Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
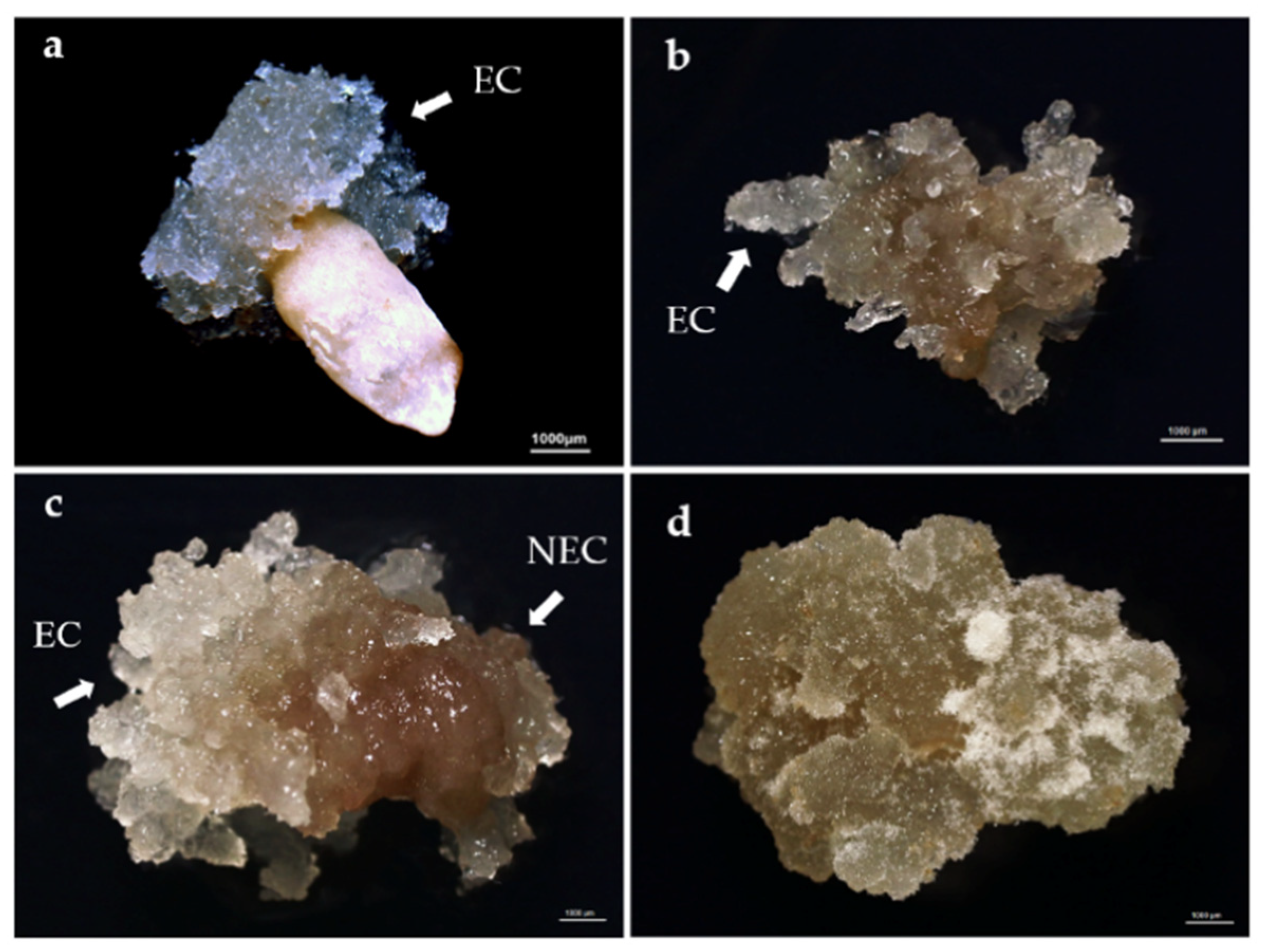
2.2. Initiation of Embryogenic Tissues
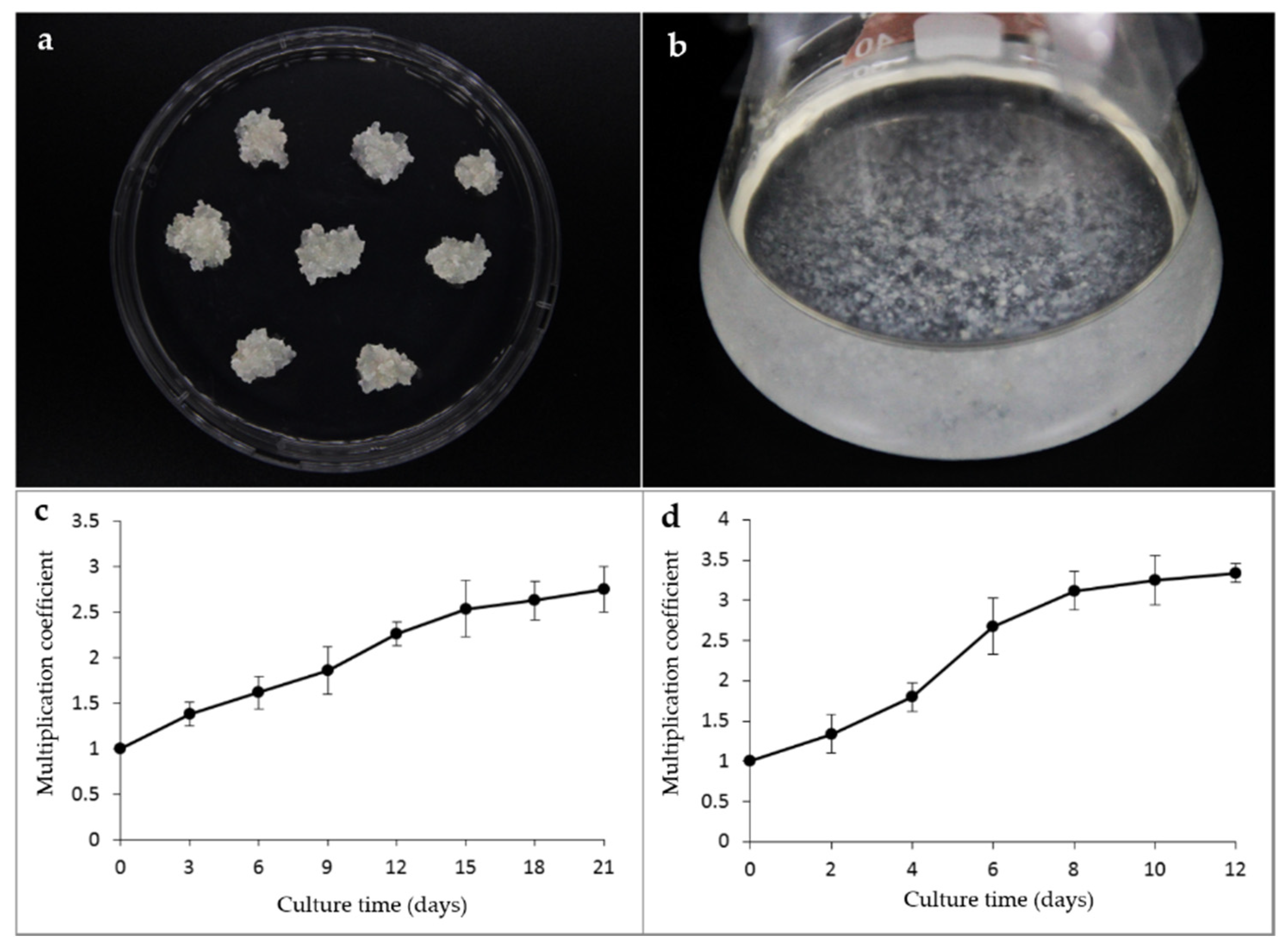
2.3. Embryogenic Tissues Proliferation
2.4. Somatic Embryo Maturation
2.4.1. Effect of ABA on Somatic Embryo Production
2.4.2. Effect of Sucrose, Phytagel and PEG4000 on Somatic Embryo Production
2.4.3. Effect of Different Tissue Inoculation Methods on Somatic Embryo Production
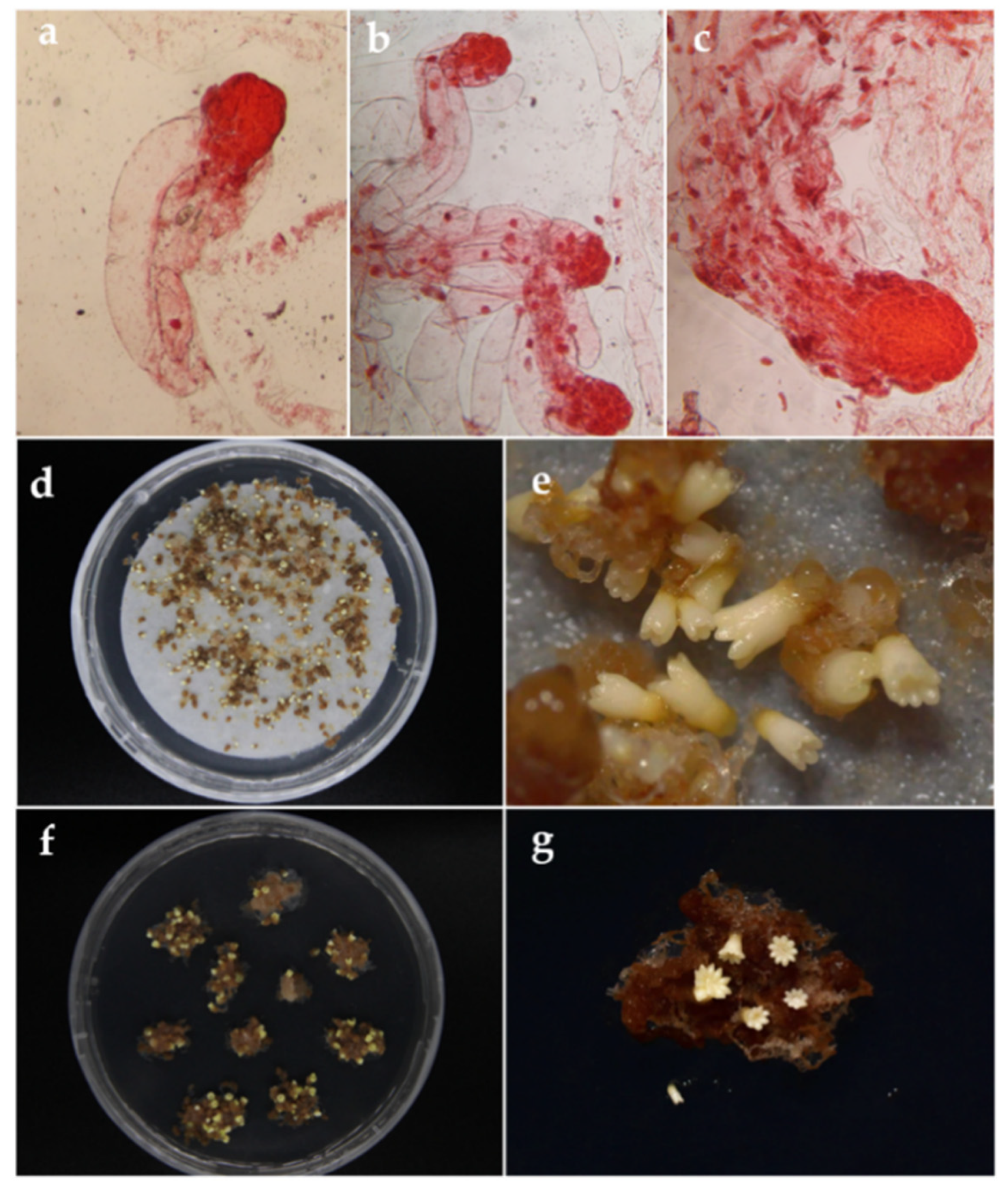
2.5. Embryo Germination and Conversion
2.6. Experiment Design and Data Analysis
3. Results
3.1. Embryogenic Tissues Initiation
3.2. Embryogenic Tissues Proliferation
3.3. Somatic Embryo Maturation
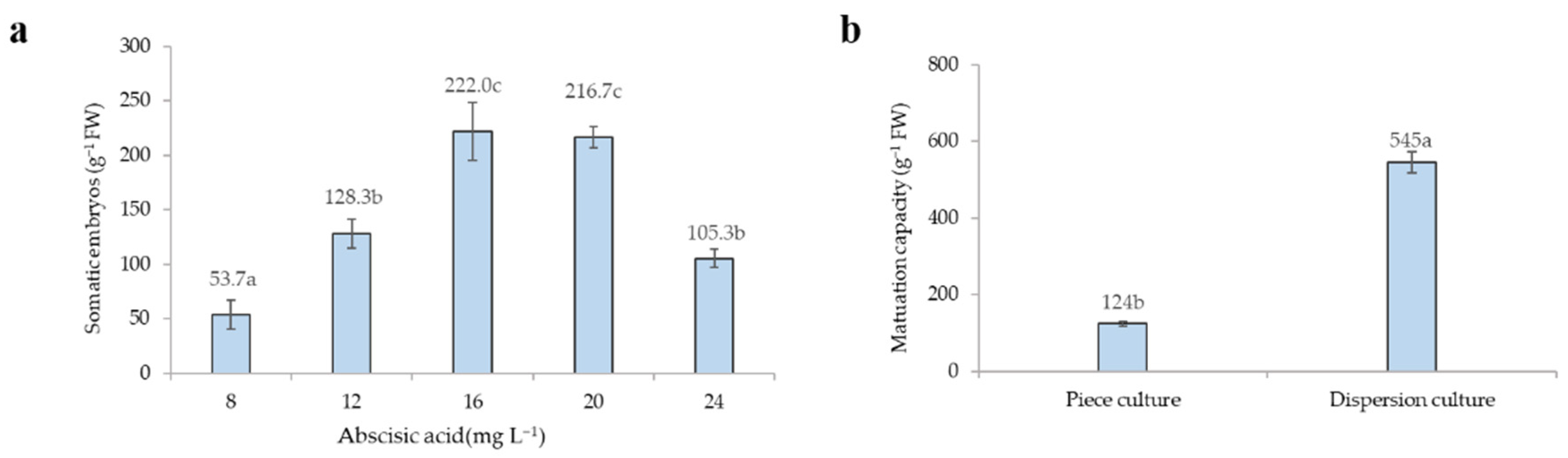
3.3.1. Effects of ABA on Somatic Embryo Maturation
3.3.2. Effects of Culture Methods on Somatic Embryo Maturation
3.3.3. Effects of Sucrose, Gellan Gum (phytagel) and PEG on Somatic Embryo Maturation
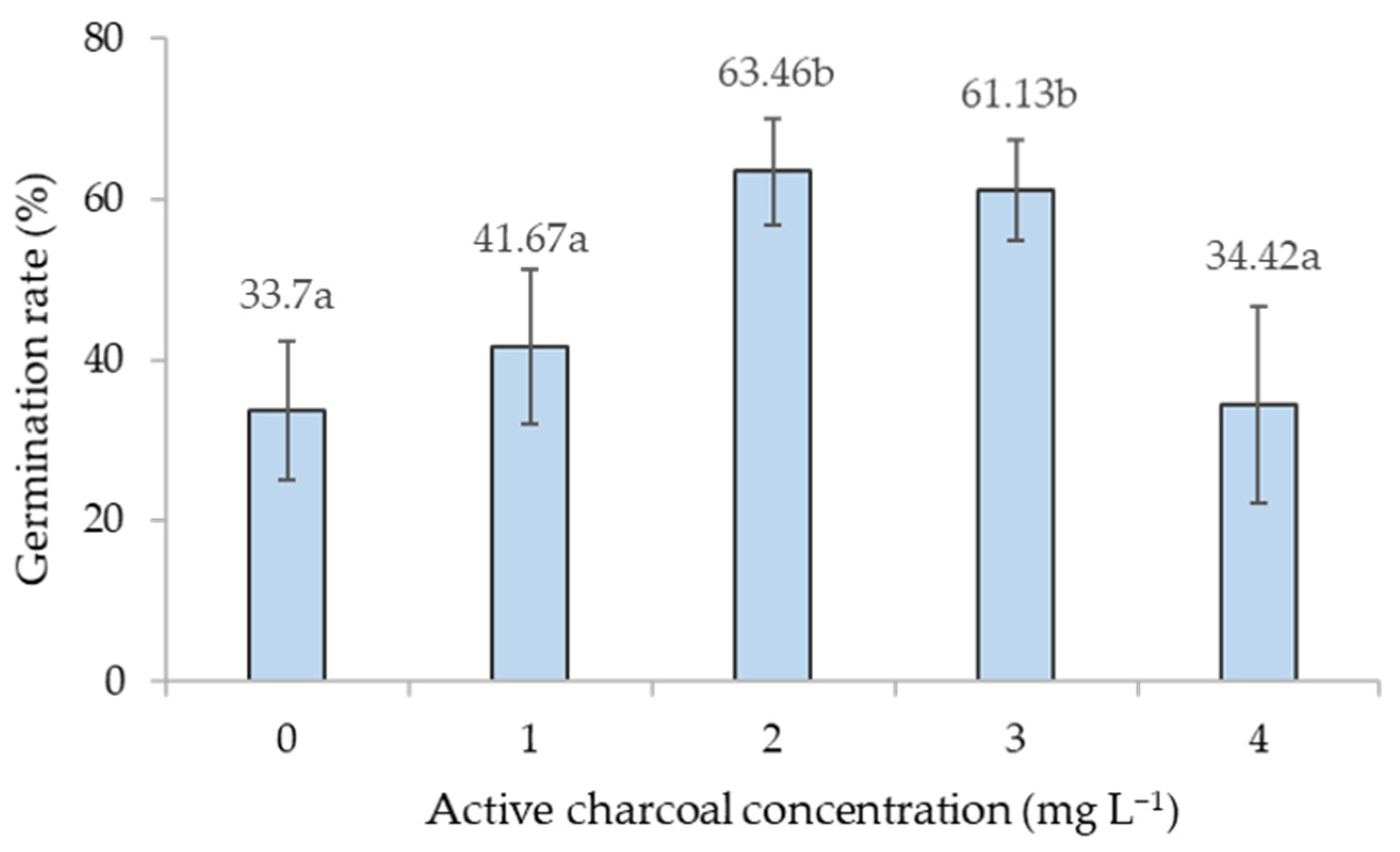
3.3.4. Somatic Embryo Germination and Conversion
4. Discussion
4.1. Embryogenic Tissues Induction and Proliferation in L. principis-rupprechtii Mayr
4.2. Somatic Embryo Maturation
4.3. Effect of Active Charcoal on Somatic Embryo Germination
4.4. Effect of Physical Post-Maturation Treatment on Somatic Embryo Germination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, X.; Sun, Z.; Sun, S. A study on geographic distribution and population variation law of Larix principis-rupprechtii Mary. Nat. For. Shanxi Sci. Silvae Sin. 1992, 28, 493–501. [Google Scholar]
