Soil Aggregate-Associated Carbon Fraction Dynamics during the Process of Tea (Camellia sinensis L.) Planting in Southern Guangxi, China
Abstract
:1. Introduction
2. Materials and Methods
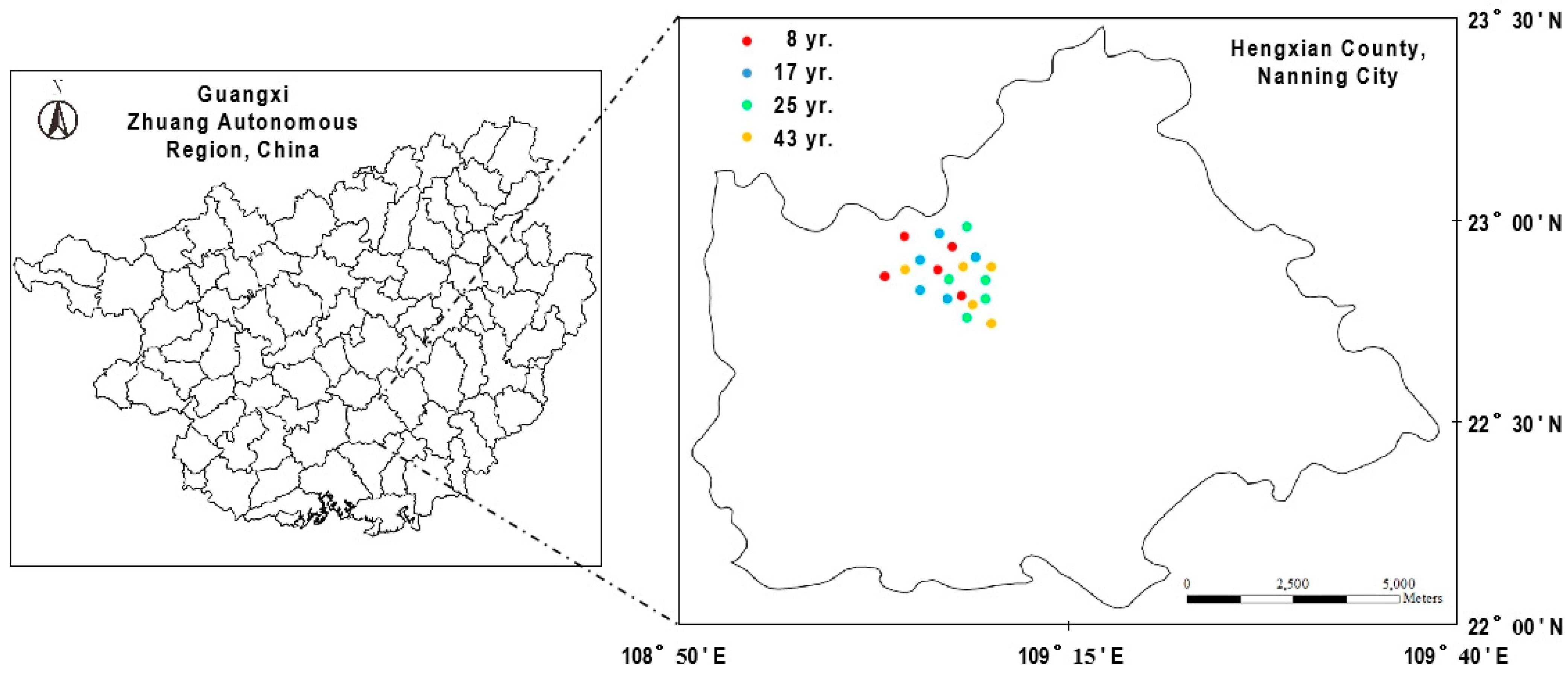
2.1. Location of the Experiment
2.2. Design of the Experiment
2.3. Sampling for Litter and Soil
2.4. Separation of Soil Aggregates
2.5. Analyses of Soil Physic-Chemical Properties
2.6. Analyses of Calculations and Statistics
3. Results
3.1. Composition and Stability of Soil Aggregates
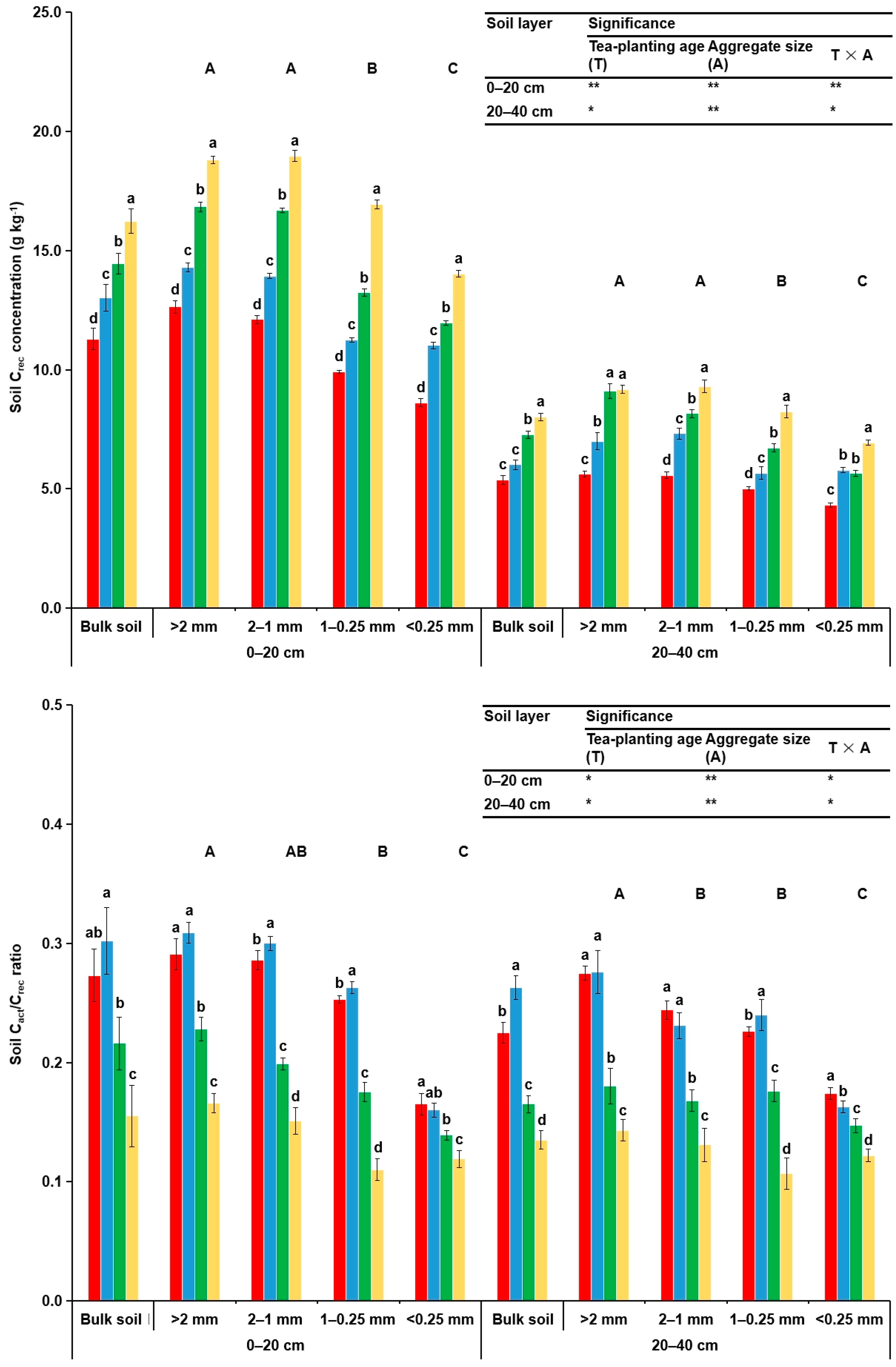
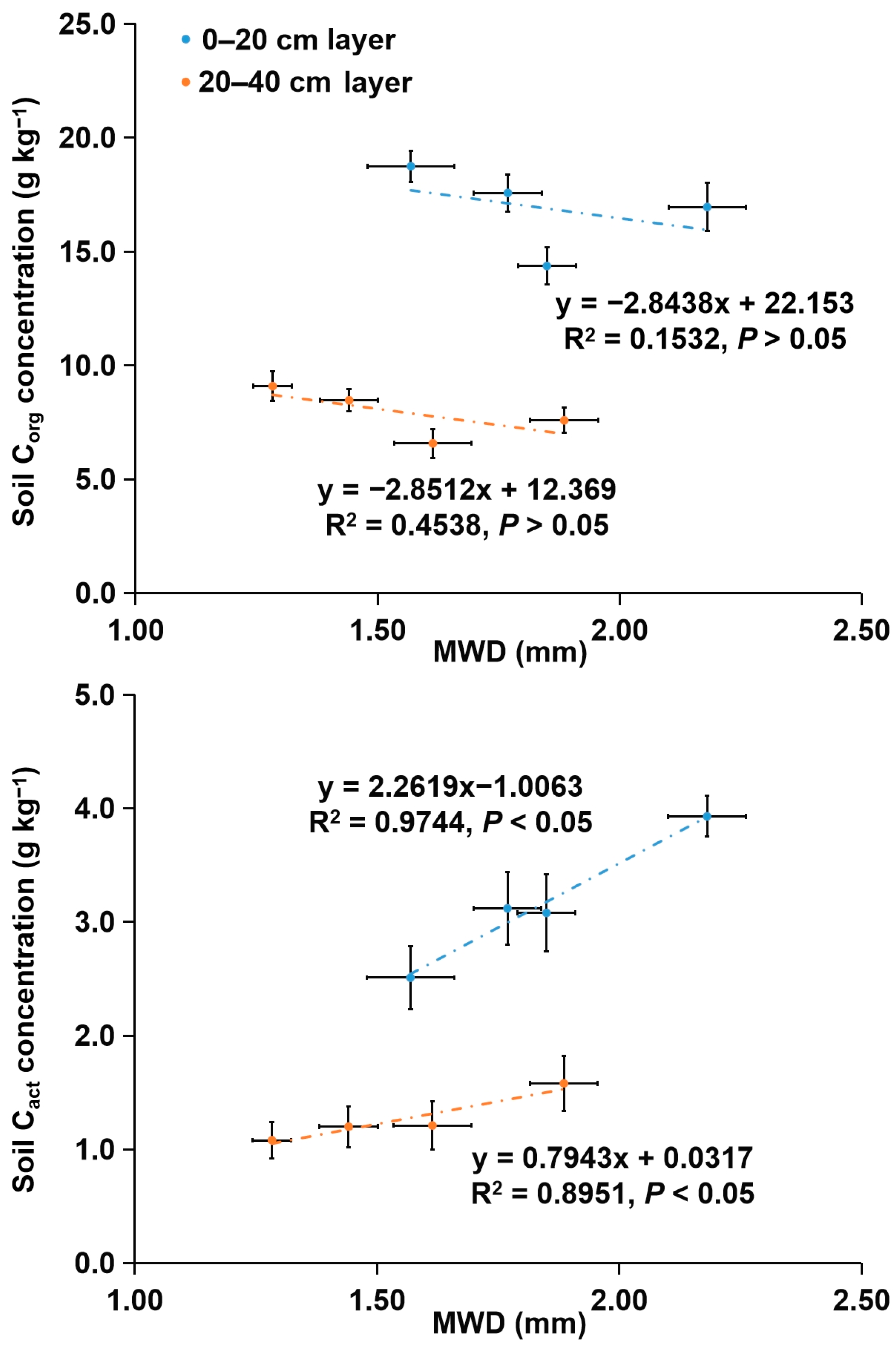
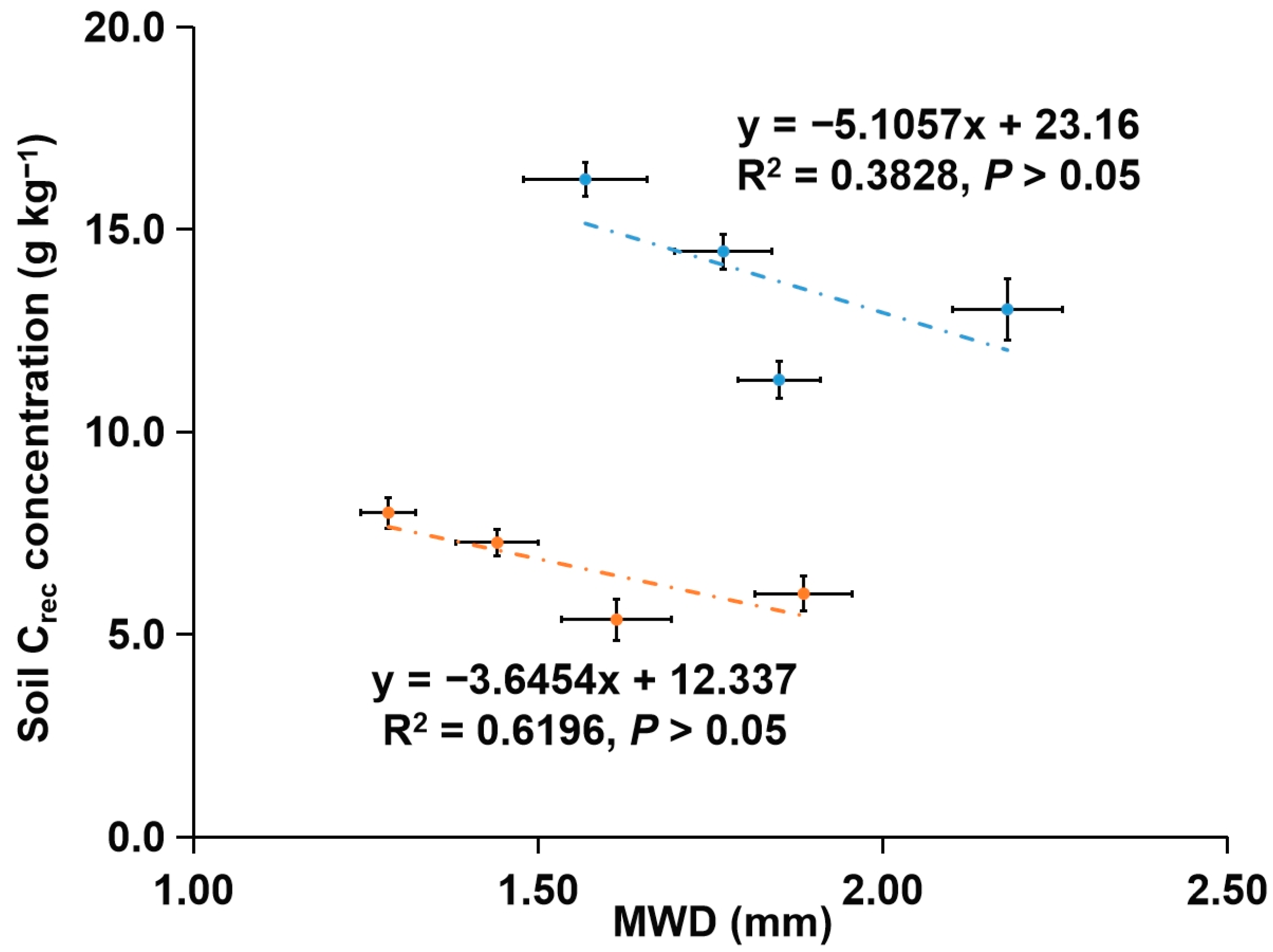
3.2. Concentrations of Corg and Its Fractions in Soil Aggregates
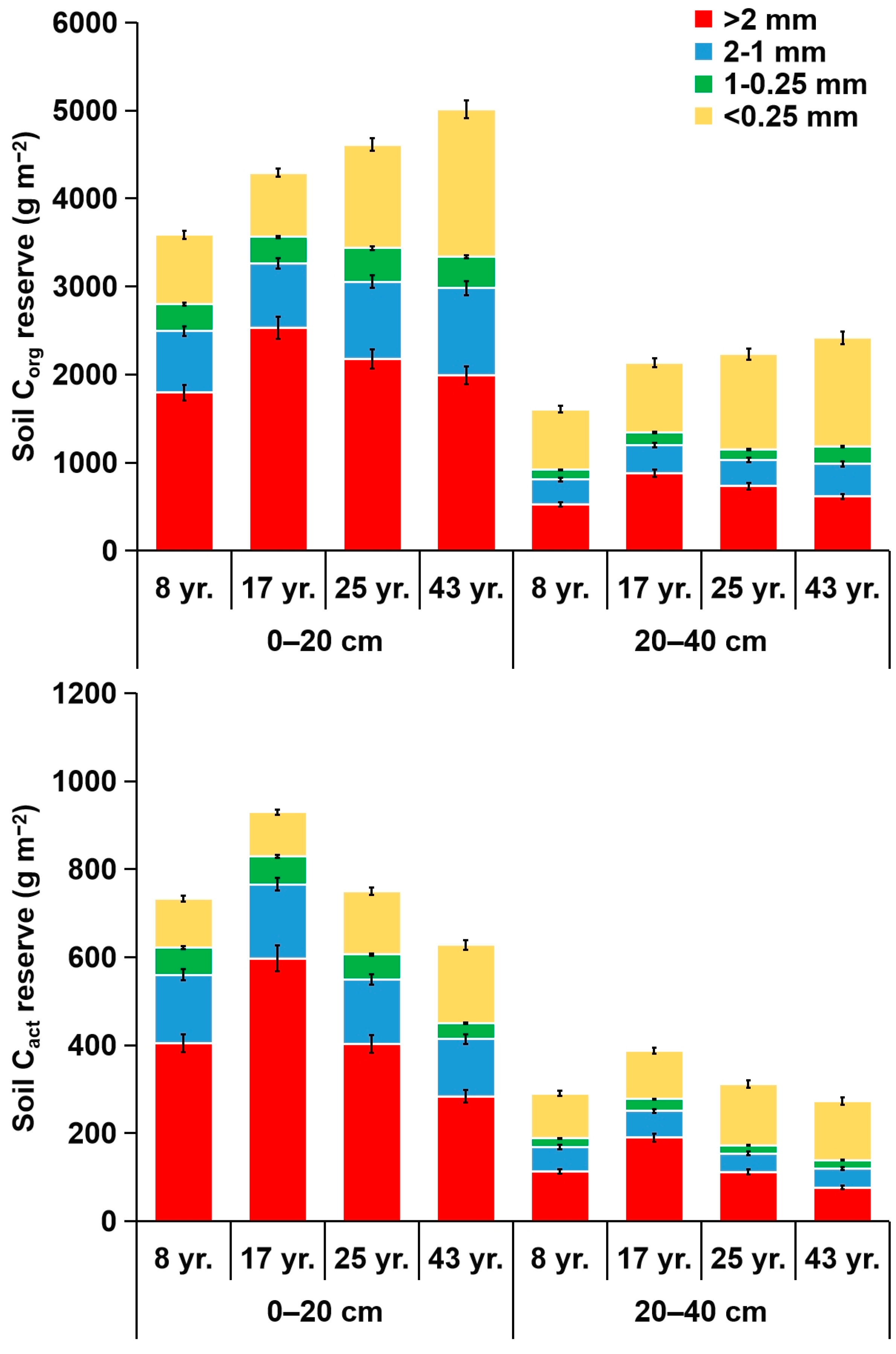
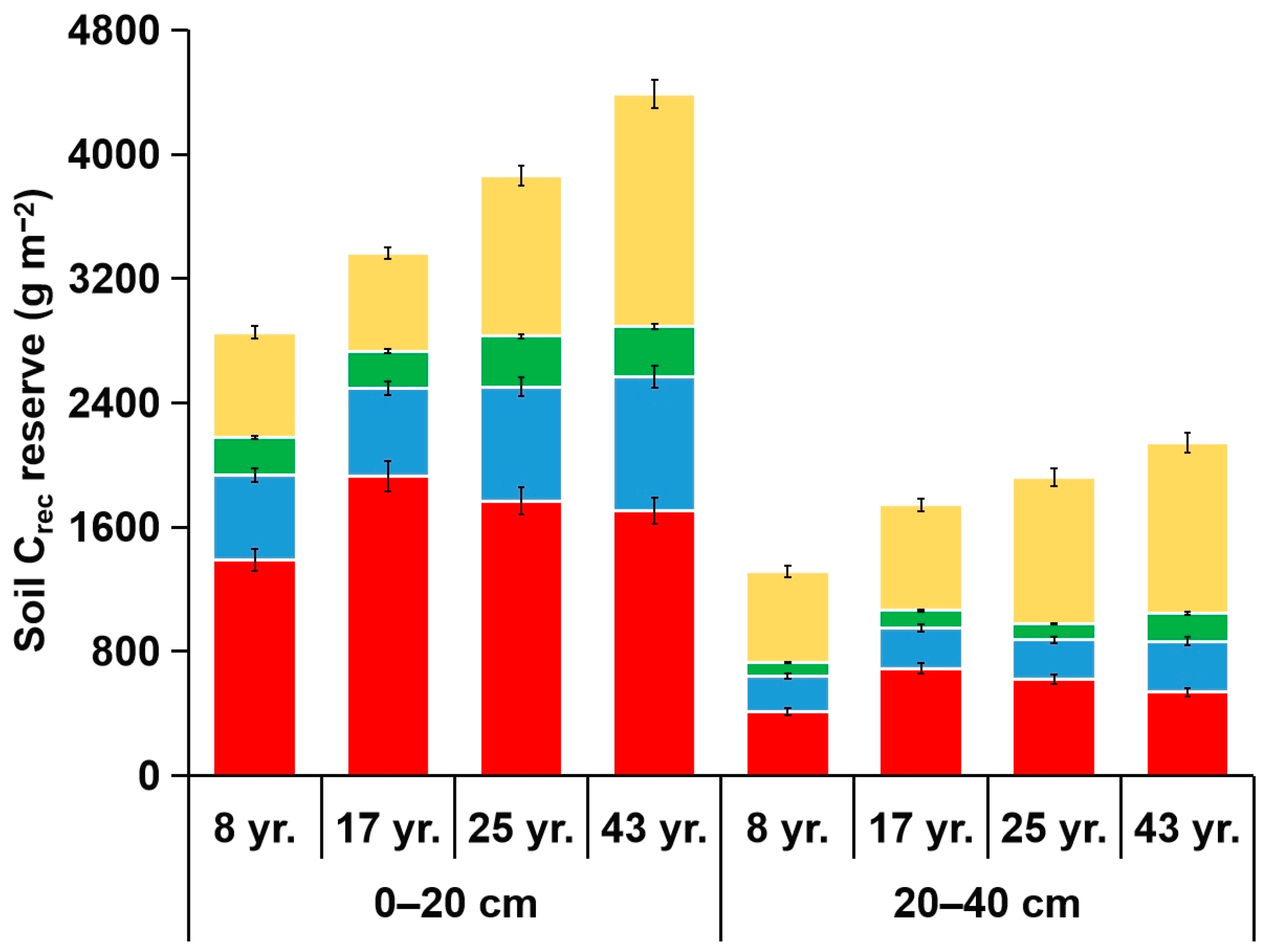
3.3. Reserves of Corg and Its Fractions in Soil Aggregates
4. Discussion
4.1. Composition and Stability of Soil Aggregates
4.2. Concentrations of Corg and Its Fractions in Soil Aggregates
4.3. Reserves of Corg and Its Fractions in Soil Aggregates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Tea Committee. An area planted with tea in various provinces at the end of each year. Ann. Bull. Stat. 2020, 47, 20. [Google Scholar]
- Jiang, J.; Jiang, W.; Jiang, W. The present situation and countermeasures of the development of tea industry in Guangxi. Popular Sci. Technol. 2018, 20, 72–73. [Google Scholar] [CrossRef]
- Bleuler, M.; Farina, R.; Francaviglia, R.; Di Bene, C.; Napoli, R.; Marchetti, A. Modelling the impacts of different carbon sources on the soil organic carbon stock and CO2 emissions in the Foggia province (Southern Italy). Agric. Syst. 2017, 157, 258–268. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Chen, M. Carbon flow analysis of China’s agro-ecosystem from 1980 to 2013: A perspective from substance flow analysis. J. Environ. Sci. 2017, 55, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Mizuta, K.; Taguchi, S.; Sato, S. Soil aggregate formation and stability induced by starch and cellulose. Soil Biol. Biochem. 