Defoliation, Recovery and Increasing Mortality in Italian Forests: Levels, Patterns and Possible Consequences for Forest Multifunctionality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Data Analysis
3. Results and Discussion
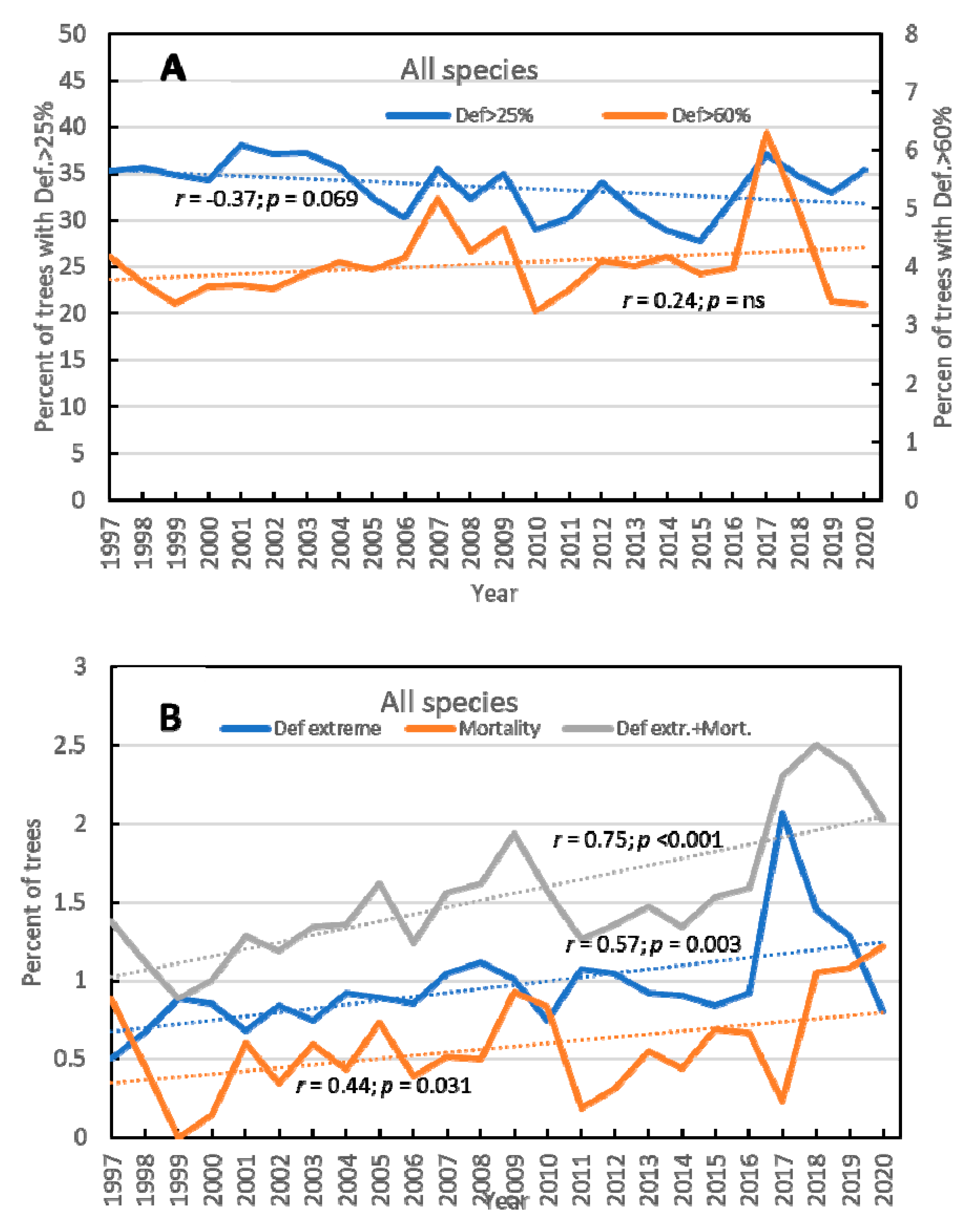
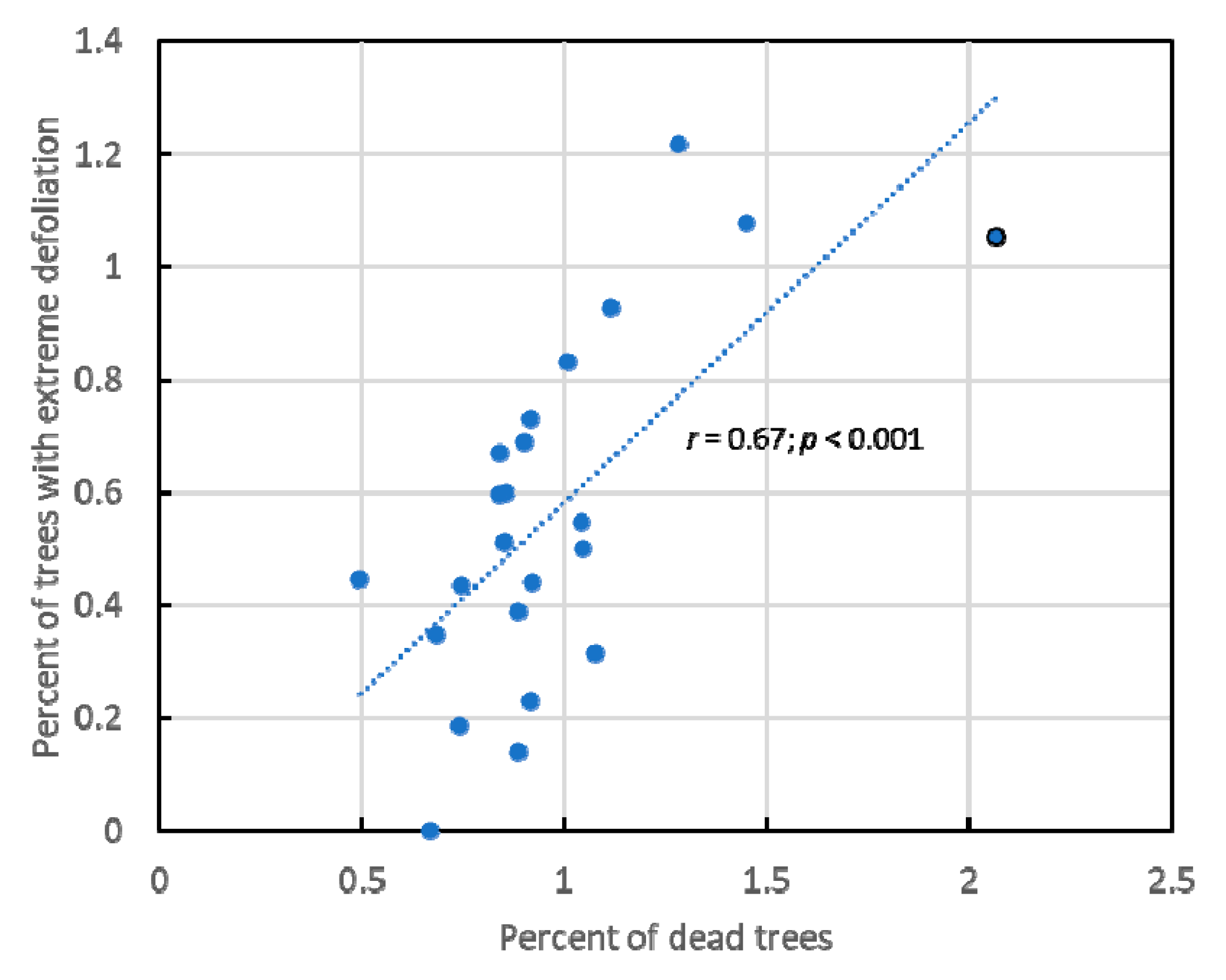
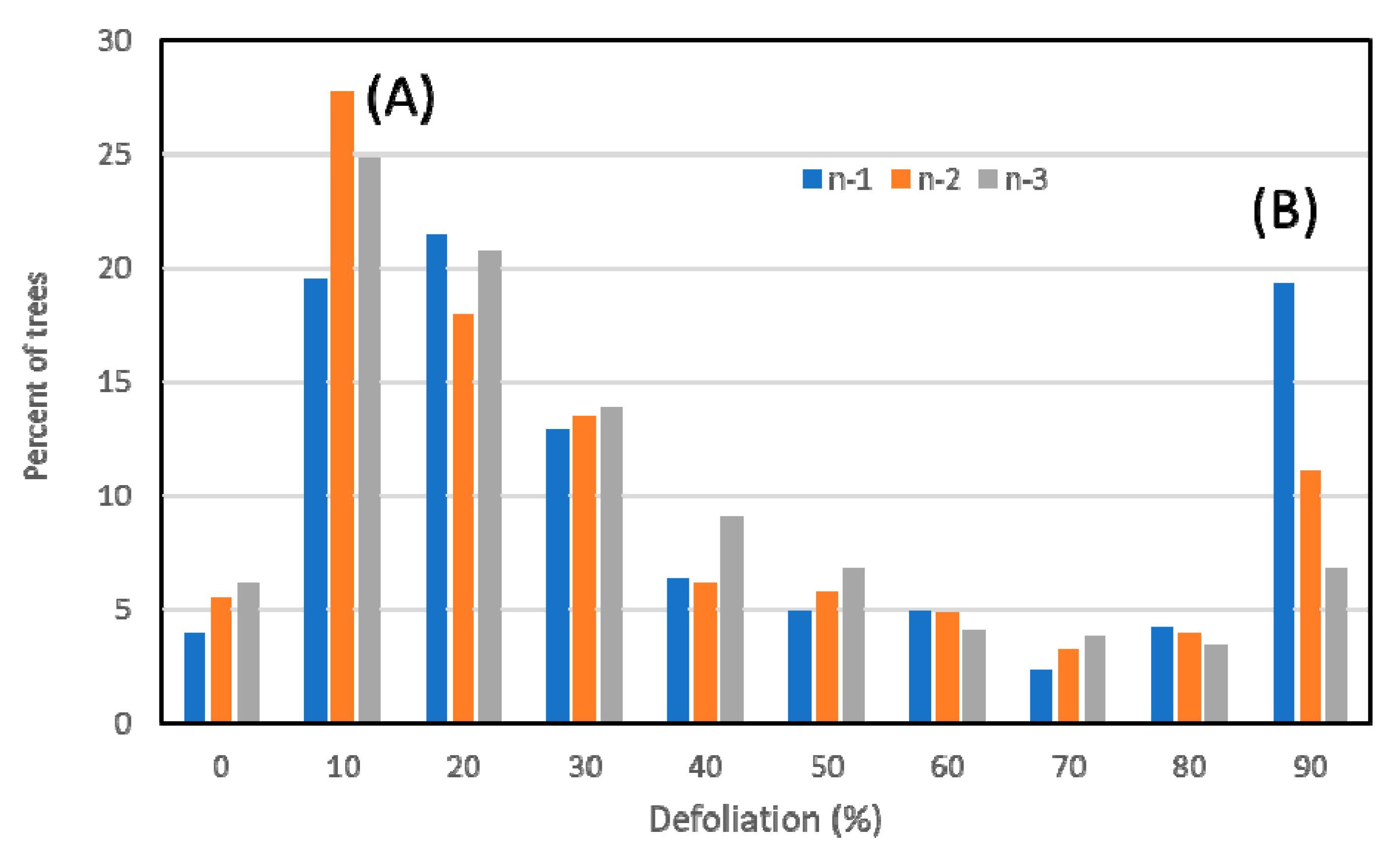
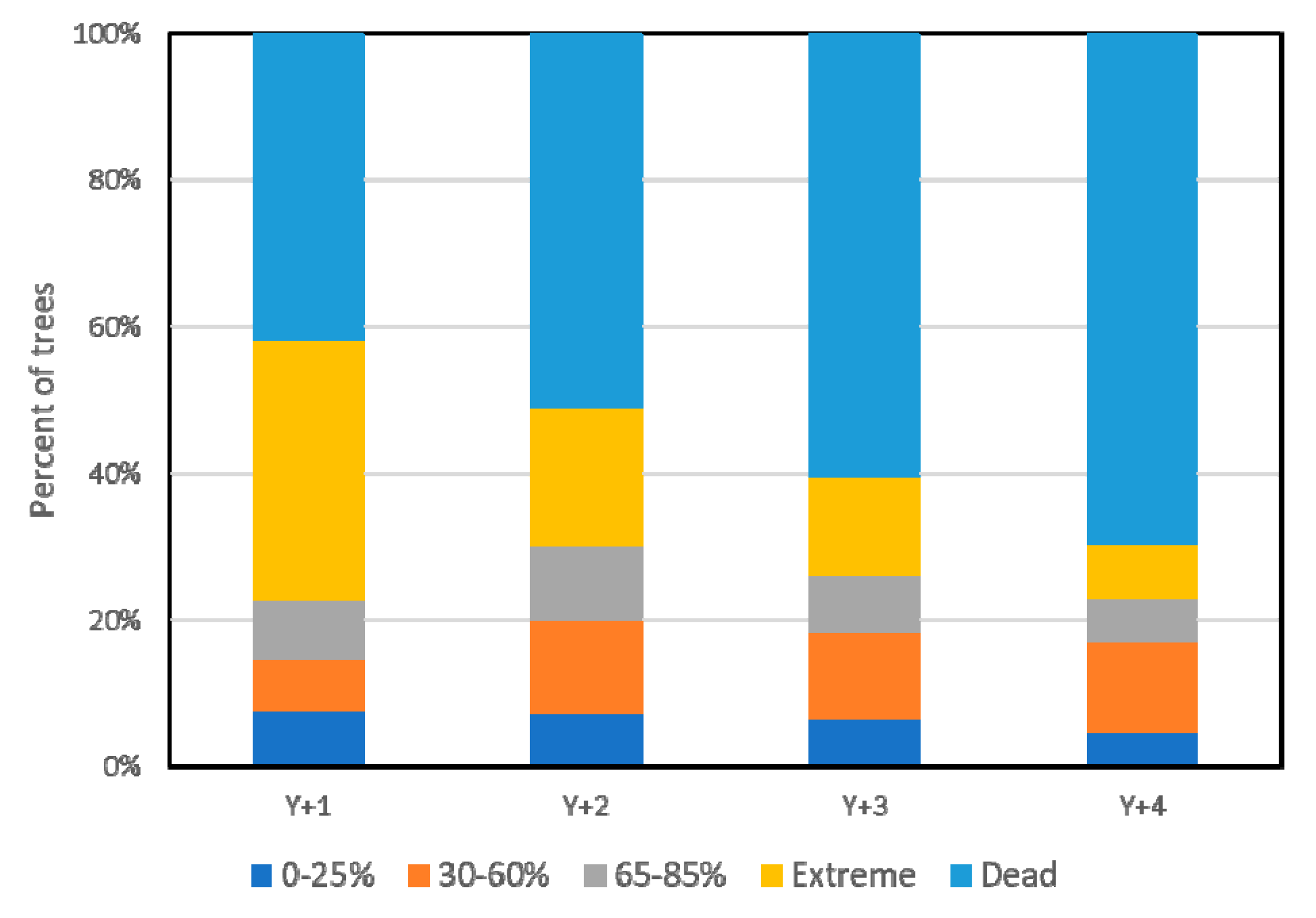
3.1. Overall Results
3.2. Results Per Species
3.3. What Have We Learned?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferretti, M.; Fisher, R. Forest Monitoring, Methods for Terrestrial Investigations in Europe with an Overview of North America and Asia; Develop. Environ. Sci. 12 Series; Krupa, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; p. 507. [Google Scholar]
- Iacopetti, G.; Bussotti, F.; Selvi, F.; Maggino, F.; Pollastrini, M. Forest ecological heterogeneity determines contrasting relationships between crown defoliation and tree diversity. For. Ecol. Manag. 2019, 448, 321–329. [Google Scholar] [CrossRef]
- Toïgo, M.; Nicolas, M.; Jonard, M.; Croisé, L.; Nageleisene, L.M.; Jactel, H. Temporal trends in tree defoliation and response to multiple biotic and abiotic stresses. For. Ecol. Manag. 2020, 477, 118476. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Camin, F.; Calderisi, M.; Ferretti, M. Consistent response of crown transparency shoot growth and leaf traits on Norway spruce (Picea abies (L.) H. Karst.) trees along an elevation gradient in northern Italy. Ecol. Ind. 2016, 60, 1041–1044. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofolini, F.; Cristofori, A.; Pollastrini, M.; Camin, F.; Ferretti, M. A multi-proxy approach reveals common and species-specific features associated with tree defoliation in broadleaved species. For. Ecol. Manag. 2020, 467, 118–151. [Google Scholar] [CrossRef]
- Pollastrini, M.; Feducci, M.; Bonal, D.; Fotelli, M.; Gessler, A.; Gossiord, C.; Guyot, V.; Jactel, H.; Nguyen, D.; Radoglou, K.; et al. Physiological significance of forest tree defoliation: Results from a survey in a mixed forest in Tuscany (central Italy). For. Ecol. Manag. 2016, 361, 170–178. [Google Scholar] [CrossRef]
- Tallieu, C.; Badeau., V.; Allard., D.; Nageleisen, L.M.; Bréda., N. Year-to-year crown condition poorly contributes to ring width variations of beech trees in French ICP level I network. For. Ecol. Manag. 2020, 465, 118071. [Google Scholar] [CrossRef]
- Ferretti, M.; Bacaro, G.; Brunialti, G.; Calderisi, M.; Croisé, L.; Frati, L.; Nicolas, M. Tree canopy defoliation can reveal growth decline in mid-latitude temperate forests. Ecol. Ind. 2021, 127, 107749. [Google Scholar] [CrossRef]
- Michel, A.; Prescher, A.K.; Schwärzel, K. Forest Condition in Europe: The 2020 Assessment. In ICP Forests Technical Report under the UNECE Convention on Long-Range Transboundary Air Pollution (Air Convention); Thünen Institute: Eberswalde, Germany, 2020. [Google Scholar]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, H.; Schuldt, B.; Sanders, T.G.M.; Macinnis-Ng, C.; Boehmer, H.J.; Allen, C.D.; Bolte, A.; Crowther, T.W.; Hansen, M.C.; Medlyn, B.E.; et al. Monitoring global tree mortality patterns and trends. Report from the VW symposium ‘Crossing scales and disciplines to identify global trends of tree mortality as indicators of forest health’. New Phytol. 2018, 217, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Franklin, J.F.; Shugart, H.H.; Harmon, M.E. Tree death as an ecological process. BioScience 1987, 37, 550–556. [Google Scholar] [CrossRef]
- Harmon, M.E.; Bell, D.M. Mortality in Forested Ecosystems: Suggested Conceptual Advances. Forests 2020, 11, 572. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- Das, A.J.; Stephenson, N.L.; Davis, K.P. Why do trees die? Characterizing the drivers of background tree mortality. Ecology 2016, 97, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Rohner, B.; Kumar, S.; Liechti, K.; Gessler, A.; Ferretti, M. Tree vitality indicators revealed a rapid response of beech forests to the 2018 drought. Ecol. Ind. 2020, 120, 106903. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Harrison, S.P.; Adams, H.D.; Kelley, D.I.; Li, G.; Tissue, D.T.; Dawson, T.E.; Fensham, R.; Medlyn, B.E.; Palmer, A.; et al. Drought and resprouting plants. New Phytol. 2015, 206, 583–589. [Google Scholar] [CrossRef]
- ICP-Forests. 30 Years of Monitoring the Effects of Long Range Transboudary Air Pollution on Forests in Europe and beyond. 2016. Available online: http://icp-forests.net/ (accessed on 10 July 2021).
- Brunetti, M.; Buffoni, L.; Mangianti, F.; Maugeri, M.; Nanni, T. Temperature, precipitation and extreme events during the last century in Italy. Glob. Plan. Chang. 2004, 40, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, M.; Maugeri, M.; Monti, F.; Nanni, T. Temperature and precipitation variability in Italy in the last two centuries from homogenised instrumental time series. Int. J. Clim. 2006, 26, 345–381. [Google Scholar] [CrossRef]
- Fratianni, S.; Acquaotta, F. The Climate of Italy. In Landscapes and Landforms of Italy; Soldati, M., Marchetti, M., Eds.; Springer: Cham, Switzerland; pp. 29–38.
- Bartolini, G.; Betti, G.; Gozzini, B.; Iannuccilli, M.; Magno, R.; Messeri, G.; Spolverini, N.; Torrigiani, T.; Vallorani, R.; Grifoni, D. Spatial and temporal changes in dry spells in a Mediterranean area: Tuscany (central Italy), 1955–2017. Int. J. Clim. 2021, 1, 1–22. [Google Scholar]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Fontana, G.; Toreti, A.; Ceglar, A.; De Sanctis, G. Early heat waves over Italy and their impacts on durum wheat yields. Nat. Hazards Earth Syst. Sci. 2015, 15, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Lorenzini, G.; Pellegrini, E.; Campanella, A.; Nali, C. It’s not just the heat and the drought: The role of ozone air pollution in the 2012 heat wave. Agrochimica 2014, 58, 40–42. [Google Scholar]
- Brilli, L.; Moriondo, M.; Ferrise, R.; Dibari, C.; Bindi, M. Climate change and Mediterranean crops: 2003 and 2012, two possible examples of the near future. Agrochimica 2014, 58, 20–33. [Google Scholar]
- Rita, A.; Camarero, J.J.; Nolè, A.; Borghetti, M.; Brunetti, M.; Pergola, N.; Serio, C.; Vicente-Serrano, S.M.; Tramutoli, V.; Ripullone, F. The impact of drought spells on forests depends on site conditions: The case of 2017 summer heat wave in southern Europe. Glob. Chang. Biol. 2020, 26, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Dalponte, M.; Marzini, S.; Solano-Correa, Y.T.; Tonon, G.; Vescovo, L.; Gianelle, D. Mapping Forest windthrows using high spatial resolution multispectral satellite images. Int. J. Appl. Earth Obs. Geoinf. 2020, 93, 102206. [Google Scholar] [CrossRef]
- Dobbertin, M.; Brang, P. Crown defoliation improves tree mortality models. For. Ecol. Manag. 2001, 141, 271–284. [Google Scholar] [CrossRef]
- Eichhorn, J.; Roskams, P.; Potocic, N.; Timermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletkovic, I.; Schröck, H.W.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Coordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; p. 54. ISBN 978-3-86576-162-0. Available online: http://www.icp-forests.org/Manual.htm (accessed on 10 July 2021).
- Bussotti, F.; Bettini, D.; Cenni, E.; Ferretti, M.; Sarti, C.; Nibbi, R.; Capretti, P.; Stergulc, F.; Tiberi, R. PARTE 2—Valutazione della condizione delle chiome. In Procedure di Rilievo Nelle Aree di Saggio e Valutazione della Condizione delle Chiome. Manuale di Campagna; Ministero delle Politiche Agricole, Alimentari e Forestali: Roma, Italy, 2016. [Google Scholar]
- Gasparini, P.; Di Cosmo, L.; Rizzo, M. PARTE 1—Procedure di rilievo nelle aree di saggio di Livello I. In Procedure di Rilievo nelle Aree di Saggio e Valutazione della Condizione delle Chiome. Manuale di Campagna; Ministero delle Politiche Agricole, Alimentari e Forestali: Roma, Italy, 2016. [Google Scholar]
- Müller, E.; Stierlin, H.R. Tree Crown Photos. In Sanasilva; Swiss Federal Institute for Forest Snow and Landscape Research: Birmensdorf, Switzerland, 1990. [Google Scholar]
- Ferretti, M. (Ed.) Mediterranean Forest Trees. IA Guide for Crown Assessment; CEC-UN/ECE: Brussels, Belgium; Geneva, Switzerland, 1994. [Google Scholar]
- Ferretti, M.; Bussotti, F.; Cenni, E.; Cozzi, A. Implementation of Quality Assurance procedures in the Italian programs of forest condition monitoring. Water Air Soil Poll. 1999, 116, 371–376. [Google Scholar] [CrossRef]
- Bussotti, F.; Cozzi, A.; Cenni, E.; Bettini, D.; Sarti, C.; Ferretti, M. Measurement errors in monitoring tree crown conditions. J. Environ. Monit. 2009, 11, 769–773. [Google Scholar] [CrossRef]
- Magno, R.; De Filippis, T.; Di Giuseppe, E.; Pasqui, M.; Rocchi, L.; Gozzini, B. Semi-Automatic Operational Service for Drought Monitoring and Forecasting in the Tuscany Region. Geosciences 2018, 8, 49. [Google Scholar] [CrossRef] [Green Version]
- Pollastrini, M.; Puletti, N.; Selvi, F.; Iacopetti, G.; Bussotti, F. Widespread crown defoliation after a drought and heat wave in the forests of Tuscany (central Italy) and their recovery—A case study from summer 2017. Front. For. Glob. Chan. 2019, 2, 74. [Google Scholar] [CrossRef] [Green Version]
- Puletti, N.; Mattioli, W.; Bussotti, F.; Pollastrini, M. Monitoring the effects of extreme drought events on forest health by Sentinel-2 imagery. J. Appl. Remot. Sen. 2019, 13, 020501. [Google Scholar] [CrossRef]
- Bertini, G.; Ferretti, F.; Fabbio, G.; Raddi, S.; Magnani, F. Quantifying tree and volume mortality in Italian forests. For. Ecol. Manag. 2019, 444, 42–49. [Google Scholar] [CrossRef]
- Neumann, M.; Mues, V.; Moreno, A.; Hasenauer, H.; Seidl, R. Climate variability drives recent tree mortality in Europe. Glob. Chang. Biol. 2017, 23, 4788–4797. [Google Scholar] [CrossRef]
- Etzold, S.; Ziemińska, K.; Rohner, B.; Bottero, A.; Bose, A.K.; Ruehr, N.K.; Zingg, A.; Rigling, A. One Century of Forest Monitoring Data in Switzerland Reveals Species and Site-Specific Trends of Climate-Induced Tree Mortality. Front. Plant Sci. 2019, 10, 307. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Taccoen, A.; Piedallu, C.; Seynave, I.; Perez, V.; Gégout-Petit, A.; Nageleisen, L.M.; Bontemps, J.D.; Gégout, J.C. Background mortality drivers of European tree species: Climate change matters. Proc. R. Soc. 2019, 286, 20190386. [Google Scholar] [CrossRef] [Green Version]
- Senf, C.; Pflugmacher, D.; Yang, Z.; Sebald, J.; Knorn, J.; Neumann, M.; Hostert, P.; Seidl, R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat. Commun. 2018, 9, 4978. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Benito, P.; Lines, E.R.; Gómez-Aparicio, L.; Zavala, M.A.; Coomes, D.A. Patterns and drivers of tree mortality in Iberian forests: Climatic effects are modified by competition. PLoS ONE 2013, 8, e56843. [Google Scholar] [CrossRef] [Green Version]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 84–103. [Google Scholar] [CrossRef]
- Brun, P.; Psomas, A.; Ginzler, C.; Thuiller, W.; Zappa, M.; Zimmermann, N.E. Large-scale early-wilting response of Central European forests to the 2018 extreme drought. Glob. Chang. Biol. 2020, 26, 7021–7035. [Google Scholar] [CrossRef] [PubMed]
- Bigler, C.; Gavin, D.G.; Gunning, C.; Veblen, T.T. Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos 2007, 116, 1983–1994. [Google Scholar] [CrossRef]
- Furniss, T.J.; Larson, A.J.; Kane, V.R.; Lutz, J.A. Wildfire and drought moderate the spatial elements of tree mortality. Ecosphere 2020, 11, e03214. [Google Scholar] [CrossRef]
- Trugman, A.T.; Detto, M.; Bartlett, M.K.; Medvigy, D.; Anderegg, W.R.L.; Schwalm, C.; Schaffer, B.; Pacala, S.W. Tree carbon allocation explains forest drought-kill and recovery patterns. Ecol. Lett. 2018, 21, 1552–1560. [Google Scholar] [CrossRef]
- Battisti, A.; Benvegnù, I.; Colombari, F.; Haack, R.A. Invasion by the chestnut gall wasp in Italy causes significant yield loss in Castanea sativa nut production. Agric. For. Entomol. 2014, 16, 75–79. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as cause of oak decline in Central Europe. Forest Path. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. iFor. Biogeosci. For. 2017, 10, 796–806. [Google Scholar] [CrossRef] [Green Version]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gentilesca, T.; Oliva, J.; Redondo, M.A.; Ripullone, F. Drought and Phytophthora Are Associated With the Decline of Oak Species in Southern Italy. Front. Plant Sci. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Minerbi, S.; Cescatti, A.; Cherubini, P.; Hellrigl, K.; Markart, G.; Saurer, M.; Mutinelli, G. Scots Pine dieback in the Isarco Valley due to severe drought in the summer of 2003. Forest Obs. 2006, 2/3, 89–144. [Google Scholar]
- Vacchiano, G.; Garbarino, M.; Borgogno, M.; Mondino, E.; Motta, R. Evidences of drought stress as a predisposing factor to Scots pine decline in Valle d’Aosta (Italy). Eur. J. Forest Res. 2012, 131, 989–1000. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Eilmann, B.; Weber, P.; Rigling, A.; Eckstein, D. Growth reactions of Pinus sylvestris L. and Quercus pubescens Willd. to drought years at a xeric site in Valais, Switzerland. Dendrochronologia 2006, 23, 121–132. [Google Scholar] [CrossRef]
- Cenni, E.; Bussotti, F.; Galeotti, L. The decline of Pinus nigra Arn. reforestation stand on a limestone substrate: The role of nutritional factors examined by means of foliar diagnosis. Ann. Sci. For. 1998, 55, 567–576. [Google Scholar] [CrossRef]
- Netherer, S.; Kandasamy, D.; Jirosová, A.; Kalinová, B.; Schebeck, M.; Schlyter, F. Interactions among Norway spruce, the bark beetle Ips typographus and its fungal symbionts in times of drought. J. Pest Sci. 2021, 94, 591–614. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Zhou, Z.; Wang, C. Defoliation-induced tree growth declines are jointly limited by carbon source and sink activities. Sci. Total Environ. 2021, 762, 143077. [Google Scholar] [CrossRef]
- Galiano, L.; Martinez-Vilalta, J.; Sabaté, S.; Lloret, F. Determinants of drought induced effects on crown condition and their relationship with depletion of carbon reserve in a Mediterranean holm oak forest. Tree Physiol. 2012, 32, 478–489. [Google Scholar] [CrossRef] [Green Version]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2018, 38, 1718–1736. [Google Scholar] [CrossRef] [Green Version]
- Spinoni, J.; Naumann, G.; Vogt, J.V. Pan-European seasonal trends and recent changes of drought frequency and severity. Glob. Plan. Chang. 2017, 148, 113–130. [Google Scholar] [CrossRef]
- Hari, V.; Rakovec, O.; Markonis, Y.; Hanel, M.; Kumar, R. Increased future occurrences of the exceptional 2018–2019 Central European drought under global warming. Sci. Rep. 2020, 10, 12207. [Google Scholar] [CrossRef] [PubMed]
- Moravec, V.; Markonis, Y.; Rakovec, O.; Svoboda, M.; Trnka, M.; Kumara, R.; Hanel, M. Europe under multi-year droughts: How severe was the 2014–2018 drought period? Environ. Res. Lett. 2021, 16, 034062. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Observing climate change impacts on European forests: What works and what does not in ongoing long-term monitoring networks. Front. Plant Sci. 2017, 8, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussotti, F.; Pollastrini, M. Traditional and novel indicators of climate change impacts on European forest trees. Forests 2017, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Bussotti, F.; Pollastrini, M. Revisiting the concept of stress in forest trees at the time of global change and issues for stress monitoring. Plant Stress 2021, 2, 100013. [Google Scholar] [CrossRef]
| Species | N | Def > 25% | Def > 60% | Def > 85% | Def 100% | Def 85–100% | Dead/Extr |
|---|---|---|---|---|---|---|---|
| Castanea sativa Mill. | 475.08 | 61.36 | 12.98 | 3.52 | 1.08 | 4.60 | 0.31 |
| Fagus sylvatica L. | 1099.04 | 30.21 | 2.96 | 0.92 | 0.42 | 1.34 | 0.45 |
| Ostrya carpinifolia Scop. | 329.75 | 34.91 | 6.07 | 1.49 | 0.34 | 1.83 | 0.23 |
| Quercus cerris L. | 589.96 | 25.08 | 1.06 | 0.22 | 0.49 | 0.71 | 2.18 |
| Quercus ilex L. | 220.13 | 23.27 | 1.12 | 0.11 | 0.08 | 0.19 | 0.72 |
| Quercus pubescens Willd. | 718.88 | 51.82 | 5.08 | 0.98 | 0.46 | 1.44 | 0.47 |
| Larix decidua L. | 348.00 | 19.93 | 2.17 | 0.65 | 0.59 | 1.24 | 0.90 |
| Picea abies (L.) Karst. | 544.29 | 19.39 | 1.79 | 0.28 | 0.50 | 0.78 | 1.74 |
| Pinus nigra Arn. | 192.88 | 24.92 | 2.86 | 0.33 | 0.75 | 1.08 | 2.24 |
| Pinus sylvestris L. | 205.46 | 34.19 | 3.42 | 0.58 | 1.06 | 1.64 | 1.85 |
| Species | Def > 25% | Def > 60% | Def > 85% | Def 100% | 85 + 100% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Castanea sativa | 0.829 | *** | 0.820 | *** | 0.733 | *** | 0.577 | ** | 0.751 | *** |
| Fagus sylvatica | −0.178 | ns | 0.196 | ns | 0.314 | ns | 0.250 | ns | 0.389 | (*) |
| Ostrya carpinifolia | 0.208 | ns | −0.101 | ns | −0.136 | ns | 0.422 | * | 0.043 | ns |
| Quercus cerris | –0.497 | * | 0.299 | ns | 0.310 | ns | −0.036 | ns | 0.055 | ns |
| Quercus ilex | −0.680 | *** | −0.494 | * | −0.066 | ns | −0.094 | ns | −0.093 | ns |
| Quercus pubescens | −0.729 | *** | −0.468 | * | 0.366 | (*) | −0.029 | ns | 0.311 | ns |
| Larix decidua | 0.208 | ns | −0.101 | ns | −0.136 | ns | 0.422 | * | 0.043 | ns |
| Picea abies | 0.240 | ns | 0.439 | * | 0.308 | ns | 0.584 | ** | 0.722 | *** |
| Pinus nigra | −0.090 | ns | −0.499 | * | 0.208 | ns | 0.469 | * | 0.519 | ** |
| Pinus sylvestris | 0.417 | * | 0.524 | ** | 0.224 | ns | 0.202 | ns | 0.305 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bussotti, F.; Papitto, G.; Di Martino, D.; Cocciufa, C.; Cindolo, C.; Cenni, E.; Bettini, D.; Iacopetti, G.; Pollastrini, M. Defoliation, Recovery and Increasing Mortality in Italian Forests: Levels, Patterns and Possible Consequences for Forest Multifunctionality. Forests 2021, 12, 1476. https://doi.org/10.3390/f12111476
Bussotti F, Papitto G, Di Martino D, Cocciufa C, Cindolo C, Cenni E, Bettini D, Iacopetti G, Pollastrini M. Defoliation, Recovery and Increasing Mortality in Italian Forests: Levels, Patterns and Possible Consequences for Forest Multifunctionality. Forests. 2021; 12(11):1476. https://doi.org/10.3390/f12111476
Chicago/Turabian StyleBussotti, Filippo, Giancarlo Papitto, Domenico Di Martino, Cristiana Cocciufa, Claudia Cindolo, Enrico Cenni, Davide Bettini, Giovanni Iacopetti, and Martina Pollastrini. 2021. "Defoliation, Recovery and Increasing Mortality in Italian Forests: Levels, Patterns and Possible Consequences for Forest Multifunctionality" Forests 12, no. 11: 1476. https://doi.org/10.3390/f12111476
APA StyleBussotti, F., Papitto, G., Di Martino, D., Cocciufa, C., Cindolo, C., Cenni, E., Bettini, D., Iacopetti, G., & Pollastrini, M. (2021). Defoliation, Recovery and Increasing Mortality in Italian Forests: Levels, Patterns and Possible Consequences for Forest Multifunctionality. Forests, 12(11), 1476. https://doi.org/10.3390/f12111476