- Ahn, C.H.; Tull, A.R.; Montello, P.M.; Merkle, S.A. A clonal propagation system for Atlantic white cedar (Chamaecyparis thyoides) via somatic embryogenesis without the use of plant growth regulators. Plant Cell Tissue Organ Cult. 2017, 130, 91–101. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial Seeds (Principle, Aspects and Applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.G.; Maheswaran, G. Somatic embryogenesis: Factors influencing coordinated behaviour of cells as an embryogenic group. Ann. Botany 1986, 4, 443–462. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.X.; Shang, G.D.; Wu, L.Y.; Xu, G.Z.; Zhao, X.Y.; Wang, J.W. Chromatin Accessibility Dynamics and a Hierarchical Transcriptional Regulatory Network Structure for Plant Somatic Embryogenesis. Dev. Cell. 2020, 54, 742–757. [Google Scholar] [CrossRef]
- Montalbán, I.A.; García-Mendiguren, O.; Moncaleán, P. Somatic Embryogenesis in Pinus sp. Methods Mol. Biol. 2016, 1359, 405–415. [Google Scholar]
- Hakman, I.; Fowke, L.C.; Von Arnold, S. The development of somatic embryos in tissue cultures initiated from immature embryos of Picea abies (Norway Spruce). Plant Sci. 1985, 38, 53–59. [Google Scholar] [CrossRef]
- Pullman, G.S.; Bucalo, K. Pine Somatic Embryogenesis Using Zygotic Embryos as Explants. Methods Mol. Biol. 2010, 267–291. [Google Scholar] [CrossRef]
- Bettinger, P.; Clutter, M.; Siry, J.; Kane, M.; Pait, J. Broad implications of southern pine clonal forestry on planning and management of forests. Int. For. Rev. 2009, 11, 331–345. [Google Scholar] [CrossRef]
- Gupta, P.K.; Timmis, R. Mass propagation of conifer trees in liquid cultures-progress toward commercialization. Plant Cell Tissue Organ Cult. 2005, 81, 339–346. [Google Scholar] [CrossRef]
- Klimaszewska, K. Plantlet development from immature zygotic embryos of hybrid larch through somatic embryogenesis. Plant Sci. 1989, 63, 95–103. [Google Scholar] [CrossRef]
- Von Aderkas, P.; Klimaszewska, K.; Bonga, J.M. Haploid and diplod embryogenesis in Larix leptolepis, L. decidua and their reciprocal hybrids. Can. J. For. Res. 1990, 20, 9–14. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Devantier, Y.; Lachance, D.; Lelu, M.A.; Charest, P.J. Larix laricina (Tamarack) somatic embryogenesis and genetic transformation. Can. J. For. Res. 1997, 27, 538–550. [Google Scholar] [CrossRef]
- Thompson, R.G.; Von Aderkas, P. Somatic embryogenesis and plant regeneration from immature embryos of western larch. Plant Cell Rep. 1992, 11, 379–385. [Google Scholar] [CrossRef]
- Lü, S.F.; Zhang, S.G.; Qi, L.W.; Sun, X.M.; Wang, J.F. Somatic embryogenesis from immature embryos of Larix kaempferi. Sci. Silvae Sinicae 2005, 41, 48–52. [Google Scholar]
- Song, Y.; Zhen, C.; Zhang, H.; Li, S. Embryogenic Callus Induction and Somatic Embryogenesis from Immature Zygotic Embryos of Larix olgensis. Sci. Silvae Sin. 2016, 52, 45–54. [Google Scholar]
- Xie, Z.L.; Li, D.; Chen, Y.S.; Yu, X.J.; Gu, X.; He, C.Z. Induction of Embryonic Callus of Pinus yunnanensis. J. Southwest For. Univ. Nat. Sci. 2019, 39, 28–34. [Google Scholar]
- Yang, H.H.; Wang, Z.W.; Yang, J.L. Research Progress on Somatic Embryogenesis in Pinaceae. J. Temp. For. Res. 2021, 4, 1–8. [Google Scholar]
- Song, Y.; Li, S.; Zhang, H.; Bai, X.; Dong, H. Establishment and Optimization of Embryogenic Callus Suspension Culture System of Larix. Sci. Silvae Sin. 2018, 54, 146–154. [Google Scholar]
- Pullman, G.S.; Johnson, S.; Peter, G.; Cairney, J.; Xu, N. Improving loblolly pine somatic embryo maturation: Comparison of somatic and zygotic embryo morphology, germination, and gene expression. Plant Cell Rep. 2003, 21, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Biddington, N.L. The influence of ethylene in plant tissue culture. Plant Growth Reg. 1992, 11, 173–187. [Google Scholar] [CrossRef]