2015, 87, 90–96. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Xie, J.; Wang, Y.; Liu, X. Accumulation of organic carbon and its association with macro-aggregates during 100 years of oasis formation. CATENA 2018, 172, 770–780. [Google Scholar] [CrossRef]
- Voltolini, M.; Taş, N.; Wang, S.; Brodie, E.; Ajo-Franklin, J.B. Quantitative characterization of soil micro-aggregates: New opportunities from sub-micron resolution synchrotron X-ray microtomography. Geoderma 2017, 305, 382–393. [Google Scholar] [CrossRef] [Green Version]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Medinski, T.V.; Freese, D.; Böhm, C.; Slazak, A. Soil carbon fractions in short rotation poplar and black locust coppices, Germany. Agrofor. Syst. 2014, 88, 505–515. [Google Scholar] [CrossRef]
- Modak, K.; Ghosh, A.; Bhattacharyya, R.; Biswas, D.R.; Das, T.K.; Das, S.; Singh, G. Response of oxidative stability of aggregate-associated soil organic carbon and deep soil carbon sequestration to zero-tillage in subtropical India. Soil Tillage Res. 2019, 195, 104370. [Google Scholar] [CrossRef]
- Li, G.-L.; Pang, X.-M. Effect of land-use conversion on C and N distribution in aggregate fractions of soils in the southern Loess Plateau, China. Land Use Policy 2010, 27, 706–712. [Google Scholar] [CrossRef]
- Zeng, L.; Li, J.; Zhou, Z.; Yu, Y. Optimizing land use patterns for the grain for Green Project based on the efficiency of ecosystem services under different objectives. Ecol. Indic. 2020, 114, 106347. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, W.; Su, S.; Weng, M.; Cai, Z. Biophysical and socioeconomic determinants of tea expansion: Apportioning their relative importance for sustainable land use policy. Land Use Policy 2017, 68, 438–447. [Google Scholar] [CrossRef]
- Li, W.; Zheng, Z.; Li, T.; Zhang, X.; Wang, Y.; Yu, H.; He, S.; Liu, T. Effect of tea plantation age on the distribution of soil organic carbon fractions within water-stable aggregates in the hilly region of Western Sichuan, China. CATENA 2015, 133, 198–205. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, Z.; Li, T.; Zhang, X.; He, S.; Wang, Y.; Liu, T.; Li, W. Dynamics of soil organic carbon mineralization in tea plantations converted from farmland at Western Sichuan, China. PLoS ONE 2017, 12, e0185271. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Tang, L.; Ye, S. Dynamics of soil bacterial community diversity and composition at aggregate scales in a chronosequence of tea gardens. CATENA 2021, 206, 105486. [Google Scholar] [CrossRef]
- Wang, S.; Ye, S. Dynamics of bacterial function characteristics in soil aggregates during the tea-planting process. Geoderma 2020, 377, 114609. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; Part 3, No. 5; pp. 961–1010. [Google Scholar]
- Bremner, J.M. Nitrogen-total. In Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; Part 3, No. 5; pp. 1085–1121. [Google Scholar]
- Six, J.; Conant, R.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Kurmi, B.; Nath, A.J.; Lal, R.; Das, A.K. Water stable aggregates and the associated active and recalcitrant carbon in soil under rubber plantation. Sci. Total. Environ. 2020, 703, 135498. [Google Scholar] [CrossRef]
- Nimmo, J.R.; Perkins, K.S. Aggregate stability and size distribution methods of soil analysis. In Physical Methods; Dane, J.H., Ed.; ASA and SSSA: Madison, WI, USA, 1996; Part 4, No. 5; pp. 317–328. [Google Scholar]
- Eynard, A.; Schumacher, T.; Lindström, M.; Malo, D. Effects of agricultural management systems on soil organic carbon in aggregates of Ustolls and Usterts. Soil Tillage Res. 2005, 81, 253–263. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, S.; Ghoshal, N. Impact of land use change on soil aggregate dynamics in the dry tropics. Restor. Ecol. 2017, 25, 962–971. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Douelle, A.; Kwaw-Mensah, D. Soil microaggregate and macroaggregate decay over time and soil carbon change as influenced by different tillage systems. J. Soil Water Conserv. 2014, 69, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Fang, S.; Chen, H.Y.; Zhu, R.; Peng, S.; Ruan, H. Soil Aggregation and Organic Carbon Dynamics in Poplar Plantations. Forests 2018, 9, 508. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Tan, W.; Chen, X. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res. 2018, 185, 10. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Zheng, Z. Distribution of microbial biomass and activity within soil aggregates as affected by tea plantation age. CATENA 2017, 153, 29. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, Z.; Li, T.; He, S.; Zhang, X.; Wang, Y.; Liu, T. Effect of tea plantation age on the distribution of glomalin-related soil protein in soil water-stable aggregates in southwestern China. Environ. Sci. Pollut. Res. 2018, 26, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, C.; Gu, F.; Wang, Y.; Chen, J. Soil aggregate stability improves greatly in response to soil water dynamics under natural rains in long-term organic fertilization. Soil Tillage Res. 2018, 184, 281–290. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, Z.; Li, T.; He, S.; Zhang, X.; Wang, Y.; Liu, T. Distribution of inorganic phosphorus fractions in water-stable aggregates of soil from tea plantations converted from farmland in the hilly region of western Sichuan, China. J. Soils Sediments 2017, 18, 906–916. [Google Scholar] [CrossRef]
- Yin, J.; Zheng, Z.; Li, T.; Zhang, X.; He, S.; Wang, Y.; Yu, H.; Liu, T. Effect of tea plantation age on the distribution of fluoride and its fractions within soil aggregates in the hilly region of Western Sichuan, China. J. Soils Sediments 2016, 16, 2128–2137. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Luna, L.; Miralles, I.; Andrenelli, M.C.; Gispert, M.; Pellegrini, S.; Vignozzi, N.; Solé-Benet, A. Restoration techniques affect soil organic carbon, glomalin and aggregate stability in degraded soils of a semiarid Mediterranean region. CATENA 2016, 143, 256–264. [Google Scholar] [CrossRef]
- Li, C.; Cao, Z.; Chang, J.; Zhang, Y.; Zhu, G.; Zong, N.; He, Y.; Zhang, J.; He, N. Elevational gradient affect functional fractions of soil organic carbon and aggregates stability in a Tibetan alpine meadow. CATENA 2017, 156, 139–148. [Google Scholar] [CrossRef]
- Xue, B.; Huang, L.; Huang, Y.; Zhou, F.; Li, F.; Kubar, K.A.; Li, X.; Lu, J.; Zhu, J. Roles of soil organic carbon and iron oxides on aggregate formation and stability in two paddy soils. Soil Tillage Res. 2019, 187, 161–171. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Jastrow, J. Physical and chemical protection in hierarchical soil aggregates regulates soil carbon and nitrogen recovery in restored perennial grasslands. Soil Biol. Biochem. 2013, 61, 31. [Google Scholar] [CrossRef]
- Gentile, R.; Vanlauwe, B.; Six, J. Integrated Soil Fertility Management: Aggregate carbon and nitrogen stabilization in differently textured tropical soils. Soil Biol. Biochem. 2013, 67, 124–132. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Loss, A.; Lourenzi, C.R.; dos Santos, E.; Mergen, C.A.; Benedet, L.; Pereira, M.; Piccolo, M.D.C.; Brunetto, G.; Lovato, P.E.; Comin, J.J. Carbon, nitrogen and natural abundance of 13C and 15N in biogenic and physicogenic aggregates in a soil with 10 years of pig manure application. Soil Tillage Res. 2017, 166, 52–58. [Google Scholar] [CrossRef]
- Varvel, G.; Wilhelm, W. No-tillage increases soil profile carbon and nitrogen under long-term rainfed cropping systems. Soil Tillage Res. 2011, 114, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Ostertag, R.; Marin-Spiotta, E.; Silver, W.L.; Schulten, J. Litterfall and Decomposition in Relation to Soil Carbon Pools Along a Secondary Forest Chronosequence in Puerto Rico. Ecosystems 2008, 11, 701–714. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2012, 19, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fierer, N.; Schimel, J.P.; A Holden, P. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Su, Y.Z.; Liu, W.J.; Yang, R.; Chang, X.X. Changes in Soil Aggregate, Carbon, and Nitrogen Storages Following the Conversion of Cropland to Alfalfa Forage Land in the Marginal Oasis of Northwest China. Environ. Manag. 2009, 43, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef] [Green Version]




| Soil Layer (cm) | Tea-Planting Age (Year) | Soil | ||||
|---|---|---|---|---|---|---|
| Bulk Density (g cm−3) | Sand (2–0.02 mm) (%) | Silt (0.02–0.002 mm) (%) | Clay (<0.002 mm) (%) | pH | ||
| 0–20 | 8 | 1.29 ± 0.03 a | 30.18 ± 0.14 a | 36.21 ± 0.28 a | 33.61 ± 0.16 a | 4.57 ± 0.03 a |
| 17 | 1.27 ± 0.04 a | 31.24 ± 0.22 a | 36.33 ± 0.24 a | 32.43 ± 0.17 a | 4.51 ± 0.02 a | |
| 25 | 1.30 ± 0.03 a | 30.84 ± 0.16 a | 35.89 ± 0.21 a | 33.27 ± 0.18 a | 4.32 ± 0.01 b | |
| 43 | 1.31 ± 0.02 a | 29.31 ± 0.23 a | 37.46 ± 0.11 a | 33.23 ± 0.09 a | 4.17 ± 0.02 c | |
| 20–40 | 8 | 1.34 ± 0.04 a | 29.00 ± 0.32 a | 38.68 ± 0.36 a | 32.32 ± 0.25 a | 4.62 ± 0.01 a |
| 17 | 1.36 ± 0.03 a | 27.40 ± 0.48 a | 39.42 ± 0.21 a | 33.18 ± 0.36 a | 4.58 ± 0.03 a | |
| 25 | 1.41 ± 0.05 a | 30.46 ± 0.57 a | 38.47 ± 0.19 a | 31.07 ± 0.45 a | 4.45 ± 0.02 b | |
| 43 | 1.37 ± 0.02 a | 28.89 ± 0.32 a | 38.22 ± 0.34 a | 32.89 ± 0.27 a | 4.26 ± 0.04 c | |
| Soil Layer (cm) | Tea-Planting Age (Year) | Distribution of Soil Aggregates (%) | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| >2 mm | 2–1 mm | 1–0.25 mm | <0.25 mm | T | A | T × A | ||
| 0–20 | 8 | 42.57 ± 3.27 b | 17.46 ± 1.38 a | 9.56 ± 0.89 a | 30.41 ± 2.88 b | ** | ** | * |
| 17 | 53.19 ± 4.03 a | 15.91 ± 0.94 a | 8.38 ± 0.93 a | 22.52 ± 3.04 c | ||||
| 25 | 40.41 ± 2.26 b | 16.94 ± 1.24 a | 9.43 ± 1.02 a | 33.22 ± 3.53 b | ||||
| 43 | 34.62 ± 2.13 c | 17.38 ± 1.16 a | 7.25 ± 0.78 a | 40.75 ± 4.27 a | ||||
| A | C | D | B | |||||
| 20–40 | 8 | 27.42 ± 3.07 b | 15.21 ± 3.05 a | 6.65 ± 0.89 a | 50.72 ± 3.68 b | ** | ** | * |
| 17 | 36.19 ± 2.85 a | 13.17 ± 2.04 a | 7.33 ± 1.02 a | 43.31 ± 4.02 c | ||||
| 25 | 24.18 ± 2.63 bc | 11.01 ± 1.17 a | 5.46 ± 0.98 a | 59.35 ± 3.84 a | ||||
| 43 | 21.36 ± 3.41 c | 12.89 ± 2.18 a | 7.98 ± 2.87 a | 57.77 ± 2.35 a | ||||
| B | C | D | A | |||||
| Item | Soil Layer (cm) | Tea-Planting Age (Year) | Percentage Contribution (%) | Significance | |||||
|---|---|---|---|---|---|---|---|---|---|
| >2 mm | 2–1 mm | 1–0.25 mm | <0.25 mm | T | A | T × A | |||
| Corg | 0–20 | 8 | 49.97 ± 2.47 b | 19.55 ± 1.97 a | 8.54 ± 0.47 a | 21.94 ± 1.47 c | ** | ** | ** |
| 17 | 58.87 ± 3.13 a | 17.05 ± 2.01 a | 7.04 ± 0.85 a | 17.03 ± 2.03 d | |||||
| 25 | 47.11 ± 2.05 b | 19.11 ± 2.34 a | 8.26 ± 0.35 a | 25.52 ± 1.87 b | |||||
| 43 | 39.65 ± 3.41 c | 19.83 ± 1.03 a | 7.13 ± 0.27 a | 33.39 ± 1.55 a | |||||
| A | C | D | B | ||||||
| 20–40 | 8 | 32.71 ± 2.03 b | 17.58 ± 1.02 a | 6.81 ± 0.38 b | 42.90 ± 2.67 b | * | ** | * | |
| 17 | 41.20 ± 3.27 a | 15.13 ± 1.38 ab | 6.56 ± 0.64 b | 37.11 ± 3.57 c | |||||
| 25 | 32.80 ± 1.98 b | 13.25 ± 1.47 b | 5.44 ± 0.57 b | 48.50 ± 3.41 a | |||||
| 43 | 25.37 ± 2.67 c | 15.37 ± 2.13 ab | 8.25 ± 0.49 a | 51.01 ± 2.97 a | |||||
| B | C | D | A | ||||||
| Cact | 0–20 | 8 | 55.11 ± 3.02 b | 21.25 ± 1.35 a | 8.44 ± 0.51 a | 15.19 ± 2.41 b | ** | ** | * |
| 17 | 64.23 ± 3.74 a | 18.17 ± 2.03 a | 6.78 ± 0.91 bc | 10.83 ± 2.36 c | |||||
| 25 | 53.82 ± 2.48 b | 19.50 ± 2.44 a | 7.55 ± 0.54 b | 19.12 ± 3.06 b | |||||
| 43 | 45.21 ± 3.28 c | 20.74 ± 1.09 a | 5.66 ± 0.63 c | 28.39 ± 3.36 a | |||||
| A | B | C | B | ||||||
| 20–40 | 8 | 38.93 ± 2.98 b | 19.07 ± 2.58 a | 6.93 ± 0.36 a | 35.07 ± 1.29 c | * | ** | * | |
| 17 | 48.92 ± 2.06 a | 15.59 ± 3.03 ab | 6.98 ± 0.51 a | 28.51 ± 3.45 d | |||||
| 25 | 35.91 ± 3.84 b | 13.66 ± 1.02 b | 5.83 ± 0.84 a | 44.60 ± 2.48 b | |||||
| 43 | 28.03 ± 2.51 c | 15.75 ± 1.48 ab | 7.03 ± 0.69 a | 49.19 ± 1.03 a | |||||
| A | B | C | A | ||||||
| Crec | 0–20 | 8 | 48.65 ± 2.03 b | 19.12 ± 1.05 a | 8.57 ± 1.03 a | 23.67 ± 2.34 b | ** | ** | ** |
| 17 | 57.39 ± 3.05 a | 16.75 ± 1.47 b | 7.12 ± 0.89 a | 18.74 ± 3.21 c | |||||
| 25 | 45.81 ± 1.47 b | 19.03 ± 2.31 a | 8.40 ± 1.21 a | 26.77 ± 0.89 b | |||||
| 43 | 38.86 ± 2.36 c | 19.69 ± 1.98 a | 7.34 ± 0.58 a | 34.11 ± 2.14 a | |||||
| A | C | D | B | ||||||
| 20–40 | 8 | 31.33 ± 2.47 b | 17.26 ± 2.03 a | 6.79 ± 0.47 b | 44.63 ± 3.48 b | * | ** | * | |
| 17 | 39.49 ± 2.03 a | 15.03 ± 1.35 ab | 6.47 ± 0.68 b | 39.02 ± 1.02 c | |||||
| 25 | 32.30 ± 1.89 b | 13.19 ± 2.36 b | 5.38 ± 0.39 b | 49.14 ± 3.21 b | |||||
| 43 | 25.03 ± 3.47 c | 15.32 ± 2.49 ab | 8.40 ± 0.88 a | 51.24 ± 2.19 a | |||||
| B | C | D | A | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; He, X.; Ye, S.; Wang, S. Soil Aggregate-Associated Carbon Fraction Dynamics during the Process of Tea (Camellia sinensis L.) Planting in Southern Guangxi, China. Forests 2021, 12, 1530. https://doi.org/10.3390/f12111530
Zhang C, He X, Ye S, Wang S. Soil Aggregate-Associated Carbon Fraction Dynamics during the Process of Tea (Camellia sinensis L.) Planting in Southern Guangxi, China. Forests. 2021; 12(11):1530. https://doi.org/10.3390/f12111530
Chicago/Turabian StyleZhang, Chao, Xinxin He, Shaoming Ye, and Shengqiang Wang. 2021. "Soil Aggregate-Associated Carbon Fraction Dynamics during the Process of Tea (Camellia sinensis L.) Planting in Southern Guangxi, China" Forests 12, no. 11: 1530. https://doi.org/10.3390/f12111530
APA StyleZhang, C., He, X., Ye, S., & Wang, S. (2021). Soil Aggregate-Associated Carbon Fraction Dynamics during the Process of Tea (Camellia sinensis L.) Planting in Southern Guangxi, China. Forests, 12(11), 1530. https://doi.org/10.3390/f12111530






