Comparison of Intra-Annual Xylem and Phloem Formation of Picea crassifolia Stands at Two Latitudes in Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Sample Preparation
2.3. Cellular Measurements
2.4. Meteorological Data Collection
2.5. Statistical Analysis
2.5.1. Growth Dynamic Fitting and Biological Parameters Computing
2.5.2. The Between-Site Comparison of Xylem and Phloem Formation
3. Results
3.1. Climatic Differences between Sites
3.2. Dynamics of Xylem Formation between Sites
3.3. Dynamics of Phloem Formation between Sites
3.4. Comparison of Phloem and Xylem Relationship between Sites
4. Discussion
4.1. Differences in Xylem Formation between Sites
4.2. Difference in Phloem Formation between Sites
4.3. Differences in Xylem and Phloem Relationships between Sites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Huang, J.G.; Alam, S.A.; Zhai, L.; Dawson, A.; Stadt, K.J.; Comeau, P.G. Drought causes reduced growth of trembling aspen in western Canada. Glob. Chang. Biol. 2017, 23, 2887–2902. [Google Scholar] [CrossRef]
- Csillery, K.; Kunstler, G.; Courbaud, B.; Allard, D.; Lassegues, P.; Haslinger, K.; Gardiner, B. Coupled effects of wind-storms and drought on tree mortality across 115 forest stands from the Western Alps and the Jura mountains. Glob. Chang. Biol. 2017, 23, 5092–5107. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II And III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Stanturf, J. Forest plantations and conservation of natural forests. Ecosyst. For. Plant 2008, 1676–1680. [Google Scholar]
- Snyder, P.K.; Delire, C.; Foley, J.A. Evaluating the influence of different vegetation biomes on the global climate. Clim. Dyn. 2004, 23, 279–302. [Google Scholar] [CrossRef]
- Lutz, J.A.; Larson, A.J.; Swanson, M.E.; Freund, J.A. Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 2012, 7, e36131. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Evans, M.N.; Liu, X.; Wang, W.; Xu, G.; Wu, G.; Zhang, L. Spatial patterns of precipitation-induced moisture availability and their effects on the divergence of conifer stem growth in the western and eastern parts of China’s semi-arid region. For. Ecol. Manag. 2019, 451, 117524. [Google Scholar] [CrossRef]
- Yan, M.-J.; Zhang, J.-G.; He, Q.-Y.; Shi, W.-Y.; Otsuki, K.; Yamanaka, N.; Du, S. Sapflow-based stand transpiration in a semiarid natural oak forest on China’s loess plateau. Forests 2016, 7, 227. [Google Scholar] [CrossRef] [Green Version]
- Locosselli, G.M. The cambium activity in a changing world. Trees 2017, 32, 1–2. [Google Scholar] [CrossRef]