- Jean-Paul, R.; Alain, L.; Jean, F. Role of ethylene on induction and expression of carrot somatic embryogenesis: Relationship with polyamine metabolism. Plant Sci. 1994, 103, 223–229. [Google Scholar]
- Qi, L.W.; Han, Y.F.; Li, L.; Ewald, D.; Han, S.Y. Study on effect of ABA, PEG4000 and AgNO3 on number of somatic embryos of Larix principis-rupprechtii by 311-A regression design. Chin. J. Biotech. 2001, 17, 84–89. [Google Scholar]
- Song, Y.; Li, S.; Bai, X.; Zhang, H. Screening and verification of the factors influencing somatic embryo maturation of Larix olgensis. J. For. Res. 2018, 29, 1581–1589. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Wang, H.; Shen, H.; Yang, L. Optimization of maturation process for somatic embryo production and cryopreservation of embryogenic tissue in Pinus koraiensis. Plant Cell Tissue Organ Cult. 2021, 144, 185–194. [Google Scholar] [CrossRef]
- Li, D. Study on Larch Zygotic Embryo Development and Somatic Embryo Induction; Beijing Forestry University: Beijing, China, 2013. [Google Scholar]
- Klimaszewska, K.; Lachance, D.; Pelletier, G.; Lelu, A.-M.; Seguin, A. Regeneration of transgenic Picea glauca, P. Mariana, and P. abies after cocultivation of embryogenic tissue with Agrobacterium tumefaciens. Vitr. Cell. Dev. Biol. 2001, 37, 748–755. [Google Scholar] [CrossRef]
- Litvay, J.D.; Verma, D.C.; Johnson, M.A. Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of wild carrot (Daucus carota L.). Plant Cell Rep. 1985, 4, 325–328. [Google Scholar] [CrossRef]
- Park, Y.S.; Barrett, J.D.; Bonga, J.M. Application of somatic embryogenesis in high-value clonal forestry: Deployment, genetic control, and stability of cryopreserved clones. Vitr. Cell. Dev. Biol. 1998, 34, 231–239. [Google Scholar] [CrossRef]
- Zhang, C.X.; Qian, L.; Kong, L. Induction, development and maturation of somatic embryos in Bunge’s pine (Pinus bungeana Zucc. ex Endl.). Plant Cell Tissue Organ Cult. 2007, 91, 273–280. [Google Scholar] [CrossRef]
- Chen, S. Optimization of Somatic Embryogenesis in Picea likiangensis (Franch.) Pritz and Proteomic Analysis on SEs at Developmental Stages; Yunnan University: Yunnan, China, 2010. [Google Scholar]
- Park, Y.S.; Lelu-Walter, M.A.; Harvengt, L.; Trontin, J.F.; Maceacheron, I.; Klimaszewska, K.; Bonga, J.M. Initiation of somatic embryogenesis in Pinus banksiana, P. strobus, P. pinaster, and P. sylvestris at three laboratories in Canada and France. Plant Cell Tissue Organ Cult. 2006, 86, 87–101. [Google Scholar] [CrossRef]
- Pullman, G.S.; Bucalo, K. Pine somatic embryogenesis: Analyses of seed tissue and medium to improve protocol development. New For. 2014, 45, 353–377. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Hosoi, Y. Progress in Somatic Embryogenesis of Japanese Pines. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.; Taylor, H.F. Assessment of the changes of 2,4-dichlorophenoxyacetic acid concentrations in plant tissue culture media in the presence of activated charcoal. Plant Cell Tissue Organ Cult. 1990, 20, 165–172. [Google Scholar]
- Pullman, G.S.; Gupta, P.K.; Timmis, R.; Carpenter, C.; Kreitinger, M.; Welty, E. Improved Norway spruce somatic embryo development through the use of abscisic acid combined with activated carbon. Plant Cell Rep. 2005, 24, 271–279. [Google Scholar] [CrossRef]
- Rai, M.K.; Shekhawat, N.S.; Harish Gupta, A.K.; Jaiswal, U. The role of abscisic acid in plant tissue culture—a review of recent progress. Plant Cell Tissue Organ Cult. 2011, 106, 179–190. [Google Scholar] [CrossRef]
- Hugo, P.F.F.; Leila, N.V.; Catarina, C.P. Glutathione and abscisic acid supplementation influences somatic embryo maturation and hormone endogenous levels during somatic embryogenesis in Podocarpus lambertii Klotzsch ex Endl. Plant Sci. 2016, 253, 98–106. [Google Scholar]
- LW, Q. Studies on the somatic embryogenesis and establishment of experimental system in Larixprincipis-Rupprechtii. Acta Biol. Exp. Sinica. 2000, 33, 358–365. [Google Scholar]
- Lelu-Walter, M.A.; Gautier, F.; Eliásová, K.I.; Sanchez, L.; Teyssier, C.; Lomenech, A.M.; Metté, C.L.; Hargreaves, C.; Trontin, J.F.O.; Reeves, C. High gellan gum concentration and secondary somatic embryogenesis: Two key factors to improve somatic embryo development in Pseudotsuga menziesii. Plant Cell Tissue Organ Cult. 2018, 132, 137–155. [Google Scholar] [CrossRef]
- Gupta, P.K.; Pullman, G.S. Method for reproducing coniferous plants by somatic embryogenesis using abscisic acid and osmotic potential variation. US Patent 5036007, 18 September 1990. [Google Scholar]
- Klimaszewska, K.; Bernier-Cardou, M.; Cyr, D.R.; Sutton, B.C.S. Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. Vitr. Cell. Dev. Biol. Plant 2000, 36, 279–286. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Smith, D.R. Maturation of SEs of Pinus strobus is promoted by a high concentration of gellan gum. Physiol. Plant. 2010, 100, 949–957. [Google Scholar] [CrossRef]
- Yang, J.; Gui, Y.; Yang, Y. Somatic embryogenesis and plantlet regeneration in mature zygotic embryos of Picea Meyeri. Acta Bot. Sinica. 1997, 39, 315–321. [Google Scholar]
- Lelu, M.; Bastien, C.; Drugeault, A. Somatic embryogenesis and plantet development in Pinus sylvestris and Pinus pinaster on medium with and without growth regulators. Physiol. Plant. 1999, 105, 719–728. [Google Scholar] [CrossRef]
- Lelu-Walter, M.; Pâques, L.E. Simplified and improved somatic embryogenesis of hybrid larches (Larix × eurolepis and Larix × marschlinsii). Perspectives for breeding. Ann. For. Sci. 2009, 66, 104. [Google Scholar] [CrossRef]
- Hosoi, Y.; Maruyama, T.E. Plant regeneration from embryogenic tissue of Pinus luchuensis Mayr, an endemic species in Ryukyu Island, Japan. Plant Biotechnol. 2012, 29, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Teyssier, C.; Grondin, C.; Bonhomme, L.; Lomenech, A.M.; Vallance, M.; Morabito, D.; Label, P.; Lelu-Walter, M.A. Increased gelling agent concentration promotes somatic embryo maturation in hybrid larch (Larix × eurolepsis): A 2-DE proteomic analysis. Physiol. Plant. 2011, 141, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Attree, S.M.; Fowke, L.C. Embryogeny of gymnosperms: Advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult. 1993, 35, 1–35. [Google Scholar] [CrossRef]
- Durzan, D.J.; Gupta, P.K. Biotechnology of somatic polyembryogenesis and plantlet regeneration in loblolly pine. BioTechnology 1987, 5, 147–151. [Google Scholar]
- Becwar, M.R.; Nagmani, R.; Wann, S.R. Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda). Can. J. For. Res.-Rev. Can. De. Rech. For. 1990, 20, 810–817. [Google Scholar] [CrossRef]
- Salajova, T.; Salaj, J. Somatic embryogenesis in Pinus nigra: Embryogenic tissue initiation, maturation and regeneration ability of established cell lines. Biol. Plant 2005, 49, 333–339. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Hosoi, Y.; Ishii, K. Propagation of Japanese red pine (Pinus densiflora Zieb. etZucc.) via somatic embryogenesis. Propag Ornam Plant. 2005, 5, 199–204. [Google Scholar]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryogenesis and plant regeneration in yakutanegoyou, Pinus armandii Franch. var. amamiana (Koidz.) Hatusima, an endemic and endangered species in Japan. Vitr. Cell. Dev. Biol. Plant 2007, 43, 28–34. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Park, Y.; Overton, C.; Maceacheron, I. Optimized Somatic Embryogenesis in Pinusstrobus L. Vitr. Cell. Dev. Biol. Plant 2001, 37, 392–399. [Google Scholar] [CrossRef]
- Lelu-Walter, M.; Bernier-Cardou, M.; Klimaszewska, K. Simplified and improved somatic embryogenesis for clonal propagation of Pinuspinaster (Ait.). Plant Cell Rep. 2006, 25, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-H.; Choi, Y.-E. In vitro clonal propagation and stable cryopreservation system for Platycladus orientalis via somatic embryogenesis. Plant Cell Tissue Organ Cult. 2017, 131, 513–523. [Google Scholar] [CrossRef]
- Kim, Y.W.; Moon, H.K. Enhancement of somatic embryogenesis and plant regeneration in Japanese larch (Larix leptolepis). Plant Cell Tissue Organ Cult. 2007, 88, 241–245. [Google Scholar] [CrossRef]
- Pan, M.J.; van Staden, J. The use of charcoal in vitro culture—A review. Plant Growth Reg. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- von Aderkas, P.; Label, P.; Lelu, M.A. Charcoal affects early development and hormonal concentrations of SEs of hybrid larch. Tree Physiol. 2002, 22, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Winkle, S.C.; Pullman, G.S. The combined impact of pH and activated carbon on the elemental composition of a liquid conifer embryogenic tissue initiation medium. Plant Cell Rep. 2003, 22, 303–311. [Google Scholar] [CrossRef]
- van Winkle, S.C.; Johnson, S.; Pullman, G.S. The impact of Gelrite and activated carbon on the elemental composition of two conifer embryogenic tissue initiation media. Plant Cell Rep. 2003, 21, 1175–1182. [Google Scholar] [CrossRef]
- Rodríguezgacio, M.D.C.; Matillavázquez, M.A.; Matilla, A.J. Seed dormancy and ABA signaling: The breakthrough goes on. Plant Signal. