Abstract
The herbaceous vegetation and forest stand characteristics in European beech forests growing in the Polish part of the Eastern Carpathians have changed over the last 40 years. This has been influenced by many factors, including land-use change, forest management and climate change. This study investigates changes in forest cover and structure and the associated changes in herbaceous layer plant communities and seeks to elucidate whether and how beech forest herbaceous layer communities have been affected by climate change. The study used information from archival and current land cover maps, semi-permanent sampling plots, forest management plans for the Forest Districts of Brzozów, Lesko and Ustrzyki Dolne and meteorological weather station data compiled for three study periods of herbaceous vegetation (1970s, 2000s, 2010s). In the study area, the regular shelterwood system was changed to an irregular shelterwood system that produces stands with a complex overstorey structure. The results revealed the important role of light availability in shaping the species composition of the herbaceous layer in semi-natural Carpathian beech forests, which was strongly related to the course of management activities. An overall decrease in the number of species during the 2010s is linked to the ageing of beech forests, increased intensity of management activities in ageing stands, competition from understorey vegetation and lower soil moisture that can be linked to climate change. Our study partially supports the existing findings that more manipulative forest management systems can play an important role in countering the current and expected effects of climate change on the forest ecosystem because of the low degree of spatial differentiation of the stand’s structure (developmental stages). Therefore, foresters managing the structure of stands should strive to create a forest structure with high variability of developmental stages on a regional scale.
1. Introduction
Forests are highly complex terrestrial ecosystems that have important environmental, social and economic functions [1,2,3,4]. Forests shape climate, regulate the water cycle, prevent floods and protect soil from erosion. Forests provide wood and other non-timber forest products, such as mushrooms and herbs. They also provide conditions that support a large number of species, conserving genetic resources and protecting ecosystem services [5,6,7,8].
Forests, like other ecosystems, are shaped by direct and indirect human activities [9,10]. Direct activities include the modification of stand structure by forest management, the spread of invasive species, timber harvesting and the use of non-timber forest services and functions [1,11,12]. The effects of indirect human activities include climate change, land-use change, changes in forest cover over time, as well as nitrogen accumulation and air pollution [13,14,15,16,17].
It is likely that changes In the case of European beech forests in the Polish part of the Eastern Carpathians can be attributed primarily to altered land use (abandonment of agricultural land and its afforestation), changes in forest management systems and climate change. The sudden decrease in population density after World War II as a result of political displacement played a key role in the conversion of agricultural land to forest [18,19]. Before World War II, the population density in the Polish part of the Eastern Carpathians was 100 people per 100 ha of agricultural land [20]. In contrast, in post-war censuses, these areas contained less than 30% of the pre-war population [21]. This decrease in population density resulted in the abandonment of agricultural land, allowing forests to develop through natural succession or by afforestation [22,23,24,25]. As a result, forest cover has increased and forest fragmentation decreased, which should benefit the maintenance and spread of herbaceous forest species, especially species with slow dispersal rates, e.g., myrmecochores [26,27].
The change in land use is also linked with the abandonment of non-agricultural forest uses by the surrounding farming population. After World War II, practices such as firewood collection, raking forest floor litter and use of forests as grazing land for livestock gradually disappeared in the Polish region of the Eastern Carpathians [28,29]. The decrease in the intensity of incidental forest use by the surrounding population was primarily due to the aforementioned population displacement. In subsequent years, the intensification of livestock farming and the legal prohibition of such activities have contributed to increases in forest area [29,30]. One of the consequences of decreased non-agricultural uses of the forest has been increased organic matter accumulation on the forest floor, which should increase the amount of nutrients available to shallow-rooting herbaceous species [31,32,33].
Before World War II, Poland’s forests were heavily exploited. Timber harvesting exceeded growth, and mainly targeted the oldest stands, which were felled on vast areas, often using clearcutting [34]. In the Polish part of the Eastern Carpathians, between 1950 and 1990, the regular shelterwood system (with a stand regeneration period of 10–20 years, resulting in single-storey stands with little age differentiation) began to be applied. Then, in the second half of the 1990s, the regular shelterwood system was replaced by the irregular shelterwood system [35,36]. In the irregular shelterwood system, felling and subsequent regeneration take place over a period of 30–50 years. In this management system, it is possible to use a variety of cut types adapted to the needs of the species for regeneration (edge cuts on groups and streaks, shelterwood and selected cuts, and even complete cuts on small areas). The cuts in a given forest sub-compartment usually take place every 3–6 years, resulting in stands with a complex overstorey structure and a large diversity of stand ages. Irregular shelterwood management is accompanied by long-term changes in the amount of light reaching the forest floor. The resulting gaps in the stand, which are widened during successive cuttings, are successively filled by understorey species. Thus, the competitive impact of understorey growth mainly by European beech (Fagus sylvatica L.) growing in dense clumps seems to have an important impact on herbaceous vegetation in beech forests. Beech appears to be a strong competitor for nutrients and water [37]. Forest management practices (size of harvested area, type of cut, length of regeneration period) have significant impacts on the diversity of its communities because they change short-term and long-term habitat conditions (mainly the amount of light reaching the forest floor and humidity) and create favourable conditions or limitations for seed dispersal. In the Polish part of the Eastern Carpathians, the proportion of tree stands over 100 years old is 38.5% (Forest Districts of Brzozów, Lesko and Ustrzyki Dolne). In this type of ageing beech stands, tree species diversity is lower, with beech being a strong competitor with other tree species, displacing them in later stages of forest development [38]. This affects the quality of forest litter, with changes in the amount of available nutrients and the acidification of topsoil [39,40].
The final factor affecting herbaceous layer plant communities addressed in this study is climate change. In recent decades, the impact of climate change on European beech forests has been considered primarily in the context of changes in forest stand structure. Droughts and low amounts of rainfall reduce the annual growth of trees. It was also found that diversified species composition may contribute to better stand stability during climate change [41,42,43,44,45]. In the Carpathians, between 1881 and 2009, the annual mean temperature has increased by about 1.6 °C [46]. Higher temperatures cause faster soil warming in the spring and this promotes the earlier onset of plant growth, increasing the proportion of thermophilic species in plant communities [47,48]. The decline in precipitation in recent years translates into lower topsoil moisture [49]. Lower water availability during the growing season can affect productivity and shift the species composition of forest ecosystems [50]. Zellweger et al. [48] indicated that the microclimate dependent on the tree and shrub cover has a greater influence on forest herbaceous vegetation than the macroclimate. Hence, managing stands so that the total tree cover and shrub layer do not decrease in the coming years is likely to mitigate some of the changes caused by rising temperatures, which may reduce the spread of thermophilic species.
The aim of this study was to follow (1) changes in the cover and structure of forest stands in the Polish Eastern Carpathians and (2) changes in beech forest herbaceous layer plant communities occurring against this background. Additionally, this study addresses question (3) of whether and to what extent herbaceous layer plant communities may be affected by climate change.
2. Materials and Methods
2.1. Study Area
The study area encompassed three Forest Districts—Brzozów, Lesko and Ustrzyki Dolne, in the Polish Eastern Carpathians (49°33′6.900″ N; 22°20′42.225″ E, Figure 1), with a total area of 1731.15 km2. Most of this area is located in the mesoregion Sanocko-Turczańskie Mountains, Dynów Foothills and Bukowiec Foothills. The soils are dominated by brown soils formed from Carpathian flysch [51,52]. In the period 1966–2018, the average annual temperature was 7.7 °C, with an annual rainfall of 820.8 mm (data from the Lesko station, 420 m a.s.l. [53]). Beech forms the dominant forest type, with a phytosociological classification of Dentario glandulosae Fagetum Klika 1927 em. Mat. 1964. Beech forests in the Polish part of the Carpathians cover 25.3% of the total land area and the species is one of the most important in this region [54].
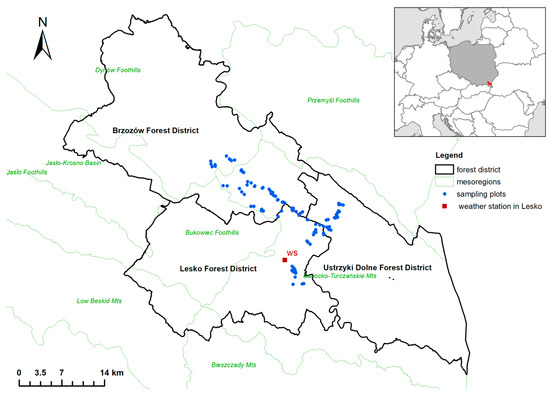
Figure 1.
Location of the study site.
Over 90% of the stands within the area have protective functions, mainly reducing soil erosion and impoverishment and protecting surface and groundwater. This area is a part of the Natura 2000 network (‘‘Ostoja Góry Słonne” PLH180013 and “Góry Słonne” PLB180003), including the Słonne Mountains Landscape Park and thirteen nature reserves.
2.2. Data Collection
Data from the three Forest Districts, Brzozów, Lesko and Ustrzyki Dolne, were used to assess stand structure and forest use. We chose these Forest Districts because they encompassed semi-permanent sampling plots intended for long-term observation of vegetation in the fertile mountain beech forests (Figure 1, [40,51]).
Changes in forest cover were evaluated using maps of spatial data of forest distribution in the Polish Carpathians in the 1860s, 1930s and 1970s, developed as part of the FORECOM project [24]. Forest cover in the 2000s and 2010s was determined on the basis of data from the CORINE Land Cover project [55], where satellite images were used to plot land use. Vector graphics denoting forests in each year were trimmed to the study area and forest area and forest cover in subsequent periods were calculated, with changes visualised using ArcMap 10.7 (ESRI, Redlands, CA, USA).
In order to analyse changes in age and species structure of forest stands, data of forest area by age class for dominant species was obtained from forest management plans for three time periods (adjusted to the herbaceous vegetation research periods) for each Forest District: Brzozów (1976, 2007, 2017), Lesko (1976, 2009, 2019) and Ustrzyki Dolne (1977, 2009, 2019). Age class was assigned based on the age of the dominant tree species in 20-year age-class categories (I—1–20 years; II—21–40 years; III—41–60 years; IV—61–80 years; V—81–100; VI—101–120; VII—more than 121 years). Stands in the regeneration phase and in the “for-regeneration” class, i.e., in which felling is about to be carried out, are designated “RP” (regeneration period).
To track changes in herbaceous layer plant communities and percentage cover by tree species, we used data from phytosociological relevés covering a period of 40 years made using the Braun–Blanquet method [56] in three research periods (in the years 1972–1973, 2005–2007 and 2017–2018). These datasets were collected from 67 semi-permanent sampling plots used for long-term observation of vegetation [40,51].
Because of the fine-grained differentiation of microclimatic conditions in forests on mountain areas, we placed great emphasis on the accuracy of the location of resurveyed plots. To do it, during the reestablishment of the sampling plots in the 2000s, we used the 1970s source materials ([51] and a 1:45,000 scale unpublished map that was drawn by Dzwonko during his study) and marked them by geographic coordinates. During every resampling period, we verified the location of sampling plots using descriptions (exposition inclination and altitude) given by Dzwonko [51]. To make the phytosociological data from three research periods comparable, the vegetation records during resampling were taken from plots with the same area and during the growing season, as in the 1970s. Moreover, we checked if the differentiation in localization of sampling plots could have an impact on results. We found that most of the sampling plots were located in a relatively narrow gradient of a.s.l. and inclination (500–650 m a.s.l. and 5–15 degrees) and quite evenly spaced in relation to the main exposures (NSEW, 12, 15, 10, 11). Therefore we did not address the issue of the impact of microhabitats on changes in the herbaceous layer when generalizing the materials of field observations.
To evaluate changes in climate, meteorological data from 1966 to 2018 provided by the Institute of Meteorology and Water Management—National Research Institute [53] from the Lesko station (420 m a.s.l.) were used. Average temperatures and precipitation were calculated for ten-year periods (1966–1975; 1999–2008; 2009–2018). The range of years coincides with the ten-year periods covered by forest management plans, during which phytosociological surveys were carried out on semi-permanent sampling plots. Climate data for the months of the growing season, i.e., April to August, were used to compare research periods, similar to Bosela et al. [43] and Thom et al. [57].
2.3. Analysis of Forest Vegetation
Changes in herbaceous layer plant communities in beech forests were analysed using data generated from phytosociological relevés from the 1970s, 2000s and 2010s. Total cover of the tree and shrub layer defined according to Ewald et al. [58] (Total_Cov), herbaceous cover (Herb_Cov), number of herbaceous species (Number_Herb) and number of seedlings (Number_Seedl) of woody species in the study plots were compared for each period of research. Ecological groups of species were identified and compared, specifically for (1) ancient forest plant species (Number_Ancient) (as described by Hermy et al. [59] and Dzwonko and Loster [60], these are forest plant species that are very slow to colonize a habitat and whose presence indicates long-term site stability) and (2) species characteristic of beech forests (S_beech_forest) as described in phytosociological nomenclature by Matuszkiewicz [61]. Additionally, for each phytosociological relevé, the number of species representing fast- and slow-dispersing species groups was calculated. Fast-dispersing species include anemochores (ANE)—dispersal by wind; endozoochores (END)—dispersal by animals via digestion; epizoochores (EPI)—dispersal by adhesion on animals. Slow-dispersing species include myrmecochores (MYR)—dispersal by ants; hydrochores (HYD)—dispersal by water; baro- and autochores (BAR and AUT)—passive and active dispersal by plants [59,60]. In addition, for each phytosociological relevé from the three study periods, the Ellenberg indicator values (EIVs, [62]) were calculated based on species presence/absence in phytosociological relevés. Among the EIVs, the Ellenberg indicators for light (L), temperature (T), soil moisture (F), soil reaction (R), and soil nitrogen (N) were selected. These indicators enabled changes in habitat conditions to be determined indirectly [63]. To calculate the Total_cov, Braun–Blanquet cover-abundance values of each species in the tree and shrub layer were converted to average per cent cover values.
For identifying species indicative of a given research period, we used indicator species analysis [64]. For this purpose, we used herbaceous plants with a total attendance of at least 5%. The statistical significances of the species indicator values (IndVal) were estimated by 9999 random permutations of plots across sampling periods. We expected that the ecological preferences of the obtained groups of indicator species would contribute to the identification of the drivers of changes in forest vegetation.
Differences between scores obtained for the 1970s, 2000s and 2010s were tested using repeated measures tests. Depending on data distribution, either the ANOVA or the Friedman test was applied, followed by a posteriori Tukey’s or Wilcoxon’s test with Bonferroni correction, respectively. Statistical analyses were calculated using PAST software version 4.03 (Øyvind Hammer, Natural History Museum, University of Oslo, Oslo, Norway) [65].
The analyses did not take into account early spring herbaceous species, which were excluded to avoid errors resulting from shifts in the onset of the spring season. To avoid errors resulting from incorrect identification of similar species, Senecio nemorensis and S. fuchsii, as well as similar ferns species from the genus Dryopteris, were combined into one group.
3. Results
3.1. Changes in Forest Cover
Forest cover within the study boundaries almost doubled from 1860 to 2018 (from 43,679 ha to 82,408 ha, Figure 2). The greatest increase in forest cover occurred between the 1930s and 1970s. During this period, forest cover increased from 26.1% to 42.8%, which was mainly due to depopulation processes. In subsequent years, forest cover continued to grow, but at a much slower rate, reaching 47.6% in the 2010s.
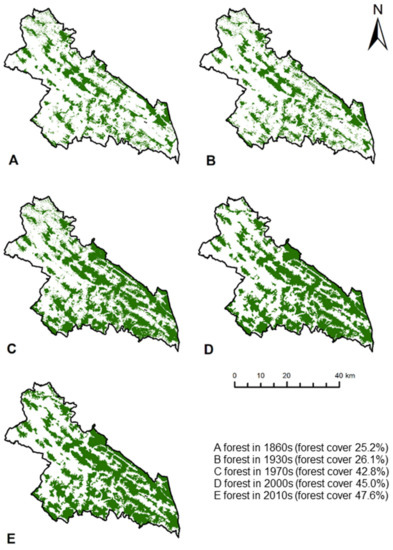
Figure 2.
Changes in forest cover over time.
3.2. Changes in Stand Structure and Forest Management
The age structure and dominant species of forest stands changed significantly from the 1970s to 2010s (Figure 3). In the 1970s, stands in the 21–40-year age class dominated (32.6%). In the 2000s, most stands fell within the 41–60-year class, followed by regenerating (RP) stands (27.3%). In the 2000s and 2010s, RP stands accounted for the largest area (27.3% and 28.6%, respectively).
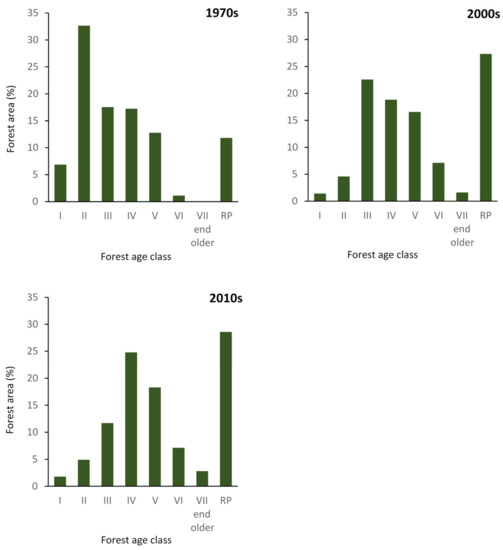
Figure 3.
Proportion of forest area by age class (RP—stands in the regeneration period) in the 1970s, 2000s and 2010s.
Between the 1970s and 1990s forests in Brzozów, Lesko and Ustrzyki Dolne Forest Districts were regenerated using the regular shelterwood system. This created even-aged, one-storied stands, which can be seen in Figure 3 for the 1970s, where the differentiation of age classes III–V was small. At the end of the 1990s, management was changed to the group shelterwood and irregular shelterwood systems. In subsequent years, management was primarily by the irregular shelterwood system, which produces irregular stand structures even on small spatial scales. A clear age differentiation was evident in the 2010s, with a large share of stands still classified as regenerating. The predominance of regenerating stands will continue in the coming decades because the irregular shelterwood system is characterized by a long regeneration period (lasting from 30 to 50 years).
3.3. Changes in Beech Forest Communities
Based on data from forest management plans, between the 1970s and 2010s the share of stands with beech as the dominant species increased from 27.6% to 34.1% (Table 1). These results are confirmed by data from study plots, where the percentage cover of beech between the 1970s and 2010s increased on average from 47.4% to 54.9% (Table 2). Moreover, between the 2000s and 2010s, the average percentage cover of admixture tree species changed, with fir (Abies alba) increasing from 10.4% to 14.1% while sycamore (Acer pseudoplatanus) decreased from 8.6% to 7.3% and ash (Fraxinus excelsior) from 2.7% to 0.1% (Table 2).

Table 1.
Percentages of dominant tree species in forest stands during the 1970s, 2000s and 2010s. Data are from Forest Management Plans. The table includes species whose percentage share was greater than 0.1.

Table 2.
Mean percentage of canopy tree species in semi-permanent sampling plots.
In the 2010s there was less herbaceous layer cover (Table 3) and a lower mean number of herbaceous plant species per study plot than in previous study periods. The average number of ancient forest and beech forest characteristic species also decreased significantly compared to the 1970s and 2000s. In addition, the average number of species with fast and slow seed dispersal in the 2010s was lower than in the 1970s. However, species with slow-dispersing seeds showed significant differences between all study periods, with the highest number of slow dispersal species found in the 2000s.

Table 3.
Changes in variables obtained based on research at semi-permanent sampling plots over time. Values with different superscript letters differed significantly based on Tukey’s or Wilcoxon’s posteriori tests at the p level, at least p ≤ 0.05. F and Chi2—ANOVA and Friedman test score, respectively.
Analysis of habitat conditions based on Ellenberg indicator values showed that in the 2010s more light reached the forest floor and there was less soil moisture than in the 1970s and 2000s. In addition, in the 2000s and 2010s, the soil reaction was lower than in the 1970s (Table 3).
The indicator species analysis showed a large difference between the diagnostic species not only in terms of species composition (Figure 4) but also habitat requirements. In the 1970s, the diagnostic species were characterized by low light requirements (e.g., Mercurialis perennis, Oxalis acetosella, Polygonatum multiflorum) and moderate nitrogen demand (e.g., Chrysosplenium alternifolium, Geranium phaeum, Petasites albus, Phyteuma spicatum). In the 2000s, the diagnostic species had slightly higher light requirements and high nitrogen demand (e.g., Anthriscus nitida, Stellaria nemorum, Veronica montana). Their composition included species related to gaps formed in the stand during partial cutting of trees, such as Galeopsis speciosa and Rubus idaeus. In the 2010s, species of clearings with high demand for light and nitrogen dominated among diagnostic species (e.g., Rubus hirtus, Rumex obtusifolius, Senecio fuschii and S. nemorensis).
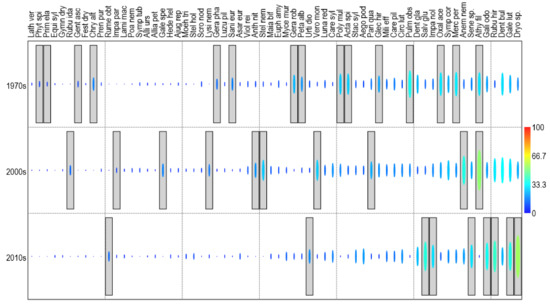
Figure 4.
Results of indicator species analysis. Values of indicator value of species (IndVal%) for study periods are presented. The statistical significances (p < 0.05) of the indicator values have been boxed.
3.4. Climatic Conditions
The average temperature during the growing season (April to August), gradually increased over the three study periods: in the 1970s it was 13.7 °C, in the 2000s 14.6 °C and in the 2010s 15.1 °C (Figure 5). The average temperature increase between the 1970s and 2010s was 1.4 °C. Average precipitation during the growing season was significantly lower (463.8 mm) in the 2010s than in the 1970s (516.3 mm) or 2000s (513.7 mm) (Figure 6).
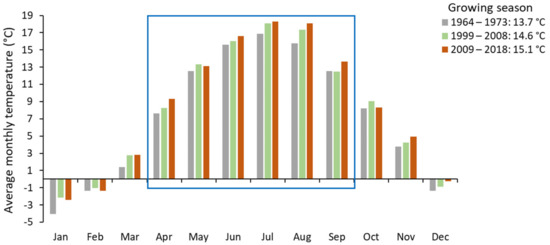
Figure 5.
Average monthly temperature in the periods 1966–1975, 1999–2008 and 2009–2018 based on climate data from the Lesko weather station (420 m a.s.l.). The blue frame encloses months falling within the growing season.
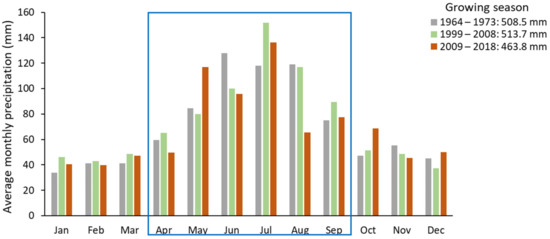
Figure 6.
Average monthly precipitation in the periods 1966–1975, 1999–2008 and 2009–2018 based on climate data from the Lesko weather station (420 m a.s.l.). The blue frame encloses months falling within the growing season.
4. Discussion
4.1. Impact of Changes in Forest Area
Forest cover in the Brzozów, Lesko and Ustrzyki Dolne Forest Districts increased from 25.2% to 47.6% from the 1860s to 2010s, which is much higher than the average forest cover of 29.6% in Poland overall [66]. Increased forest cover has been generally observed since the 19th century in all European mountain ranges due to the abandonment of agricultural land as a result of progressive socio-economic changes (e.g., unprofitability of agricultural production in mountain areas, population migration to towns and employment opportunities outside the agricultural sector). Slow regeneration of forest vegetation has begun on abandoned agricultural land or has been accelerated by afforestation programs [23,67,68].
Changes in forest cover in the study area largely correspond to those observed overall in the Polish Carpathians, where forest cover increased from 27% to 47% from the mid-19th century to the 2010s [69,70]. This has resulted in the establishment of extensive forest complexes contributing to reduced forest fragmentation, especially in the southern and south-eastern parts of the Polish Carpathians [25]. New forest areas have been created in places where previously a different type of land use was exercised (e.g., fields, meadows, midfield afforestation). These areas were afforested with Scots pine (Pinus sylvestris), and in areas left to natural succession, the main forest tree species was grey alder (Alnus incana) [23,28]. Our study suggests that increased forest area and progressive reconstruction of species composition over time in stands on former farmland, followed by the restoration of forest habitats, had a positive effect on the species composition of herbaceous layer plant communities. Changes in herbaceous layer plant communities over time showed a greater number of slow-dispersing and ancient forest species in the 2000s than in the 1970s. The optimum conditions for these species in the 2000s are related to the dominant, optimum stage of stand development. In the 2010s, despite an increase in forest cover, there was a decrease in the number of beech and slow-dispersing species, which is probably related to the increased intensity of management in ageing stands [71].
4.2. Impact of Changes in Stand Structure and Forest Management
Stand spatial structure consists of the vertical and horizontal organisation of the trees. Stand structure affects herbaceous plant species composition and influences habitat conditions and microclimate [31,32,72]. In commercial forests, stand structure is shaped by the silvicultural system [36]. A change in management to one that more closely mimics natural forest regeneration processes results in a significant modification of spatial structure [73,74]. In the Polish part of the Eastern Carpathians, at the end of the 1990s, the regular shelterwood system that created single-storey and single-aged stands was replaced by the irregular shelterwood system, producing more diverse stand ages and overstorey structures [35]. In the 2000s and 2010s, compared to the 1970s, there was a significant increase in the proportion of stands of age class VI and those designated in the regeneration period (RP) and a decrease in younger stands of age classes II and III. Mean stand age changed from the 1970s to the 2010s from 85.3 to 113.0 years [71]. In timber production forests, older stand ages are accompanied by increased disturbance as a result of natural changes in forest development and intensified silvicultural activities related to felling and stand regeneration. As a result of natural forest ageing, tree species diversity decreases. In stands within the study area, decreased occurrence of valuable admixture species, i.e., sycamore and ash, may contribute to nutrient depletion and acidification of forest soils [39,75]. The results presented in this paper provide evidence of this outcome, since while there was no decrease in nutrient content (EIVs N), there was an increase in soil acidity (EIVs R) between the 1970s and 2010s.
Tree crown cover decreased over time in beech stands, from 87.3% in the 1970s to 84.0% in the 2000s and 77.6% in the 2010s. However, the resulting overstorey gaps were filled relatively quickly by understorey species, with cover reaching 27.2% in the 2010s [71]. As a result, there was only a slight, non-significant decrease in total tree and undergrowth layer cover (Total_Cov) between the three study periods. Although a reasonably constant level of total tree and shrub layer cover could be expected to translate into unaltered light and moisture conditions, there were changes in the light and moisture conditions on the forest floor, with increased available light (EIVs L) and decreased soil moisture (EIVs F) in the 2010s.
Valuable indicators of drivers of changes in forest vegetation turned out to be diagnostic species. They clearly showed the strong relationship between the herbaceous plant communities and the habitat conditions changing with the development of the forest stand. As the age of the stand increased (development of the stand), the amount of light reaching the forest floor increased as a result of self-thinning or management activities. The increasing amount of light contributed to a faster release of nutrients from the accumulating dead organic debris on the forest floor [76]. Hence, the herbaceous vegetation of the 2000s was characterized by far more occurrence of mesotrophic species of deciduous forests than in the 1970s. The intensification of treatments related to the renewal of mature stands in the 2010s significantly increased thinning of tree crowns and the inflow of light to the forest floor. This was well reflected in the development of species of clearings with high demand for light and nitrogen. The result of the indicator species analysis revealed the important role of light availability in shaping the species composition of the herbaceous layer in the Carpathian beech forests. Depauw et al. [77] came to similar conclusions when analysing temperate forests across Europe. In the studied Carpathian beech forests, despite their semi-natural character, the availability of light is strongly related to the course of management activities. It seems, therefore, that changes in herbaceous layer communities largely depend on forest management.
In commercial forests, herbaceous layer species composition is influenced by activities such as vehicle traffic and soil disturbance during tree felling and extraction [78]. Thus, in beech forests evaluated for this study, in the 2010s, the intensity of forest management associated with tree felling and increased stand regeneration processes may have translated into an overall decrease in the number of herbaceous plant species.
4.3. Impact of Climate Change
Forests have a particularly strong influence on the microclimate at all times of the year, with the growing season being the key period. Stand structure that influences forest microclimate can partially compensate for the negative effects of heat and drought and slow down thermophilization (i.e., increasing relative abundances of warm-adapted or warm-tolerant species combined with the disappearance of cold-adapted species) of plant communities [48,57]. In spite of the moderating influences of tree canopies on microclimates, climate change directly or indirectly affects forest plants and their communities. This impact is increased when crown thinning alters the local microclimate, increasing temperature and decreasing moisture at the forest floor [48,57]. Bosela et al. [43] found that in diverse forest ecosystems (multi-species stands with complex spatial structure), trees can better withstand the higher temperatures associated with a warming climate. Therefore, the decrease in the tree crown cover [71] and the simplification of stand species structure observed in investigated beech forests may, with time, increase the negative impacts of warming on the functioning of herb layer communities.
Over the last 40+ years, the average growing season temperature has increased and the average precipitation has decreased. In the 2000s, when the majority of stands were at the optimum forest development stage [79] and the intensity of management activities was moderate, the impact of climate change may have been buffered by the dense canopy layer. In the 2010s, these stands reached commercial ages and the intensity of felling increased, resulting in lower tree layer cover and much higher shrub layer cover [71]. The temperature in forest stands might be expected to change with increased felling, however, a comparison of EIVs T indices between the study periods suggests that the forest floor temperature did not greatly change. This can be attributed to the buffering role of the developing shrub layer. The decrease in growing season precipitation during the 2010s is another important result of this study, which in addition to changes in stand structure, was responsible for decreased soil moisture (EIVs F) during the 2010s. Lower soil moisture in the 2010s clearly contributed to altered herbaceous species composition in beech forests by eliminating plants requiring soils with high moisture [71].
Our study supports the conclusion that more manipulative forest management systems can play an important role in countering the current and expected effects of climate change on the forest ecosystem [80]. The diverse vertical structure of the forest can lower the temperature inside the forest and reduce the humidity [81]. Therefore, implemented in the 1990s, the irregular shelterwood system allows for greater flexibility in responding to climate change by growing multi-storey stands of unequal age, which consist of trees of various sizes. In an irregular shelterwood system, the timing of cutting and the selection of harvested trees can be much better adapted to the changing climate than in a regular shelterwood system. For example, in an irregular shelterwood system, a longer renewal period allows the canopy of trees to be kept much more compact during the replacement of generations than when removing mature trees with a regular shelterwood system. However, in the period of final felling of mature stands, even more manipulative management systems may not be sufficient to counter climate change. In the study area, in the 2010s, most of the stands reached the final stage of development associated with intensive tree felling and renewal processes [79]. In this case, the small, spatial differentiation of the stands in terms of structure (developmental stages) seems to be responsible for the decline in the species richness of the herbaceous layer, including species of moisture habitats. Hence, we suggest that, while managing the structure of stands, foresters should strive to create a forest structure with high variability of developmental stages on a regional scale.
Author Contributions
Conceptualization, A.B.-P. and T.D.; methodology, A.B.-P. and T.D.; formal analysis, A.B.-P. and T.D.; investigation, A.B.-P. and T.D.; data curation, T.D.; writing—original draft preparation, A.B.-P. and T.D.; writing—review and editing, A.B.-P. and T.D.; visualisation, A.B.-P.; supervision, T.D.; funding acquisition, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for these studies was provided by the statutory fund of the University of Rzeszów.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article. Additional data are available on request from the data curator.
Acknowledgments
We would like to thank Zbigniew Dzwonko for providing the original maps with the locations of archival phytosociological relevés, and the Regional Directorate of the State Forests in Krosno for granting access to the management and administrative specification documentation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Führer, E. Forest functions, ecosystem stability and management. For. Ecol. Manag. 2000, 132, 29–38. [Google Scholar] [CrossRef]
- Święcicki, Z. (Ed.) Instrukcja Urządzania Lasu. Cz. 1. Instrukcja Sporządzania Planu Urządzenia Lasu dla Nadleśnictwa; CILP: Warsaw, Poland, 2012. (In Polish) [Google Scholar]
- Vedeld, P.; Angelsen, A.; Bojö, J.; Sjaastad, E. Forest environmental incomes and the rural poor. For. Policy Econ. 2007, 9, 869–879. [Google Scholar] [CrossRef]
- Varma, V.K.; Ferguson, I.S.; Wild, I. Decision support system for the sustainable forest management. For. Ecol. Manag. 2000, 128, 49–55. [Google Scholar] [CrossRef]
- European Forest Ecosystems–State and trends EEA Report; European Environment Agency: Copenhagen, Denmark, 2016; p. 5.
- Aggestam, F.; Konczal, A.; Sotirov, M.; Wallin, I.; Paillet, Y.; Spinelli, R.; Lindner, M.; Derks, J.; Hanewinkel, M.; Winkel, G. Can nature conservation and wood production be reconciled in managed forests? A review of driving factors for integrated forest management in Europe. J. Environ. Manage. 2000, 268, 110670. [Google Scholar] [CrossRef]
- Blicharska, M.; Angelstam, P.; Giessen, L.; Hilszczański, J.; Hermanowicz, E.; Holeksa, J.; Jacobsen, J.B.; Jaroszewicz, B.; Konczal, A.; Konieczny, A.; et al. Between biodiversity conservation and sustainable forest management—A multidisciplinary assessment of the emblematic Białowieża Forest case. Biol. Conserv. 2020, 248, 108614. [Google Scholar] [CrossRef]
- Maier, C.; Winkel, G. Implementing nature conservation through integrated forest management: A street-level bureaucracy perspective on the German public forest sector. For. Policy Econ. 2017, 82, 14–29. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Mooney, H.A.; Lubchenco, J.; Melillo, J.M. Human Domination of Earth’s Ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, J.; Nilsson, S.G.; Menozzi, P. Biodiversity, disturbances, ecosystem function and management of European forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Kindstrand, C.; Norman, J.; Boman, M.; Mattsson, L. Attitudes towards various forest functions: A comparison between private forest owners and forest officers. Scand. J. For. Res. 2008, 23, 133–136. [Google Scholar] [CrossRef]
- Kindler, E. A comparison of the concepts: Ecosystem services and forest functions to improve interdisciplinary exchange. For. Policy Econ. 2016, 67, 52–59. [Google Scholar] [CrossRef]
- Stephen, R.; Shifley, R.; Moser, K.; Nowak, D.J.; Miles, P.D.; Butler, B.J.; Aguilar, F.X.; DeSantis, R.D.; Greenfield, E.J. Five anthropogenic factors that will radically alter forest conditions and management need in the Northern United States. For. Sci. 2014, 60, 914–925. [Google Scholar] [CrossRef]
- Ortiz, J.C.; Espinosa, C.I.; Dahik, C.Q.; Mendoza, Z.A.; Ortiz, E.C.; Gusmán, E.; Weber, M.; Hildebrandt, P. Influence of Anthropogenical Factors on the Diversity and Structure of a Dry Forest in the Central Part of the Tumbesian Region (Ecuador-Perú). Forests 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Gautam, K.H.; Devoe, N.N. Ecological and anthropogenic niches of sal (Shorea robusta Gaertn. f.) forest and prospects for multiple-product forest management—A review. Forestry 2006, 79, 81–101. [Google Scholar] [CrossRef]
- Puettmann, K.J.; Wilson, S.M.; Baker, S.C.; Donoso, P.J.; Drössler, L.; Amente, G.; Harvey, B.D.; Knoke, T.; Lu, Y.; Nocentini, S.; et al. Silvicultural alternatives to conventional even-aged forest management—What limits global adoption? For. Ecosyst. 2015, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Kuemmeerle, T.; Hostert, P.; Radeloff, V.C.; Perzanowski, K.; Kruhlov, I. Post-socialist forest disturbance in the Carpathian Border region of poland, Slovakia, and Ukraine. Ecol. Appl. 2007, 17, 1279–1295. [Google Scholar] [CrossRef]
- Kuemmeerle, T.; Hostert, P.; Radeloff, V.C.; Linden, S.; Perzanowski, K.; Kruhlov, I. Cross-border Comparison of Post-socialist Farmland Abandonment in the Carpathians. Ecosystems 2008, 11, 614–628. [Google Scholar] [CrossRef]
- Kubijowicz, W. Życie Pasterskie w Beskidach Wschodnich; Prace Instytutu Geografii UJ: Kraków, Poland, 1926. (In Polish) [Google Scholar]
- Skała, M.; Wolski, J. Krajobraz (nie)pamięci–teraźniejszość nadpisująca przeszłość. Osadnictwo i ludowość. In Bojkowszczyzna Zachodnia-Wczoraj, Dziś i Jutro, Tom 2; Instytut Geografii i Przestrzennego Zagospodarowania in Stanisława Leszczyckiego Monografie: Warsaw, Poland, 2016; Volume 37, pp. 347–377. (In Polish) [Google Scholar]
- Durak, T.; Żywiec, M.; Kapusta, P.; Holeksa, J. Impact of land use and climate changes on expansion of woody species on subalpine meadows in the Eastern Carpathians. For. Ecol. Manag. 2015, 339, 127–135. [Google Scholar] [CrossRef]
- Kozak, J. Forest Cover Change in the Western Carpathians in the Past 180 Years. A Case Study in the Orawa Region in Poland. Mt. Res. Dev. 2003, 23, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Kozak, J.; Kaim, D. Forecom; Instytut Geografii i Gospodarki Przestrzennej UJ: Krakow, Poland, 2016. (In Polish) [Google Scholar]
- Kozak, J.; Ziółkowska, E.; Vogt, P.; Dobosz, M.; Kaim, D.; Kolecka, N.; Ostafin, K. Forest-Cover Increase Does Not Trigger Forest-Fragmenntation Decrease: Case Study from the Polish Carpathians. Sustainability 2018, 10, 1472. [Google Scholar] [CrossRef] [Green Version]
- Matlack, G.R.; Monde, J. Consequences of low mobility in spatially and temporally heterogeneous ecosystems. J. Ecol. 2004, 92, 1025–1035. [Google Scholar] [CrossRef]
- Matlack, G.R. Slow plants in a fast forest: Local dispersal as a predictor of species frequencies in a dynamic landscape. J. Ecol. 2005, 93, 50–59. [Google Scholar] [CrossRef]
- Marszałek, E. The forest management in the Carpathian part of Regional Directorate of the State Forest in Krosno and its influence on the protection of nature. Rocz. Bieszcz. 2011, 19, 59–75. (In Polish) [Google Scholar]
- Varga, A.; Molnár, Z.; Biró, M.; Demeter, L.; Gellény, K.; Miókovics, E.; Molnár, Á.; Molnár, K.; Ujházy, N.; Ulicsni, V.; et al. Changing year-round habitat use of extensively grazing cattle, sheep and pigs in East-Central Europe between 1940 and 2014: Consequences for conservation and policy. Agric. Ecosyst. Environ. 2016, 234, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Ustawa z dnia 28 Września 1991 r. o Lasach. Dz. U. 1991 Nr 101 poz. 444 (t.j. Dz. U. z 2020 r. poz. 1463). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19911010444 (accessed on 16 March 2021). (In Polish)
- Helmy, N.; Essl, F.; Mirtl, M.; Dirnoböck, T. Multiple environmental changes drive forest floor cegetation in a temperate mountain forest. Ecol. Evol. 2017, 7, 2155–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, M.; Weland, N.; Platner, C.; Schaefer, M.; Leuschner, C.; Thomas, F.M. Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol. Biochem. 2009, 41, 2122. [Google Scholar] [CrossRef]
- Verheyn, K.; Baeten, L.; De Frenne, P.; Bernhardt-Römermann, M.; Brunet, J.; Cornelis, J.; Decocq, G.; Dierschke, H.; Eriksson, O.; Hédl, R.; et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 2012, 100, 352–365. [Google Scholar] [CrossRef]
- Majchrowska, A. The History of State Forest and Forestry in Poland. Manag. North. Eur. For. 2018, 12, 318–358. [Google Scholar] [CrossRef]
- Jaworski, A.; Kołodziej, Z. Beech (Fagus sulvatica L.) forest of a selection structure in the Bieszczady Mountains (Southeastern Poland). J. For. Sci. 2004, 50, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, A. Hodowla Lasu. Tom, I. Sposoby Zagospodarowania, Odnawianie Lasu, Przebudowa i Przemiana Drzewostanów; Powszechne Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 2018. (In Polish) [Google Scholar]
- Ellenberg, H.; Leuscher, C. Vegetation Mitteleuropas mit den Alpen in Ökologischer, Dynamischer und Historischer Sicht; Ulmer: Stuttgart, Germany, 2010. (In German) [Google Scholar]
- Mölder, A.; Streit, M.; Schmidt, W. When beech strikes back: How strict nature conservation reduces herb-layer diversity and productivity in Central European deciduous forests. For. Ecol. Manag. 2014, 319, 51–61. [Google Scholar] [CrossRef]
- Augusto, L.; Dupouey, J.; Ranger, J. Effects of tree species on understory vegetation and environmental conditions in temperate forests. Ann. For. Sci. 2003, 60, 823–831. [Google Scholar] [CrossRef]
- Durak, T.; Holeksa, J. Biotic homogenisation and differentiation along a habitat gradient resulting from the ageing of manager beech stands. For. Ecol. Manag. 2015, 351, 47–56. [Google Scholar] [CrossRef]
- Vacek, S.; Černý, T.; Vacek, Z.; Podrázský, V.; Mikeska, M.; Králíček, I. Long-term changes in vegetation annd site conditions in beech and spruce forest of lower mountains ranges of Central Europe. For. Ecol. Manag. 2017, 398, 75–90. [Google Scholar] [CrossRef]
- Vacek, S.; Prokůpková, A.; Vacek, Z.; Bulušek, D.; Šimůnek, V.; Králíček, I.; Prausová, R.; Hájek, V. Growth response of mixed beech forests to climate change, various management and game pressure in Central Europe. J. For. Sci. 2019, 65, 331–345. [Google Scholar] [CrossRef]
- Bosela, M.; Štefančík, I.; Petráš, R.; Vacek, S. The effects of climate Warming on the growth of European beech forest depend critically on thinning strategy and site productivity. Agric. For. Meteorol. 2016, 222, 21–31. [Google Scholar] [CrossRef]
- Krupková, L.; Havránková, K.; Krejza, J.; Sedlák, P.; Marek, M.V. Impact of water scarcity on spruce and beech forests. J. For. Res. 2019, 30, 899–909. [Google Scholar] [CrossRef]
- Parpan, V.I.; Stojko, S.M.; Parpan, T.V. Phytocoenotical and ecological characterization of beech forests (Fagus sylvaticae L.) of Ukraine and possibility to expand their area due to global Warming. Infrastruktura i Ekologia Terenów Wiejskich 2013, 2, 29–44. [Google Scholar]
- Melo, M.; Lapin, M.; Kapolková, H.; Pecho, J.; Kružicová, A. Climate Trends in the Slovak Part of the Carpathians. In The Carpathians: Integrating Nature and Society Towards Sustainability. Environmental Science and Engineering; Kozak, J., Ostapowicz, K., Bytnerowicz, A., Wyżga, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 131–150. [Google Scholar] [CrossRef]
- Henrichs, S.; Winterhoff, W.; Schmidt, W. Vegetation dynamics of beech forests on limestone in central Germany over half a century–Effects of climate change, forest management, eutrophication or game browsing? Biodivers. Ecol. 2012, 4, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Van Gansbeke, P.; Verheyen, K.; Bernhardt-Römermann, M.; Baeten, L.; Hédl, R.; Berki, I.; Brunet, J.; et al. Forest microclimate dynamics drive plant responses to Warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Felsmann, K.; Baudis, M.; Kayler, Z.E.; Puhlmann, H.; Ulrich, A.; Gessler, A. Responses of the structure and function of the understory plant communities to preciptation reduction across forest ecosystems in Germany. Ann. For. Sci. 2018, 75, 3. [Google Scholar] [CrossRef] [Green Version]
- Archaux, F.; Wolters, V. Impact of summer drought on forest biodiversity: Why do we know? Ann. For. Sci. 2006, 63, 645–652. [Google Scholar] [CrossRef]
- Dzwonko, Z. Forest communities of the Gory Słonne Range (Polish Eastern Carpathians). Fragm. Flor. Geobot. 1977, 23, 161–200. [Google Scholar]
- Skiba, S.; Drewnik, M. Soil map of the polish carpathian mountains. Rocz. Bieszcz. 2003, 11, 15–20. [Google Scholar]
- Meteorological Data. Averages and Monthly Totals. Lesko. Available online: https://meteomodel.pl (accessed on 16 March 2021).
- Trampler, T.; Mąkosa, K.; Girżda, A.; Bąkowski, J.; Dmyterko, E. Siedliskowe Podstawy Hodowli Lasu; PWRiL: Warsaw, Poland, 1990. (In Polish) [Google Scholar]
- CORINE land Cover-CLC. Available online: https://clc.gios.gov.pl (accessed on 16 March 2021).
- Braun−Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde; Springer: Vienna, Austria, 1964. [Google Scholar]
- Thom, D.; Sommerfeld, A.; Sebald, J.; Hagge, J.; Müller, J.; Seidl, R. Effect of disturbance patterns and deadwood on the microclimate in European beech forests. Agric. For. Meteorol. 2020, 291, 108066. [Google Scholar] [CrossRef]
- Ewald, J.; Jehl, H.; Braun, L.; Lohberger, E. Die Vagetation des Nationalparks Bayerischer Wald als Ausdruck von Standort und Walddynarnik. Tuexenia 2011, 31, 9–38. [Google Scholar]
- Hermy, M.; Honnay, O.; Firbank, L.; Grashof−Bokdam, C.; Lawesson, J.E. An ecological comparison between ancient and other forest plant species of Europe, and the implications for forest conservation. Biol. Conserv. 1999, 91, 9–22. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S. Wskaźnikowe gatunki roślin starych lasów i ich znaczenie dla ochrony przyrody i kartografii roślinności. IGiPZ PAN. Pr. Geogr. 2001, 178, 120–132. (In Polish) [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski. [A Guide for the Identification of Polish Plant Communities]; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2001. (In Polish) [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzenin Mitteleuropa. Scripta Geobot. 1992, 18, 1–248. [Google Scholar]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology a review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Rozkrut, D. Roczniki Statystyczne Leśnictwa 2020; GUS: Warsaw, Poland, 2020. (In Polish) [Google Scholar]
- MacDonald, D.; Crabtree, J.R.; Wiesinger, G.; Dax, T.; Stamou, N.; Fleury, P.; Lazpita, J.G.; Gibon, A. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. J. Environ. Manage. 2000, 59, 47–69. [Google Scholar] [CrossRef]
- Kozak, J.; Estreguil, C.; Troll, M. Forest cover changes in the northern Carpathians in the 20th century: A slow transition. J. Land Use Sci. 2007, 2, 127–146. [Google Scholar] [CrossRef]
- Kozak, J. Forest Cover Changes and Their Drivers in the Polish Carpathian Mountains since 1800. In Reforesting Landscapes Linking Pattern and Process; Nagendra, H., Southworth, J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 253–273. [Google Scholar] [CrossRef]
- Kozak, J.; Szwagrzyk, M. Have there been forest transitions? Forest transition theory revisited in the context of the Modifiable Areal Unit Problem. R. Geogr. Soc. IBG 2016, 48, 504–512. [Google Scholar] [CrossRef]
- Bugno-Pogoda, A.; Durak, R.; Durak, T. Impact of Forest Management on the Temporal Dynamics of Herbaceous Plant Diversity in the Carpathian Beech Forests over 40 Years. Biology 2021, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Ujházy, K.; Hederová, L.; Máliš, F.; Ujházyová, M.; Bosela, M.; Čiliak, M. Overstorey dynamics controls plant diversity in age-class temperate forests. For. Ecol. Manag. 2017, 391, 96–105. [Google Scholar] [CrossRef]
- Schütz, J.P. Silvicultural tools to develop irregular and diverse forest structures. Forestry 2002, 75, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Raymond, P.; Bedard, S.; Roy, V.; Larouche, C.; Tremblay, S. The irregular shelterwood system: Review, classification, and potential application to forests affected by partial disturbances. J. For. 2009, 107, 405–413. [Google Scholar]
- Langenbruch, C.; Helfrich, M.; Flessa, H. Effects of beech (Fagus sylvatica), ash (Fraxinus excelsior) and lime (Tilia spec.) on soil chemical properties in a mixed deciduous forest. Plant Soil. 2012, 352, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Facelli, J.M.; Pickett, S.T.A. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Czerepko, J.; et al. Light availability and land-use history drive biodiversity and functional changes in forest herb layer communities. J. Ecol. 2019, 108, 1411–1425. [Google Scholar] [CrossRef]
- Bergstedt, J.; Hagner, M.; Milberg, P. Effects on vegetation composition of a modified forest harvesting and propagation method compared with clear-cutting, scarification and planting. Appl. Veg. Sci. 2008, 11, 159–168. [Google Scholar] [CrossRef]
- Durak, T.; Bugno-Pogoda, A.; Durak, R. Application of forest inventories to assess the forest developmental stages on plots dedicated to long-term vegetation studies. For. Ecol. Manag. 2021, 489, 119041. [Google Scholar] [CrossRef]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef] [Green Version]
- Kovács, B.; Tinya, F.; Ódor, P. Stand structural drivers of microclimate in mature temperate mixed forests. Agric. For. Meteorol. 2017, 234–235, 11–21. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).