- Matte Risopatron, J.P.; Sun, Y.; Jones, B.J. The vascular cambium: Molecular control of cellular structure. Protoplasma 2010, 247, 145–161. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Dinneny, J.R.; Yanofsky, M.F. Vascular patterning: Xylem or phloem? Curr. Biol. 2004, 14, R112–R114. [Google Scholar] [CrossRef] [PubMed]
- Cabon, A. Predicting Forest Responses to Climate: Integrating Water and Temperature Constraints from the Cell to the Region. Doctoral Dissertation, Autonomous University of Barcelona, Cerdanyola del Vallès, Spain, 2020. [Google Scholar]
- Petit, G.; Crivellaro, A. Comparative axial widening of phloem and xylem conduits in small woody plants. Trees 2014, 28, 915–921. [Google Scholar] [CrossRef]
- Maunoury-Danger, F.; Fresneau, C.; Eglin, T.; Berveiller, D.; Francois, C.; Lelarge-Trouverie, C.; Damesin, C. Impact of carbohydrate supply on stem growth, wood and respired CO2 delta13C: Assessment by experimental girdling. Tree Physiol. 2010, 30, 818–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, D.R. The impacts of water stress on phloem transport in Douglas-fir trees. Tree Physiol. 2014, 34, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonova, G.F.; Stasova, V.V. Seasonal development of phloem in scots pine stems. Russ. J. Dev. Biol. 2006, 37, 306–320. [Google Scholar] [CrossRef]
- Hölttä, T.; Mencuccini, M.; Nikinmaa, E. Linking phloem function to structure: Analysis with a coupled xylem-phloem transport model. J. Theor. Biol. 2009, 259, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Sevanto, S.; Holtta, T.; Holbrook, N.M. Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant Cell Environ. 2011, 34, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Gričar, J.; Čufar, K. Seasonal dynamics of phloem and xylem formation in silver fir and Norway spruce as affected by drought. Russ. J. Plant Physiol. 2008, 55, 538–543. [Google Scholar] [CrossRef]
- Prislan, P.; Gričar, J.; de Luis, M.; Smith, K.T.; Čufar, K. Phenological variation in xylem and phloem formation in Fagus sylvatica from two contrasting sites. Agric. For. Meteorol. 2013, 180, 142–151. [Google Scholar] [CrossRef]
- Deslauriers, A.; Fonti, P.; Rossi, S.; Rathgeber, C.B.K.; Gričar, J. Ecophysiology and plasticity of wood and phloem formation. In Dendroecology; Ecological Studies; Springer: Cham, Switzerland, 2017; pp. 13–33. [Google Scholar]
- Huang, J.G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Makinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef] [PubMed]
- Swidrak, I.; Gruber, A.; Oberhuber, W. Xylem and phloem phenology in co-occurring conifers exposed to drought. Trees 2014, 28, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gricar, J.; Prislan, P.; Gryc, V.; Vavrcik, H.; de Luis, M.; Cufar, K. Plastic and locally adapted phenology in cambial seasonality and production of xylem and phloem cells in Picea abies from temperate environments. Tree Physiol. 2014, 34, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuny, H.E.; Rathgeber, C.B.; Kiesse, T.S.; Hartmann, F.P.; Barbeito, I.; Fournier, M. Generalized additive models reveal the intrinsic complexity of wood formation dynamics. J. Exp. Bot. 2013, 64, 1983–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfieri, F.J.; Evert, R.F. Seasonal development of the secondary phloem in Pinus. Am. J. Bot. 1968, 55, 518–528. [Google Scholar] [CrossRef]
- Gričar, J.; Zupančič, M.; Čufar, K.; Oven, P. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci. Technol. 2007, 41, 463–475. [Google Scholar] [CrossRef]
- Miller, T.W.; Stangler, D.F.; Larysch, E.; Seifert, T.; Spiecker, H.; Kahle, H.-P. Plasticity of seasonal xylem and phloem production of Norway spruce along an elevational gradient. Trees 2020, 34, 1281–1297. [Google Scholar] [CrossRef]
- Gričar, J. Cambial cell production and structure of xylem and phloem as an indicator of tree vitality: A review. Sustain. For. Manag. Curr. Res. 2012, 111–134. [Google Scholar]
- Fink, S. Microscopical investigations on wood formation and function in diseased trees. IAWA J. 1986, 7, 351–355. [Google Scholar] [CrossRef]
- Gričar, J.; Krže, L.; Čufar, K. Number of cells in xylem, phloem and dormant cambium in silver fir (Abies alba), in trees of different vitality. IAWA J. 2009, 30, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Cuny, H.E.; Rathgeber, C.B.K.; Frank, D.; Fonti, P.; Makinen, H.; Prislan, P.; Rossi, S.; del Castillo, E.M.; Campelo, F.; Vavrcik, H.; et al. Woody biomass production lags stem-girth increase by over one month in coniferous forests. Nat. Plants 2015, 1, 15160. [Google Scholar] [CrossRef]
- Cuny, H.E.; Rathgeber, C.B. Xylogenesis: Coniferous trees of temperate forests are listening to the climate tale during the growing season but only remember the last words! Plant Physiol. 2016, 171, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Balducci, L.; Cuny, H.E.; Rathgeber, C.B.; Deslauriers, A.; Giovannelli, A.; Rossi, S. Compensatory mechanisms mitigate the effect of warming and drought on wood formation. Plant Cell Environ. 2016, 39, 1338–1352. [Google Scholar] [CrossRef]
- Begum, S.; Kudo, K.; Matsuoka, Y.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Rahman, M.H.; Nugroho, W.D.; Oribe, Y.; Jin, H.O.; et al. Localized cooling of stems induces latewood formation and cambial dormancy during seasons of active cambium in conifers. Ann. Bot. 2016, 117, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Treml, V.; Kašpar, J.; Kuželová, H.; Gryc, V. Differences in intra-annual wood formation in Picea abies across the treeline ecotone, Giant Mountains, Czech Republic. Trees 2015, 29, 515–526. [Google Scholar] [CrossRef]
- Ponti, F.; Minotta, G.; Cantoni, L.; Bagnaresi, U. Fine root dynamics of pedunculate oak and narrow-leaved ash in a mixed-hardwood plantation in clay soils. Plant Soil 2004, 259, 39–49. [Google Scholar] [CrossRef]
- Tierney, G.L.; Fahey, T.J.; Groffman, P.M.; Hardy, J.P.; Fitzhugh, R.D.; Driscoll, C.T. Soil freezing alters fine root dynamics in a northern hardwood forest. Biogeochemistry 2001, 56, 175–190. [Google Scholar] [CrossRef]
- Zimmermann, M.H. Effect of low temperature on ascent of sap in trees. Plant Physiol. 1964, 39, 568–572. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Uria, P.; Körner, C. Low temperature limits of root growth in deciduous and evergreen temperate tree species. Funct. Ecol. 2007, 21, 211–218. [Google Scholar] [CrossRef]
- Repo, T.; Mononen, K.; Alvila, L.; Pakkanen, T.T.; Hänninen, H. Cold acclimation of pedunculate oak (Quercus robur L.) at its northernmost distribution range. Environ. Exp. Bot. 2008, 63, 59–70. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Immanen, J.; Nieminen, K.; Smolander, O.P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mahonen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Andersen, S.U.; Ljung, K.; Dolezal, K.; Miotk, A.; Schultheiss, S.J.; Lohmann, J.U. Hormonal control of the shoot stem-cell niche. Nature 2010, 465, 1089–1092. [Google Scholar] [CrossRef]
- Sperling, O.; Kamai, T.; Tixier, A.; Davidson, A.; Jarvis-Shean, K.; Raveh, E.; DeJong, T.M.; Zwieniecki, M.A. Predicting bloom dates by temperature mediated kinetics of carbohydrate metabolism in deciduous trees. Agric. For. Meteorol. 2019, 276–277, 107643. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H. Intra-annual tracheid production in balsam fir stems and the effect of meteorological variables. Trees 2005, 19, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Gričar, J.; Rossi, S.; Čufar, K.; Ellison, A.M. Critical minimum temperature limits xylogenesis and maintains treelines on the southeastern Tibetan Plateau. Sci. Bull. 2017, 62, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Guada, G.; Vázquez-Ruiz, R.A.; García-González, I. Response patterns of xylem and leaf phenology to temperature at the southwestern distribution boundary of Quercus robur: A multi-spatial study. Agric. For. Meteorol. 2019, 269–270, 46–56. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Cufar, K.; Cuny, H.E.; Deslauriers, A.; Fonti, P.; Frank, D.; Gricar, J.; Gruber, A.; King, G.M.; et al. A meta-analysis of cambium phenology and growth: Linear and non-linear patterns in conifers of the northern hemisphere. Ann. Bot. 2013, 112, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.H.; Nugroho, W.D.; Nakaba, S.; Kitin, P.; Kudo, K.; Yamagishi, Y.; Begum, S.; Marsoem, S.N.; Funada, R. Changes in cambial activity are related to precipitation patterns in four tropical hardwood species grown in Indonesia. Am. J. Bot. 2019, 106, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Rossi, S.; Camarero, J.J.; Ellison, A.M.; Liang, E.; Penuelas, J. Critical temperature and precipitation thresholds for the onset of xylogenesis of Juniperus przewalskii in a semi-arid area of the north-eastern Tibetan Plateau. Ann. Bot. 2018, 121, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Rossi, S.; Gricar, J.; Liang, E.; Cufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot. 2015, 115, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Schuster, R.; Oberhuber, W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees 2013, 27, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Larcher, W. Physiological Plant Ecology. Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Gričar, J.; Prislan, P.; de Luis, M.; Gryc, V.; Hacurová, J.; Vavrčík, H.; Čufar, K. Plasticity in variation of xylem and phloem cell characteristics of Norway spruce under different local conditions. Front. Plant Sci. 2015, 6, 730. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Jiang, S.; Muhammad, A.; Kang, J.; Zhu, H.; Li, X.; Chen, L.; Zhu, L.; Huang, J.-G. Radial growth response of Picea crassifolia to climatic conditions in a dryland forest ecosystem in northwest China. Forests 2021, 12, 1382. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A new tool for sampling microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Huang, J.G.; Rossi, S.; Ma, Q.; Yu, B.; Zhai, L.; Luo, D.; Guo, X.; Fu, S.; Zhang, W. Intra-annual dynamics of xylem growth in Pinus massoniana submitted to an experimental nitrogen addition in Central China. Tree Physiol. 2017, 37, 1546–1553. [Google Scholar] [CrossRef] [Green Version]
- Abe, H.; Funada, R.; Ohtani, J.; Fukazawa, K. Changes in the arrangement of cellulose microfibrils associated with the cessation of cell expansion in tracheids. Trees 1997, 11, 328–332. [Google Scholar] [CrossRef]
- Deslauriers, A.; Giovannelli, A.; Rossi, S.; Castro, G.; Fragnelli, G.; Traversi, L. Intra-annual cambial activity and carbon availability in stem of poplar. Tree Physiol. 2009, 29, 1223–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathgeber, C.B.K.; Longuetaud, F.; Mothe, F.; Cuny, H.; Le Moguédec, G. Phenology of wood formation: Data processing, analysis and visualisation using R (package CAVIAR). Dendrochronologia 2011, 29, 139–149. [Google Scholar] [CrossRef]
- Deslauriers, A.; Morin, H.; Begin, Y. Cellular phenology of annual ring formation of Abies balsamea in the Quebec boreal forest (Canada). Can. J. For. Res. 2003, 33, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Deslauriers, A.; Morin, H. Application of the Gompertz equation for the study of xylem cell development. Dendrochronologia 2003, 21, 33–39. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Winter, B. Linear Models and Linear Mixed Effects Models in R with Linguistic Applications. arXiv 2013, arXiv:1308.5499. [Google Scholar]
- Antonova, G.F.; Stasova, V.V. Effects of environmental factors on wood formation in larch (Larix sibirica Ldb.) stems. Trees 1997, 11, 462–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Su, W.; Zhao, X.; Xu, X. Spring precipitation effects on formation of first row of earlywood vessels in Quercus variabilis at Qinling Mountain (China). Trees 2019, 33, 457–468. [Google Scholar] [CrossRef]
- Krabel, D.; Roloff, A.; Bodson, M. Influence of sucrose on seasonal cambial growth. J. Exp. Bot. 1999, 50, 25–26. [Google Scholar]
- Patrick, L.; Cable, J.; Potts, D.; Ignace, D.; Barron-Gafford, G.; Griffith, A.; Alpert, H.; Van Gestel, N.; Robertson, T.; Huxman, T.E.; et al. Effects of an increase in summer precipitation on leaf, soil, and ecosystem fluxes of CO2 and H2O in a sotol grassland in Big Bend National Park, Texas. Oecologia 2007, 151, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Gricar, J.; Zavadlav, S.; Jyske, T.; Lavric, M.; Laakso, T.; Hafner, P.; Eler, K.; Vodnik, D. Effect of soil water availability on intra-annual xylem and phloem formation and non-structural carbohydrate pools in stem of Quercus pubescens. Tree Physiol. 2019, 39, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Deslauriers, A.; Huang, J.G.; Balducci, L.; Beaulieu, M.; Rossi, S. The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiol. 2016, 170, 2072–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myburg, A.A.; Sederoff, R.R. Xylem structure and function. Encycl. Life Sci. 2001, 1–8. [Google Scholar] [CrossRef]
- Turcotte, A.; Morin, H.; Krause, C.; Deslauriers, A.; Thibeault-Martel, M. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agric. For. Meteorol. 2009, 149, 1403–1409. [Google Scholar] [CrossRef]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Lockhart, J. An analysis of irreversible plant cell elongation. J. Theor. Biol. 1965, 8, 264–275. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.-I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of increasing drought severity caused by temperature rise in southern Europe. Environ. Res. Lett. 2014, 9, 044001. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Oribe, Y.; Kubo, T.; Funada, R. Cambial sensitivity to rising temperatures by natural condition and artificial heating from late winter to early spring in the evergreen conifer Cryptomeria japonica. Trees 2010, 24, 43–52. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Graf Pannatier, E.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, D.A.; Milburn, J.A. Transport of Photoassimriates; Longman Scientific &Technical: Harlow, UK, 1989. [Google Scholar]
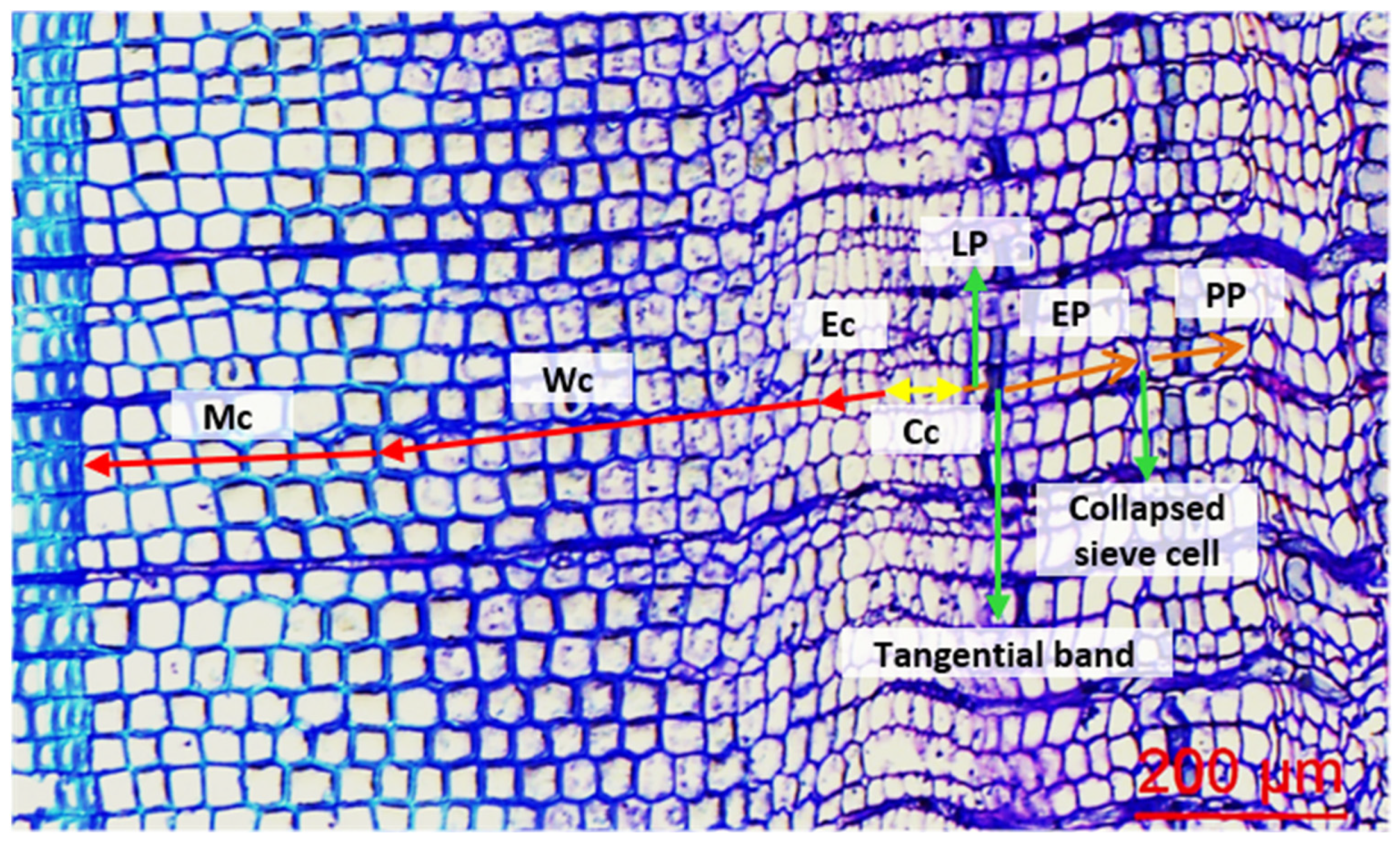

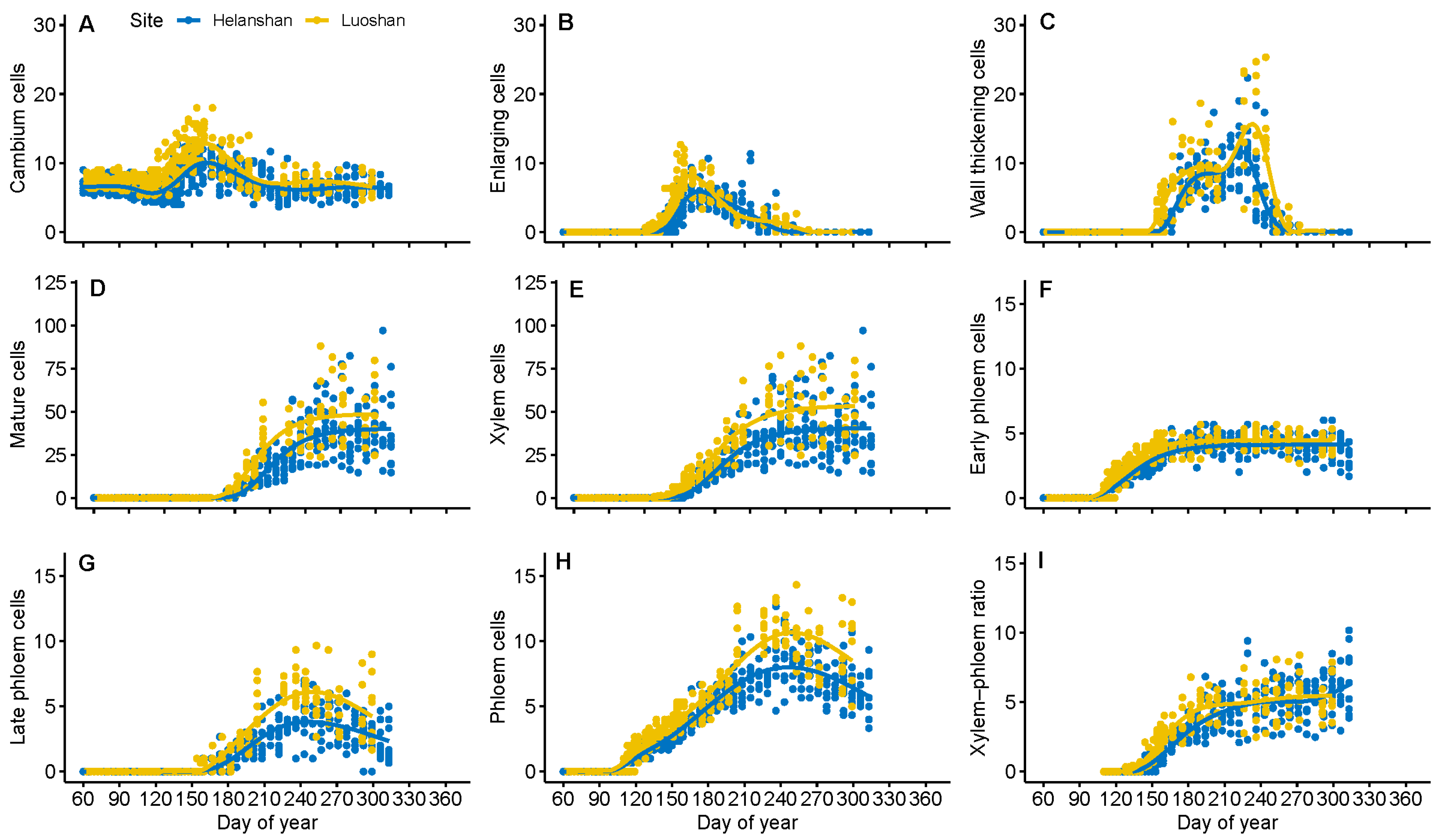
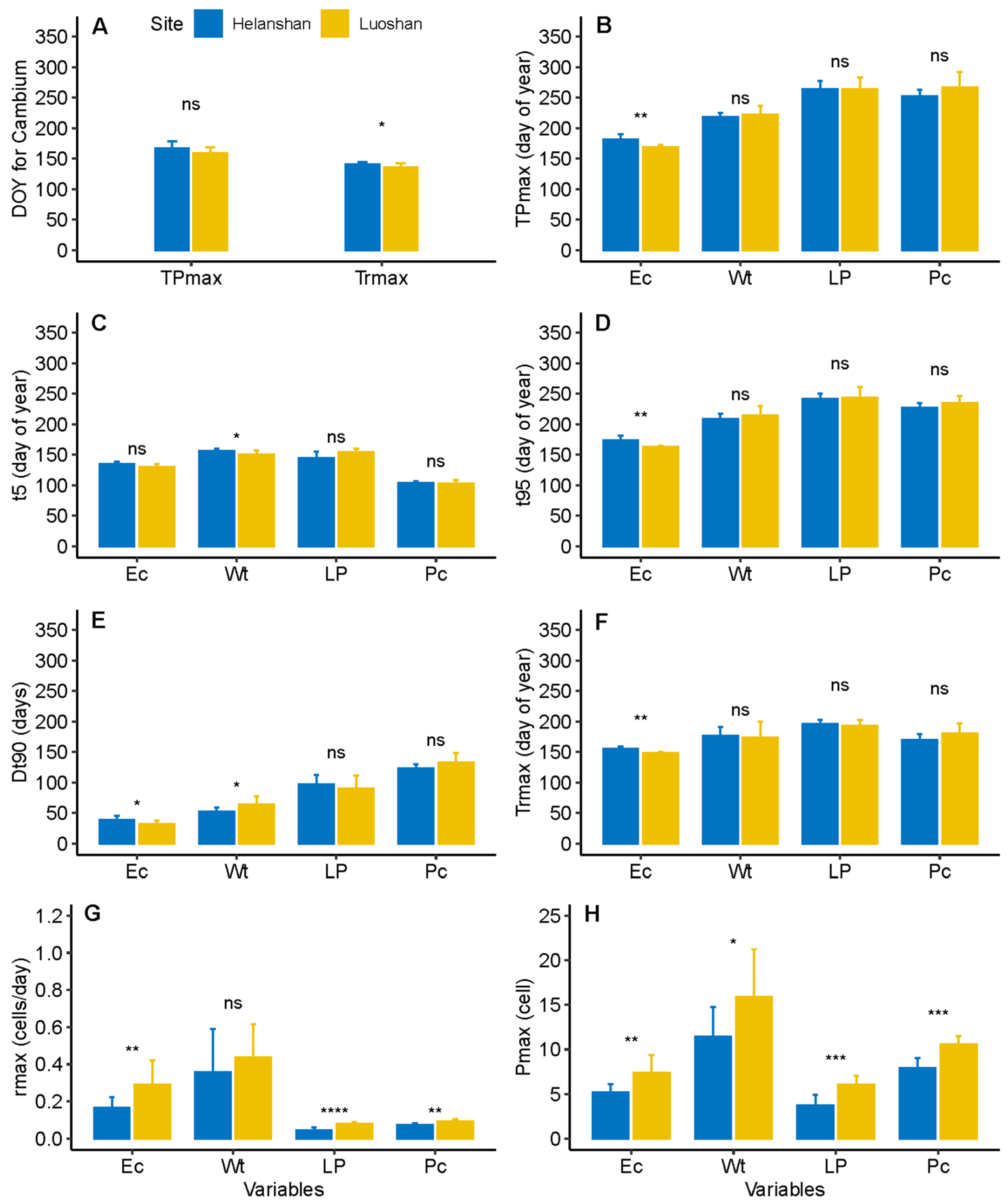

| Site | Longitude (°) | Latitude (°) | Altitude (m) | DBH (cm) |
|---|---|---|---|---|
| Helanshan | 105°54′03″ | 38°46′25″ | 2187 | 35.29 ± 3.62 a |
| Luoshan | 106°16′56″ | 37°18′18″ | 2479 | 31.55 ± 4.87 a |
| Critical Timings | Estimate | Std. Error | t-Value | p-Value |
|---|---|---|---|---|
| bE | −11.36 | 2.11 | −5.37 | 0.001 |
| bL | −6.58 | 1.77 | −3.71 | 0.008 |
| bM | −3.26 | 2.13 | −1.53 | 0.169 |
| cE | 13.76 | 3.80 | 3.62 | 0.009 |
| cL | 4.63 | 4.85 | 0.96 | 0.371 |
| dE | 25.11 | 5.10 | 4.92 | 0.002 |
| dL | 11.26 | 4.43 | 2.54 | 0.039 |
| dX | 15.92 | 5.65 | 2.82 | 0.026 |
| Variables | Source of Variation | F-Value | p-Value |
|---|---|---|---|
| Cambial cells | Site | 30.76 | <0.0001 |
| T | 5.23 | 0.006 | |
| Site × T | 2.49 | 0.084 | |
| Enlarging cells | Site | 3.03 | 0.082 |
| T | 6.34 | 0.002 | |
| Site × T | 118.56 | <0.0001 | |
| Wall thickening cells | Site | 1.93 | 0.165 |
| T | 23.53 | <0.0001 | |
| Site × T | 16.61 | <0.0001 | |
| Mature cells | Site | 0.62 | 0.433 |
| T | 18.91 | <0.0001 | |
| Site × T | 36.74 | <0.0001 | |
| Xylem cells | Site | 2.23 | 0.136 |
| T | 204.53 | <0.0001 | |
| Site × T | 6.35 | 0.002 | |
| Early phloem cells | Site | 25.77 | <0.0001 |
| T | 10.28 | <0.0001 | |
| Site × T | 108.75 | <0.0001 | |
| Late phloem cells | Site | 9.40 | 0.002 |
| T | 198.67 | <0.0001 | |
| Site × T | 14.30 | <0.0001 | |
| Phloem cells | Site | 3.05 | 0.080 |
| T | 18.21 | <0.0001 | |
| Site × T | 85.60 | <0.0001 |
| Variables | Time Period | Estimate | Std. Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Cambial cells | April–May | 0.45 | 0.50 | 0.91 | 0.366 |
| June–August | 1.19 | 0.33 | 3.60 | <0.001 | |
| September–November | 0.41 | 0.27 | 1.50 | 0.134 | |
| Enlarging cells | April–May | −5.51 | 0.41 | −13.48 | <0.0001 |
| June–August | 2.81 | 0.59 | 4.77 | <0.0001 | |
| Wall thickening cells | April–May | −5.79 | 0.49 | −11.78 | <0.0001 |
| June–August | 3.53 | 1.28 | 2.75 | 0.006 | |
| Mature cells | April–May | 0.03 | 0.08 | 0.40 | 0.690 |
| June–August | −25.98 | 4.09 | −6.35 | <0.0001 | |
| September–November | 10.89 | 3.63 | 3.00 | 0.003 | |
| Xylem cells | April–May | −12.72 | 0.65 | −19.64 | <0.0001 |
| June–August | −3.78 | 3.75 | −1.01 | 0.313 | |
| September–November | 10.89 | 3.63 | 3.00 | 0.003 | |
| Early phloem cells | April–May | −4.13 | 0.16 | −25.20 | <0.0001 |
| June–August | 0.18 | 0.09 | 1.98 | 0.048 | |
| Late phloem cells | June–August | 0.31 | 0.43 | 0.73 | 0.464 |
| September–November | 2.12 | 0.47 | 4.52 | <0.0001 | |
| Phloem cells | April–May | −4.88 | 0.18 | −27.07 | <0.0001 |
| June–August | 0.51 | 0.45 | 1.12 | 0.264 | |
| September–November | 2.80 | 0.48 | 5.81 | <0.0001 |
| Site | Dependent Variable | Fixed Effect | Estimate | Std. Error | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Helanshan | cL | tipEP | −2.17 | 0.85 | −2.54 | 0.035 |
| cL | t95EP | −0.92 | 0.35 | −2.59 | 0.032 | |
| dL | TipEP | −2.37 | 0.81 | −2.91 | 0.020 | |
| dL | t95EP | −0.90 | 0.37 | −2.42 | 0.042 | |
| dX | 95EP | −1.29 | 0.45 | −2.84 | 0.022 | |
| Luoshan | bE | rmaxLP | −477.18 | 168.14 | −2.84 | 0.030 |
| bE | r90LP | −511.05 | 152.69 | −3.35 | 0.015 | |
| bL | t5EP | 0.49 | 0.19 | 2.56 | 0.043 | |
| bL | TipEP | 0.63 | 0.24 | 2.67 | 0.037 | |
| bL | PmaxLP | −2.96 | 1.03 | −2.87 | 0.029 | |
| bM | TipEP | 0.66 | 0.18 | 3.61 | 0.011 | |
| cL | TrmaxLP | 0.98 | 0.34 | 2.85 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Li, X.; Zhao, P.; Huang, J. Comparison of Intra-Annual Xylem and Phloem Formation of Picea crassifolia Stands at Two Latitudes in Northwest China. Forests 2021, 12, 1445. https://doi.org/10.3390/f12111445
Yu B, Li X, Zhao P, Huang J. Comparison of Intra-Annual Xylem and Phloem Formation of Picea crassifolia Stands at Two Latitudes in Northwest China. Forests. 2021; 12(11):1445. https://doi.org/10.3390/f12111445
Chicago/Turabian StyleYu, Biyun, Xuebin Li, Ping Zhao, and Jianguo Huang. 2021. "Comparison of Intra-Annual Xylem and Phloem Formation of Picea crassifolia Stands at Two Latitudes in Northwest China" Forests 12, no. 11: 1445. https://doi.org/10.3390/f12111445
APA StyleYu, B., Li, X., Zhao, P., & Huang, J. (2021). Comparison of Intra-Annual Xylem and Phloem Formation of Picea crassifolia Stands at Two Latitudes in Northwest China. Forests, 12(11), 1445. https://doi.org/10.3390/f12111445






