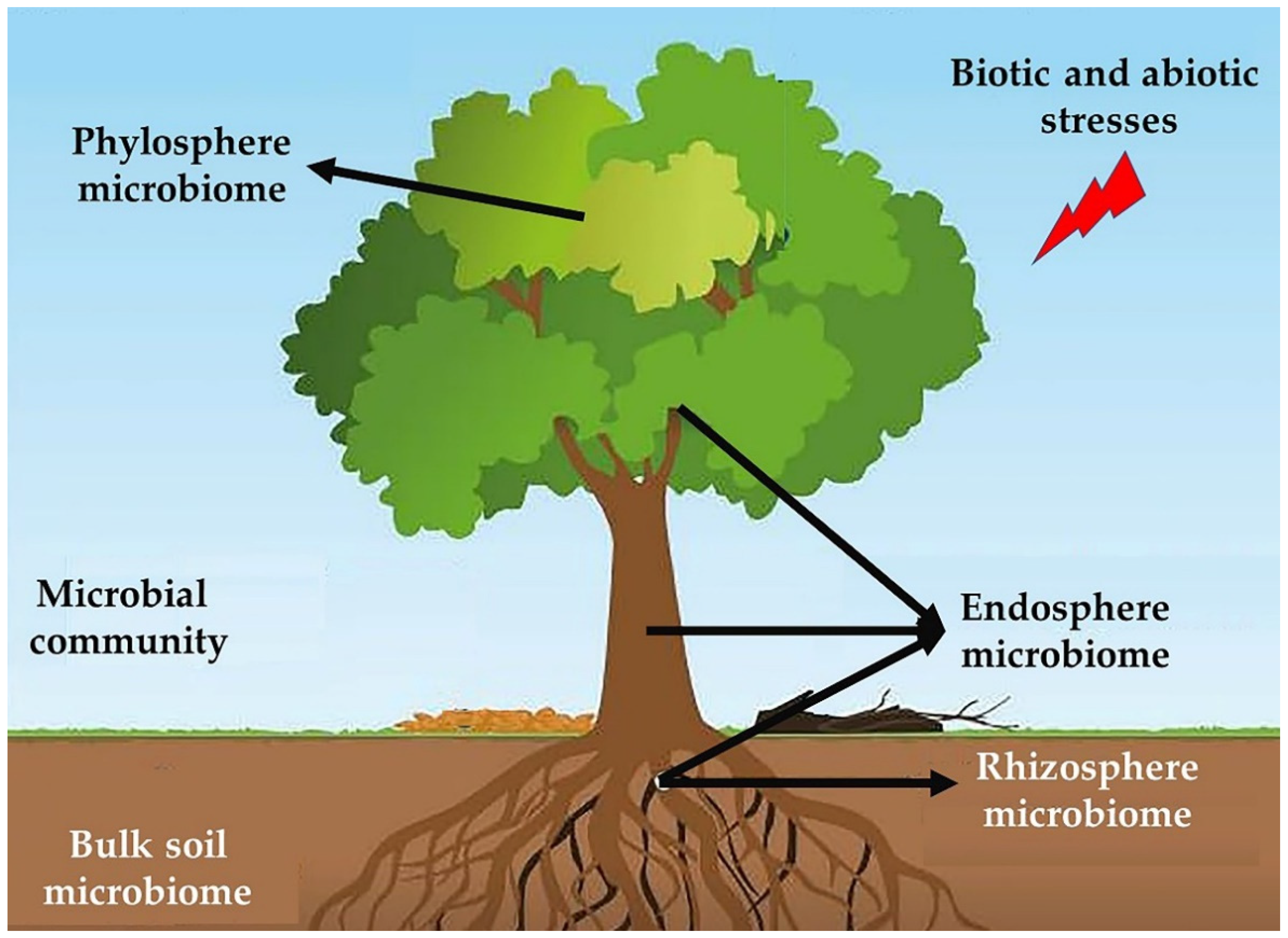
14. Role of Forest Soil Microbiota in Tree Health
15. Soil Microbiota
16. Rhizosphere
17. Phyllosphere
18. Endophytes
19. Gaps and Opportunities in Forest Microbiota Research
- -
- providing other analytical tools to complement those in use;
- -
- investigating the factors that help establish symbiotic relationships between microbes and their biological processes within an ecosystem from gene to population;
- -
- conducting in-depth multidisciplinary studies of the ecosystem associated with studies of the microbiota, and establishing the relationships between the biocenosis and the biotope (or biosphere).
20. Biofertilizer Perspectives of Microbial Inoculants
21. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Keenan, R.J.; Reams, G.A.; Achard, F.; de Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. Forest Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Bylund, H.; Schroeder, M.; Björkman, C. Forest management and natural biocontrol of insect pests. Forestry 2016, 89, 253–262. [Google Scholar] [CrossRef]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological control of emerging forest diseases: How can we move from dreams to reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. For. Health 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Cobb, R.C.; Eviner, V.T.; Rizzo, D.M. Mortality and community changes drive sudden oak death impacts on litterfall and soil nitrogen cycling. New Phytol. 2013, 200, 422–431. [Google Scholar] [CrossRef]
- Heinzelmann, R.; Rigling, D.; Prospero, S. Population genetics of the wood-rotting basidiomycete Armillaria cepistipes in a fragmented forest landscape. Fung. Biol. 2012, 116, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 49, 1426–1435. [Google Scholar] [CrossRef]
- Björkman, C.; Bylund, H.; Nilsson, U.; Nordlander, G.; Schroeder, M. Effects of new forest management on insect damage risk in a changing climate. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI International: Wallingford, UK, 2015; pp. 248–266. [Google Scholar]
- Linnakoski, R.; Forbes, K.M. Pathogens—The hidden face of wood-boring forest pest invasions. Front. Plant Sci. 2018, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Nair, K. Tropical Forest Insect Pests: Ecology, Impact, and Management; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: Using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Nowakowska, J.A.; Hsiang, T.; Patynek, P.; Stereńczak, K.; Olejarski, I.; Oszako, T. Health assessment and genetic structure of monumental Norway spruce trees during a bark beetle (Ips typographus L.) outbreak in the Białowieża Forest District, Poland. Forests 2020, 11, 647. [Google Scholar] [CrossRef]
- Goczał, J.; Oleksa, A.; Rossa, R.; Chybicki, I.; Meyza, K.; Plewa, R.; Landvik, M.; Gobbi, M.; Hoch, G.; Tamutis, V.; et al. Climatic oscillations in Quaternary have shaped the co-evolutionary patterns between the Norway spruce and its host-associated herbivore. Sci. Rep. 2020, 10, 16524. [Google Scholar] [CrossRef]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian long horned beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. J. Integr. Pest Manag. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Valenta, V.; Moser, D.; Kapeller, S.; Essl, F. A new forest pest in Europe: A review of emerald ash borer (Agrilus planipennis) invasion. J. Appl. Entomol. 2017, 141, 507–526. [Google Scholar] [CrossRef]
- Coppedge, B.R. Twig morphology and host effects on reproductive success of the twig girdler Oncideres cingulata (Say) (Coleoptera: Cerambycidae). Coleopt. Bull. 2011, 65, 405–410. [Google Scholar] [CrossRef]
- Pincebourde, S.; Ngao, J. The impact of phloem feeding insects on leaf ecophysiology varies with leaf age. Front. Plant Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, E.L.; Lanta, V.; Kozlov, M.V. Effects of sap-feeding insect herbivores on growth and reproduction of woody plants: A meta-analysis of experimental studies. Oecologia 2010, 163, 949–960. [Google Scholar] [CrossRef]
- McManus, M.; Csóka, G. History and impact of gypsy moth in North America and comparison to the recent outbreaks in Europe. Acta Silv. Lignaria Hung. 2007, 3, 47–64. [Google Scholar]
- Paini, D.R.; Mwebaze, P.; Kuhnert, P.M.; Kriticos, D.J. Global establishment threat from a major forest pest via international shipping: Lymantria dispar. Sci. Rep. 2018, 8, 13723. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Volney, W.J.A.; Fleming, R.A. Climate change and impacts of boreal forest insects. Agr. Ecosyst. Environ. 2000, 82, 283–294. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Michaelian, M. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Can. J. For. Res. 2008, 38, 1373–1384. [Google Scholar] [CrossRef]
- Dara, S.K.; Montalva, C.; Barta, M. Microbial control of invasive forest pests with entomopathogenic fungi: A review of the current situation. Insects 2019, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. In The Fungal Kingdom; Heitman, J., Howlett, B., Crous, P., Stukenbrock, E., James, T., Gow, N., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 703–726. [Google Scholar]
- Cromey, M.G.; Drakulic, J.; Beal, E.J.; Waghorn, I.A.G.; Perry, J.N.; Clover, G.R.G. Susceptibility of garden trees and shrubs to Armillaria root rot. Plant Dis. 2020, 104, 483–492. [Google Scholar] [CrossRef]
- Worrall, J.J.; Harrington, T.C.; Blodgett, J.T.; Conklin, D.A.; Fairweather, M.L. Heterobasidion annosum and H. parviporum in the southern Rocky Mountains and adjoining states. Plant Dis. 2010, 94, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Mattila, U.; Nuutinen, T. Assessing the incidence of butt rot in Norway spruce in southern Finland. Silva Fenn. 2007, 41, 29–43. [Google Scholar] [CrossRef]
- Guinet, C.; Boutigny, A.L.; Vialle, A.; Hamelin, R.C.; Frey, P.; Ioos, R. Simultaneous monitoring and quantification of Melampsora allii-populina and Melampsora larici-populina on infected poplar leaves using a duplex real-time PCR assay. Plant Pathol. 2016, 65, 380–391. [Google Scholar] [CrossRef]
- Liu, J.J.; Chan, D.; Xiang, Y.; Williams, H.; Li, X.R.; Sniezko, R.A.; Sturrock, R.N. Characterization of five novel mitoviruses in the white pine blister rust fungus Cronartium ribicola. PLoS ONE 2016, 11, e0154267. [Google Scholar] [CrossRef][Green Version]
- Cobo-Díaz, J.F.; Baroncelli, R.; Le Floch, G.; Picot, A. A novel metabarcoding approach to investigate Fusarium species composition in soil and plant samples. FEMS Microbiol. Ecol. 2019, 95, fiz084. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Kombrink, A.; Rovenich, H.; Shi-Kunne, X.; Rojas-Padilla, E.; van den Berg, G.C.M.; Domazakis, E.; de Jonge, R.; Valkenburg, D.-J.; Sánchez-Vallet, A.; Seidl, M.F.; et al. Verticillium dahliae LysM effectors differentially contribute to virulence on plant hosts. Mol. Plant Pathol. 2017, 18, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cuesta, R.; Navarro-Cerrillo, R.M.; Quero, J.L.; Ruiz-Gómez, F.J. Small-scale abiotic factors influencing the spatial distribution of Phytophthora cinnamomi under declining Quercus ilex trees. Forests 2020, 11, 375. [Google Scholar] [CrossRef]
- Jung, T.; Vettraino, A.M.; Cech, T.L.; Vannini, A. The impact of invasive Phytophthora species on European forests. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CABI: Wallingford, UK, 2013; pp. 146–158. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group (ISSG): Auckland, New Zealand, 2000. [Google Scholar]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Migliorini, D.; Ghelardini, L.; Luchi, N.; Capretti, P.; Onorari, M.; Santini, A. Temporal patterns of airborne Phytophthora spp. in a woody plant nursery area detected using real-time PCR. Aerobiologia 2019, 35, 201–214. [Google Scholar] [CrossRef]
- Belbahri, L.; Moralejo, E.; Calmin, G.; Oszako, T.; García, J.A.; Descals, E.; Lefort, F. Phytophthora polonica, a new species isolated from declining Alnus glutinosa stands in Poland. FEMS Microbiol. Lett. 2006, 261, 165–174. [Google Scholar] [CrossRef]
- Lazreg, F.; Belabid, L.; Sanchez, J.; Gallego, E.; Garrido-Cardenas, J.A.; Elhaitoum, A. First report of Globisporangium ultimum causing Pythium damping-off on Aleppo pine in Algeria, Africa, and the Mediterranean region. Plant Dis. 2013, 97, 1111. [Google Scholar] [CrossRef] [PubMed]
- Weiland, J.E.; Beck, B.R.; Davis, A. Pathogenicity and virulence of Pythium species obtained from forest nursery soils on Douglas-fir seedlings. Plant Dis. 2013, 97, 744–748. [Google Scholar] [CrossRef]
- Rosso, M.L.; Rupe, J.C.; Chen, P.; Mozzoni, L.A. Inheritance and genetic mapping of resistance to Pythium damping-off caused by Pythium aphanidermatum in ‘Archer’ soybean. Crop Sci. 2008, 48, 2215–2222. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; McDonald, H.J.; Hawke, B.G. Influence of tillage and crop rotation on the epidemiology of Pythium infections of wheat in a red-brown earth of South Australia. Soil Biol. Biochem. 1995, 27, 1065–1073. [Google Scholar] [CrossRef]
- Kannan, V.R.; Bastas, K.K.; Devi, R.S. 20 Scientific and economic impact of plant pathogenic bacteria. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; Kannan, V.R., Bastas, K.K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 369–392. [Google Scholar]
- Griffiths, H.M. Forest diseases caused by prokaryotes: Phytoplasmal and bacterial diseases. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 76–96. [Google Scholar]
- Melotto, M.; Underwood, W.; He, S.Y. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef]
- Martins, P.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in phytopathogenic bacteria: Do we know enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.E.; Wang, M.B.; Bravo, J.E.; Banta, L.M. Unmasking host and microbial strategies in the Agrobacterium-plant defense tango. Front. Plant Sci. 2015, 6, 200. [Google Scholar] [CrossRef] [PubMed]
- Kado, C.I. Crown gall. In The Plant Health Instructor; American Phytopathological Society: St. Paul, MN, USA, 2002. [Google Scholar] [CrossRef]
- Rapicavoli, J.; Ingel, B.; Blanco-Ulate, B.; Cantu, D.; Roper, C. Xylella fastidiosa: An examination of a re-emerging plant pathogen. Mol. Plant Pathol. 2018, 19, 786–800. [Google Scholar] [CrossRef]
- Chatterjee, S.; Newman, K.L.; Lindow, S.E. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Int. 2008, 21, 1309–1315. [Google Scholar] [CrossRef]
- Tebaldi, N.D.; Leite, L.N.; Marque, J.M.D.; Furlanetto, M.C.A.; Mota, L.C.B.M. Occurrence of Ralstonia solanacearum on olive tree in Brazil. Summa. Phytopathol. 2014, 40, 185. [Google Scholar] [CrossRef][Green Version]
- Coutinho, T.A.; Wingfield, M.J. Ralstonia solanacearum and R. pseudosolanacearum on Eucalyptus: Opportunists or primary pathogens? Front. Plant Sci. 2017, 8, 761. [Google Scholar] [CrossRef]
- Steele, H.; Laue, B.E.; MacAskill, G.A.; Hendry, S.J.; Green, S. Analysis of the natural infection of European horse chestnut (Aesculus hippocastanum) by Pseudomonas syringae pv. aesculi. Plant Pathol. 2010, 59, 1005–1013. [Google Scholar] [CrossRef]
- Denman, S.; Doonan, J.; Ransom-Jones, E.; Broberg, M.; Plummer, S.; Kirk, S.; Scarlett, K.; Griffiths, A.R.; Kaczmarek, M.; Forster, J.; et al. Microbiome and infectivity studies reveal complex polyspecies tree disease in Acute Oak Decline. ISME J. 2018, 12, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Amirabad, Y.; Rahimian, H.; Babaeizad, V.; Denman, S. Brenneria spp. and Rahnellavictoriana associated with acute oak decline symptoms on oak and hornbeam in Iran. For. Pathol. 2019, 49, e12535. [Google Scholar] [CrossRef]
- Snelling, J.; Tisserat, N.A.; Cranshaw, W. Kermes scale (Allokermes sp.) and the drippy nut pathogen (Brenneria quercina) associated with a decline of red oak species in Colorado. Phytopathology 2011, 101, S168. [Google Scholar]
- Sitz, R.A.; Zerillo, M.M.; Snelling, J.; Ibarra Caballero, J.; Alexander, K.; Nash, K.; Tisserat, N.A.; Cranshaw, W.S.; Stewart, J.E. Drippy blight, a disease of red oaks in Colorado, US, produced from the combined effect of the scale insect Allokermes galliformis and the bacterium Lonsdalea quercina subsp. quercina. Arboricu. Urban For. 2018, 43, 146–153. [Google Scholar]
- Sitz, R.A.; Aquino, V.M.; Tisserat, N.; Cranshaw, W.; Stewart, J.E. Insects visiting drippy blight diseased red oak trees are contaminated with the pathogenic bacterium Lonsdalea quercina. Plant Dis. 2019, 103, 1940–1946. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Ren, F.; Guo, L.; Chang, J.; Cleenwerck, I.; Ma, Y.; Wang, H. A canker disease of Populus × euramericana in China caused by Lonsdalea quercina subsp. populi. Plant Dis. 2014, 98, 368–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zlatković, M.; Tenorio-Baigorria, I.; Lakatos, T.; Tóth, T.; Koltay, A.; Pap, P.; Marković, M.; Orlović, S. Bacterial canker disease on Populus × euramericana caused by Lonsdalea populi in Serbia. Forests 2020, 11, 1080. [Google Scholar] [CrossRef]
- Li, A.; He, W. Molecular aspects of an emerging poplar canker caused by Lonsdalea populi. Front. Microbiol. 2019, 10, 2496. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Lee, I.M.; Davis, R.E.; Gundersen-Rindal, D.E. Phytoplasma: Phytopathogenic mollicutes. Ann. Rev. Microbiol. 2000, 54, 221–255. [Google Scholar] [CrossRef] [PubMed]
- Bertaccini, A.; Duduk, B.; Paltrinieri, S.; Contaldo, N. Phytoplasmas, and phytoplasma diseases: A severe threat to agriculture. Am. J. Plant Sci. 2014, 5, 1763–1788. [Google Scholar] [CrossRef]
- Marcone, C. Elm yellows: A phytoplasma disease of concern in forest and landscape ecosystems. For. Pathol. 2017, 47, e12324. [Google Scholar] [CrossRef]
- Büttner, C.; von Bargen, S.; Bandte, M.; Mühlbach, H.P. Forest diseases caused by viruses. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CABI: Wallingford, UK, 2013; pp. 50–75. [Google Scholar]
- Mühlbach, H.P.; Mielke-Ehret, N. Emaravirus. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M., Lefkowitz, E., Adams, M.J., Carstens, E.B., Eds.; Elsevier: Dordrecht, The Netherlands, 2011; pp. 767–770. [Google Scholar]
- Elbeaino, T.; Digiaro, M.; Mielke-Ehret, N.; Muehlbach, H.P.; Martelli, G.P. ICTV virus taxonomy profile: Fimoviridae. J. Gen. Virol. 2018, 99, 1478–1479. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R. Nematodes, an emerging threat to global forests: Assessment and management. Plant Pathol. J. 2012, 11, 99–113. [Google Scholar] [CrossRef]
- Vicente, C.S.; Nascimento, F.; Espada, M.; Barbosa, P.; Mota, M.; Glick, B.R.; Oliveira, S. Characterization of bacteria associated with pinewood nematode Bursaphelenchus xylophilus. PLoS ONE 2012, 7, e46661. [Google Scholar] [CrossRef]
- Umebayashi, T.; Fukuda, K.; Haishi, T.; Sotooka, R.; Zuhair, S.; Otsuki, K. The developmental process of xylem embolisms in pine wilt disease monitored by multipoint imaging using compact magnetic resonance imaging. Plant Physiol. 2011, 156, 943–951. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Anjo, S.I.; Fonseca, L.; Egas, C.; Manadas, B.; Abrantes, I. Bursaphelenchus xylophilus and B. mucronatus secretomes: A comparative proteomic analysis. Sci. Rep. 2016, 6, 39007. [Google Scholar] [CrossRef]
- Li, X.; Zhuo, K.; Luo, M.; Sun, L.; Liao, J. Molecular cloning and characterization of a calreticulin cDNA from the pinewood nematode Bursaphelenchus xylophilus. Exp. Parasitol. 2011, 128, 121–126. [Google Scholar] [CrossRef]
- Kikuchi, T.; Shibuya, H.; Aikawa, T.; Jones, J.T. Cloning and characterization of pectate lyases expressed in the esophageal gland of the pine wood nematode Bursaphelenchus xylophilus. Mol. Plant Microbe Int. 2006, 19, 280–287. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissena, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Tennekes, H.A. Time-cumulative toxicity of neonicotinoids: Experimental evidence and implications for environmental risk assessments. Int. J. Environ. Res. Public Health 2020, 17, 1629. [Google Scholar] [CrossRef] [PubMed]
- Gil García, M.D.; Martínez Galera, M.; Uclés, S.; Lozano, A.; Fernández-Alba, A.R. Ultrasound-assisted extraction based on QuEChERS of pesticide residues in honeybees and determination by LC-MS/MS and GC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 5195–5210. [Google Scholar] [CrossRef]
- Neves, A.P.; Colabuono, F.I.; Ferreira, P.A.L.; Kawakami, S.K.; Taniguchi Rubens, C.L.; Figueira, S.; Mahiques, M.M.; Montone, R.C.; Bícego, M.C. Depositional history of polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in an Amazon estuary during the last century. Sci. Total Environ. 2018, 615, 1262–1270. [Google Scholar] [CrossRef]
- Brasil, V.L.M.; Ramos Pinto, M.B.; Bonan, R.F.; Kowalski, L.P.; da Cruz Perez, D.E. Pesticides as risk factors for head and neck cancer: A review. J. Oral Pathol. Med. 2018, 47, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, W.C.; Smith, E.H.; Smith, J.R. Charles Valentine Riley: Founder of Modern Entomology; With Contributions by D.C. Weber; University of Alabama Press: Tuscaloosa, AL, USA, 2019; 214p. [Google Scholar]
- Mc Namara, L.; Griffin, C.T.; Fitzpatrick, D.; Kavanagh, K.; Carolan, J.C. The effect of entomopathogenic fungal culture filtrate on the immune response and haemolymph proteome of the large pine weevil, Hylobius abietis. Insect Biochem. Mol. Biol. 2018, 101, 1–13. [Google Scholar]
- Imoulan, A.; Hussain, M.; Kirk, P.M.; El Meziane, A.; Yao, Y.J. Entomopathogenic fungus Beauveria: Host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J. Asia Pac. Entomol. 2017, 20, 1204–1212. [Google Scholar] [CrossRef]
- Van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.-L.; Zhang, S.; Xie, X.-Q.; Shang, Y.; St. Leger, R.J.; Zhao, G.-P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Liu, Y.Q.; Min, S.H.I.; Huang, J.H.; Chen, X.X. Parasitoid wasps as effective biological control agents. J. Integr. Agr. 2019, 18, 705–715. [Google Scholar] [CrossRef]
- Barta, M.; Kautmanová, I.; Čičková, H.; Ferenčík, J.; Florián, S.; Novotný, J.; Kozánek, M. Hypocrealean fungi associated with populations of Ips typographus in West Carpathians and selection of local Beauveria strains for effective bark beetle control. Biologia 2018, 73, 53–65. [Google Scholar]
- Mayerhofer, J.; Enkerli, J.; Zelger, R.; Strasser, H. Biological control of the European cockchafer: Persistence of Beauveria brongniartii after long-term applications in the Euroregion Tyrol. BioControl 2015, 60, 617–629. [Google Scholar] [CrossRef]
- Davis, T.S.; Mann, A.J.; Malesky, D.; Jankowski, E.; Bradley, C. Laboratory and field evaluation of the entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for population management of spruce beetle, Dendroctonus rufipennis (Coleoptera: Scolytinae), in felled trees and factors limiting pathogen success. Environ. Entomol. 2018, 47, 594–602. [Google Scholar]
- Schrank, A.; Vainstein, M.H. Metarhizium anisopliae enzymes and toxins. Toxicon 2010, 56, 1267–1274. [Google Scholar]
- Kim, J.C.; Baek, S.; Park, S.E.; Kim, S.; Lee, M.R.; Jo, M.; Im, J.S.; Ha, P.; Kim, J.S.; Shin, T.Y. Colonization of Metarhizium anisopliae on the surface of pine tree logs: A promising biocontrol strategy for the Japanese pine sawyer, Monochamus alternatus. Fungal Biol. 2019, 124, 125–134. [Google Scholar] [CrossRef]
- Williams, C.D.; Dillon, A.B.; Harvey, C.D.; Hennessy, R.; Namara, L.M.; Griffin, C.T. Control of a major pest of forestry, Hylobius abietis, with entomopathogenic nematodes and fungi using eradicant and prophylactic strategies. For. Ecol. Manag. 2013, 305, 212–222. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Saleem, M.A.; Saeed, S.; Wakil, W.; Ishtiaq, M.; Ashraf, W.; Ahmed, N.; Ali, M.; Muhammad Ikram, R.; Yasin, M.; et al. Integration of entomopathogenic fungi and eco-friendly insecticides for management of red palm weevil, Rhynchophorus ferrugineus (Olivier). Saudi J. Biol. Sci. 2020, 27, 1811–1817. [Google Scholar] [CrossRef]
- Zhao, H.; Lovett, B.; Fang, W. Genetically engineering entomopathogenic fungi. Adv. Genet. 2016, 94, 137–163. [Google Scholar]
- Palyzová, A.; Svobodová, K.; Sokolová, L.; Novák, J.; Novotný, Č. Metabolic profiling of Fusarium oxysporum f. sp. conglutinans race 2 in dual cultures with biocontrol agents Bacillus amyloliquefaciens, Pseudomonas aeruginosa, and Trichoderma harzianum. Folia Microbiol. (Praha) 2019, 64, 779–787. [Google Scholar] [PubMed]
- Chen, L.; Bóka, B.; Kedves, O.; Nagy, D.; Szucs, A.; Champramary, S.; Roszik, R.; Patocskai, Z.; Münsterkötter, M.; Huynh, T.; et al. Towards the biological control of devastating forest pathogens from the genus Armillaria. Forests 2019, 10, 1013. [Google Scholar] [CrossRef]
- Kong, P.; Hong, C. Biocontrol of boxwood blight by Trichoderma koningiopsis Mb2. Crop Protect. 2017, 98, 124–127. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Wang, L.; Zhou, L.; Song, J. Study of the departure of pine wood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) from Monochamus alternatus (Coleoptera: Cerambycidae). J. Asia Pac. Entomol. 2020, 23, 981–987. [Google Scholar] [CrossRef]
- Chu, W.H.; Dou, Q.; Chu, H.L.; Wang, H.H.; Sung, C.K.; Wang, C.Y. Research advance on Esteya vermicola, a high potential biocontrol agent of pine wilt disease. Mycol. Prog. 2015, 14, 115. [Google Scholar] [CrossRef]
- Tellenbach, C.; Sieber, T.N. Do colonization by dark septate endophytes and elevated temperature affect pathogenicity of oomycetes? FEMS Microbiol. Ecol. 2012, 82, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Sipari, N.; Asiegbu, F.O. Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol. Control 2016, 99, 53–63. [Google Scholar] [CrossRef]
- Tellenbach, C.; Sumarah, M.W.; Grünig, C.R.; Miller, J.D. Inhibition of Phytophthora species by secondary metabolites produced by the dark septate endophyte Phialocephalaeuropaea. Fungal Ecol. 2013, 6, 12–18. [Google Scholar] [CrossRef]
- Oliva, J.; Messal, M.; Wendt, L.; Elfstrand, M. Quantitative interactions between the biocontrol fungus Phlebiopsis gigantea, the forest pathogen Heterobasidion annosum and the fungal community inhabiting Norway spruce stumps. For. Ecol. Manag. 2017, 402, 253–264. [Google Scholar] [CrossRef]
- Paz, I.C.P.; Santin, R.C.M.; Guimarães, A.M.; Pereira da Rosa, O.P.; Quecine, M.C.; Silva, M.C.P.; Azevedo, J.L.; Santos Matsumura, A.T. Biocontrol of Botrytis cinerea and Calonectria gracilis by eucalypts growth promoters Bacillus spp. Microb. Pathog. 2018, 121, 106–109. [Google Scholar] [CrossRef]
- Bovolini, M.P.; Lazarotto, M.; Gonzatto, M.P.; Campos de Sá, L.; Borges Junior, N. Preventive and curative control of Oidium eucalypti in Eucalyptus benthamii clonal seedlings. Revista Árvore 2018, 42, 420–504. [Google Scholar] [CrossRef]
- Cheffi, M.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Belka, M.; Vallat, A.; Rateb, M.E.; Tounsi, S.; Triki, M.A.; Belbahri, L. Olea europaea L. root endophyte Bacillus velezensis OEE1 counteracts oomycete and fungal harmful pathogens and harbours a large repertoire of secreted and volatile metabolites and beneficial functional genes. Microorganisms 2019, 7, 314. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- Jung, S.J.; Kim, N.K.; Lee, D.-H.; Hong, S.-I.; Lee, J.K. Screening and evaluation of Streptomyces species as a potential biocontrol agent against a wood decay fungus, Gloeophyllum trabeum. Mycobiology 2018, 46, 138–146. [Google Scholar] [CrossRef]
- Dahm, H.; Brzezińska, A.J.; Wrótniak-Drzewiecka, W.; Golińska, P.; Różycki, H.; Rai, M. Myxobacteria as a potential biocontrol agent effective against pathogenic fungi of economically important forest trees. Dendrobiologie 2015, 74, 13–24. [Google Scholar] [CrossRef]
- Masson, T.; Fabre, M.L.; Ferrelli, M.L.; Pidre, M.L.; Romanowski, V. Protein composition of the occlusion bodies of Epinotia aporema granulovirus. PLoS ONE 2019, 14, e0207735. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Fernández, M.M.; Diez, J.J. The use of mycoviruses in the control of forest diseases. Biocontrol. Sci. Technol. 2016, 26, 577–604. [Google Scholar] [CrossRef]
- Krstin, L.; Katanić, Z.; Ježić, M.; Poljak, I.; Nuskern, L.; Matković, I.; Idžojtić, M.; Ćurković-Perica, M. Biological control of chestnut blight in Croatia: An interaction between host sweet chestnut, its pathogen Cryphonectria parasitica and the biocontrol agent Cryphonectria hypovirus 1. Pest Manag. Sci. 2016, 73, 582–589. [Google Scholar] [CrossRef]
- Vainio, E.J.; Jurvansuu, J.; Hyder, R.; Kashif, M.; Piri, T.; Tuomivirta, T.; Poimala, A.; Xu, P.; Mäkelä, S.; Nitisa, D.; et al. Heterobasidion Partitivirus 13 mediates severe growth debilitation and major alterations in the gene expression of a fungal forest pathogen. J. Virol. 2018, 92, e01744-17. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Zoller, S.; Rigling, D. Detection and genetic characterization of a novel mycovirus in Hymenoscyphus fraxineus, the causal agent of ash dieback. Infect. Genet. Evol. 2014, 28, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Tomalak, M. Parasitic association of the mycetophagous wood nematode, Bursaphelenchus fraudulentus with the honey fungus Armillaria ostoyae. For. Pathol. 2017, 47, e12325. [Google Scholar] [CrossRef]
- Gao, S.K.; Wei, K.; Tang, Y.L.; Wang, X.Y.; Yang, Z.Q. Effect of parasitoid density on the timing of parasitism and development duration of progeny in Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2016, 97, 57–62. [Google Scholar] [CrossRef]
- Wang, X.; Wei, K.; Yang, Z.; Jennings, D.E.; Duan, J.J. Effects of biotic and abiotic factors on phenotypic partitioning of wing morphology and development in Sclerodermus pupariae (Hymenoptera: Bethylidae). Sci. Rep. 2016, 6, 26408. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.Y.; Yang, Z.Q. Effects of supplementary nutrition on parasitism ability and developmental process of a gregarious parasitoid, Sclerodermus pupariae (Hymenoptera: Bethylidae). For. Res. 2016, 29, 369–376. [Google Scholar]
- Wei, K.; Gao, S.K.; Tang, Y.L.; Wang, X.Y.; Yang, Z.Q. Determination of the optimal parasitoid-to-host ratio for efficient mass-rearing of the parasitoid, Sclerodermus pupariae (Hymenoptera: Bethylidae). J. Appl. Entomol. 2017, 141, 181–188. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Ann. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Burke, G.R. Polydnaviruses: Nature’s genetic engineers. Annu. Rev. Virol. 2014, 1, 333–354. [Google Scholar] [CrossRef]
- Strand, M.R. Teratocytes and their functions in parasitoids. Curr. Opin. Insect Sci. 2014, 6, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Forest microbiome: Diversity, complexity and dynamics. FEMS Microbiol. Rev. 2017, 41, 109–130. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Abrantes, I.; Barra Caracciolo, A.; Bevivino, A.; Ciancio, A.; Grenni, P.; Hrynkiewicz, K.; Kredics, L.; Proença, D.N. Belowground microbiota and the health of tree crops. Front. Microbiol. 2018, 9, 1006. [Google Scholar] [CrossRef]
- Mueller, U.G.; Sachs, J.L. Engineering microbiomes to improve plant and animal health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2. [Google Scholar] [CrossRef]
- Hacquard, S.; Schadt, C.W. Towards a holistic understanding of the beneficial interactions across the Populus microbiome. New Phytol. 2005, 205, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, S.; Or, D. Synthetic microbial ecology: Engineering habitats for modular consortia. Front. Microbiol. 2017, 8, 1125. [Google Scholar] [CrossRef]
- Zmora, N.; Zeevi, D.; Korem, T.; Segal, E.; Elinav, E. Taking it personally: Personalized utilization of the human microbiome in health and disease. Cell Host Microbe 2016, 19, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.C.; Rocha-Granados, M.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Lebeis, S.L. Greater than the sum of their parts: Characterizing plant microbiomes at the community-level. Curr. Opin. Plant Biol. 2015, 24, 82–86. [Google Scholar] [CrossRef]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.L.; Ling, H.; Lee, Y.S.; Chang, M.W. Microbiome engineering: Current applications and its future. Biotechnol. J. 2017, 12, 1600099. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—from metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Llado, S.; Lopez-Mondejar, R.; Baldrian, P. Forest soil bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Eloe-Fadrosh, E.A.; Ivanova, N.N.; Woyke, T.; Kyrpides, N.C. Metagenomics uncovers gaps in amplicon-based detection of microbial diversity. Nat. Microbiol. 2016, 1, 15032. [Google Scholar] [CrossRef]
- Albertsen, M.; Hugenholtz, P.; Skarshewski, A.; Nielsen, K.L.; Tyson, G.W.; Nielsen, P.H. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 2013, 31, 533–538. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Thomas, B.C.; Sharon, I.; Miller, C.S.; Castelle, C.J.; VerBerkmoes, N.C.; Wilkins, M.J.; Hettich, R.L.; Lipton, M.S.; Williams, K.H.; et al. Fermentation, hydrogen, and sulfur metabolism in multiple uncultivated bacterial phyla. Science 2012, 337, 1661–1665. [Google Scholar] [CrossRef]
- Alteio, L.V.; Schulz, F.; Seshadri, R.; Varghese, N.; Rodriguez-Reillo, W.; Ryan, E.; Goudeau, D.; Eichorst, S.A.; Malmstrom, R.R.; Bowers, R.M.; et al. Complementary metagenomic approaches improve reconstruction of microbial diversity in a forest soil. Msystems 2020, 5, e00768-19. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Garcia-Lemos, A.M.; Großkinsky, D.K.; Stokholm, M.S.; Lund, O.S.; Nicolaisen, M.H.; Roitsch, T.G.; Veierskov, B.; Nybroe, O. Root-associated microbial communities of Abies nordmanniana: Insights into interactions of microbial communities with antioxidative enzymes and plant growth. Front. Microbiol. 2019, 10, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hrynkiewicz, K.; Szymanska, S.; Piernik, A.; Thiem, D. Ectomycorrhizal community structure of Salix and Betula spp. at a saline site in central Poland in relation to the seasons and soil parameters. Water Air Soil Pollut. 2015, 226, 99. [Google Scholar] [CrossRef]
- Khabou, W.; Hajji, B.; Mohamed Zouari, M.; Rigane, H.; Ben Abdallah, F. Arbuscular mycorrhizal fungi improve growth and mineral uptake of olive tree under gypsum substrate. Ecol. Eng. 2014, 73, 290–296. [Google Scholar] [CrossRef]
- Lau, J.A.; Lennon, J.T.; Heath, K.D. Trees harness the power of microbes to survive climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 11009–11011. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2015, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Zeng, R.S.; Xu, J.F.; Li, J.; Shen, X.; Yihdego, W.J. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE 2010, 5, e13324. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.; Sbodio, A.; Tech, J.J.; Suslow, T.V.; Coaker, G.L.; Leveau, J.H.J. Leaf microbiota in an agroecosystem: Spatiotemporal variation in bacterial community composition on field-grown lettuce. ISME J. 2012, 6, 1812–1822. [Google Scholar] [CrossRef]
- Stamets, P. Mycelium Running: How Mushrooms Can Help Save the World; Random House Digital, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Burch, A.Y.; Lindow, S.E.; Post, A.F.; Belkin, S. Geographical location determines the population structure in phyllosphere microbial communities of a salt-excreting desert tree. Appl. Environ. Microbiol. 2011, 77, 7647–7655. [Google Scholar] [CrossRef]
- Peñuelas, J.; Terradas, J. The foliar microbiome. Trends Plant Sci. 2014, 19, 278–280. [Google Scholar] [CrossRef]
- Galippe, V. Note sur la présence de micro-organismes dans les tissus végétaux (deuxième note). C R Seances Soc. Biol. Fil. 1887, 39, 557–560. [Google Scholar]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Ann. Rev. Phytopathol. 2009, 49, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Christian, N.; Sullivan, C.; Visser, N.D.; Clay, K. Plant host and geographic location drive endophyte community composition in the face of perturbation. Microb. Ecol. 2016, 72, 621–632. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological control of Pseudomonas syringae pv. actinidiae (Psa), the causal agent of bacterial canker of kiwifruit, using endophytic bacteria recovered from a medicinal plant. Biol. Control 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Yaish, W.M.; Al-Harrasi, I.; Alansari, A.S.; Al-Yahyai, R.; Glick, B.R. The use of high throughput DNA sequence analysis to assess the endophytic microbiome of date palm roots grown under different levels of salt stress. Int. Microbiol. 2017, 19, 143–155. [Google Scholar]
- Yuan, Z.; Druzhinina, I.; Labbé, J.; Redman, R.; Qin, Y.; Rodriguez, R.; Zhang, C.; Tuskan, G.A.; Lin, F. Specialized microbiome of a halophyte and its role in helping non-host plants to withstand salinity. Sci. Rep. 2016, 6, 32467. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Hernández-Pacheco, C.E.; Santoyo, G. Evaluation of Bacillus and Pseudomonas to colonize the rhizosphere and their effect on growth promotion in tomato (Physalis ixocarpa Brot. ex Horm.). Rev. Chapingo Ser. Hortic. 2016, 22, 45–57. [Google Scholar] [CrossRef]
- Timm, C.M.; Pelletier, D.A.; Jawdy, S.S.; Gunter, L.E.; Henning, J.A.; Engle, N.; Lu, T.Y. Two poplar-associated bacterial isolates induce additive favorable responses in a constructed plant-microbiome system. Front. Plant Sci. 2016, 7, 497. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Smith, F.A.; Smith, S.E. What is the significance of the arbuscular mycorrhizal colonization of many economically important crop plants? Plant Soil 2011, 348, 63. [Google Scholar] [CrossRef]
- Yuan, Z.-L.; Zhang, C.; Lin, F.-C. Role of diverse non-systemic fungal endophytes in plant performance and response to stress: Progress and approaches. J. Plant Growth Regul. 2010, 29, 116–126. [Google Scholar] [CrossRef]
- Oelmüller, R.; Sherameti, I.; Tripathi, S.; Varma, A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 2009, 49, 1–17. [Google Scholar] [CrossRef]
- Narayan, O.P.; Verma, N.; Singh, A.K.; Oelmüller, R.; Kumar, M.; Prasad, D.; Kapoor, R.; Dua, M.; Johri, A.K. Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Sci. Rep. 2017, 7, 13553. [Google Scholar] [CrossRef] [PubMed]
- Likar, M.; Regvar, M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 2013, 370, 593–604. [Google Scholar] [CrossRef]
- Zachow, C.; Berg, C.; Muller, H.; Monk, J.; Berg, G. Endemic plants harbour specific Trichoderma communities with an exceptional potential for biocontrol of phytopathogens. J. Biotechnol. 2016, 235, 162–170. [Google Scholar] [CrossRef]
- Bae, H.; Roberts, D.P.; Lim, H.-S.; Strem, M.D.; Park, S.-C.; Ryu, C.-M.; Rachel Melnick, R.; Bailey, B.R. Endophytic-Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol. Plant Micr. Interact. 2011, 24, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Romeralo, C.; Witzell, J.; Romeralo-Tapia, R.; Botella, L.; Javier Diez, J. Antagonistic activity of fungal endophyte filtrates against Gremmeniella abietina infections on Aleppo pine seedlings. Eur. J. Plant Pathol. 2015, 143, 691–704. [Google Scholar] [CrossRef]
- Ben Mefteh, F.; Daoud, A.; Chenari Bouket, A.; Thissera, B.; Kadri, Y.; Cherif-Silini, H.; Eshelli, M.; Alenezi, F.N.; Vallat, A.; Oszako, T.; et al. Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 2018, 19, 1986. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.-P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal endophytes for biocontrol of ash dieback: The antagonistic potential of Hypoxylon rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Afolabi, O.G.; Hussain, M.; Qasim, M.; Wang, L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018, 217, 34–50. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.; Dennis, P.G.; Paungfoo-Lonhienne, C.; Weber, L.; Brackin, R.; Ragan, M.A.; Schmidt, S.; Hugenholgz, P. Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat. Commun. 2017, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Oter, R.; Nakano, R.T.; Dombrowski, N.; Ma, K.-W.; McHardy, A.C.; Schulze-Lefert, P. Modular traits of the Rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe 2018, 24, 155–167. [Google Scholar] [CrossRef]
- Doty, S.L.; Oakley, B.; Xin, G.; Kang, J.W.; Singleton, G.; Khan, Z.; Vajzovic, A.; Staley, J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 2009, 47, 23–33. [Google Scholar] [CrossRef]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathogenesis 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Brader, G.; Compant, G.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic potential of endophytic bacteria. Curr. Opin. Biotech. 2014, 27, 30–37. [Google Scholar] [CrossRef]
- Bordiec, S.; Paquis, S.; Lacroix, H.; Dhondt, S.; Ait Barka, E.; Kauffmann, S.; Jeandet, P.; Mazeyrat-Gourbeyre, F.; Clément, C.; Baillieul, F.; et al. Comparative analysis of defense responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J. Exp. Bot. 2011, 62, 595–603. [Google Scholar] [CrossRef]
- Denance, N.; Sanchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, B.G.; Licastro, D.; Mendonça-Previato, L.; Cámara, M.; Venturi, V. Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol. Plant Micr. Interact 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Mitter, B.N.; Pfaffenbichler, R.; Flavell, S.; Compant, L.; Antonielli, A.; Petric, A.; Sessitsch, A. A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front. Microbiol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Vainio, E.J.; Muller, M.M.; Korhonen, K.; Piri, T.; Hantula, J. Viruses accumulate in aging infection centers of a fungal forest pathogen. ISME J. 2015, 9, 497–507. [Google Scholar] [CrossRef]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Hungate, B.A.; Mau, R.L.; Schwartz, E.; Caporaso, J.G.; Dijkstra, P.; van Gestel, N.; Koch, B.J.; Liu, C.M.; McHugh, T.A.; Marks, J.C.; et al. Quantitative microbial ecology through stable isotope probing. Appl. Environ. Microb. 2015, 81, 7570–7581. [Google Scholar] [CrossRef]
- Ihrmark, K.; Bodeker, I.T.M.; Cruz-Martinez, K.; Friberg, H.; Kubartova, A.; Schenck, J.; Strid, Y.; Stenlid, J.; Brandström-Durling, M.; Clemmensen, K.E.; et al. New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 2012, 82, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2016, 24, 3315–3335. [Google Scholar] [CrossRef]
- Pandey, A.; Yarzábal, L.A. Bioprospecting cold-adapted plant growth promoting microorganisms from mountain environments. Appl. Microbiol. Biotechnol. 2019, 103, 643–657. [Google Scholar] [CrossRef]
- Rao, M.V.; Rice, R.A.; Fleischer, R.C.; Muletz-Wolz, C.R. Soil fungal communities differ between shaded and sun-intensive coffee plantations in El Salvador. PLoS ONE 2020, 15, e0231875. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Jeon, Y.-D.; Lee, O.-M.; Kim, J.-D.; Lee, N.-R.; Park, G.-T.; Son, H.-J. Characterization of a multifunctional feather-degrading Bacillus subtilis isolated from forest soil. Biodegradation 2010, 21, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR properties of cellulolytic, nitrogen-fixing, phosphate-solubilizing bacteria in rehabilitated tropical forest soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Sustaining the growth of Pinaceae trees under nutrient-limited edaphic conditions via plant-beneficial bacteria. PLoS ONE 2020, 15, e0238055. [Google Scholar] [CrossRef] [PubMed]
- Hortal, S.; Pera, J.; Parladé, J. Field persistence of the edible ectomycorrhizal fungus Lactarius deliciosus: Effect of inoculation strain, initial colonization level, and site characteristics. Mycorrhiza 2009, 19, 167–177. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. https://doi.org/10.3390/f12111579
Balla A, Silini A, Cherif-Silini H, Chenari Bouket A, Moser WK, Nowakowska JA, Oszako T, Benia F, Belbahri L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests. 2021; 12(11):1579. https://doi.org/10.3390/f12111579
Chicago/Turabian StyleBalla, Amel, Allaoua Silini, Hafsa Cherif-Silini, Ali Chenari Bouket, Warren Keith Moser, Justyna Anna Nowakowska, Tomasz Oszako, Farida Benia, and Lassaad Belbahri. 2021. "The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems" Forests 12, no. 11: 1579. https://doi.org/10.3390/f12111579
APA StyleBalla, A., Silini, A., Cherif-Silini, H., Chenari Bouket, A., Moser, W. K., Nowakowska, J. A., Oszako, T., Benia, F., & Belbahri, L. (2021). The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests, 12(11), 1579. https://doi.org/10.3390/f12111579








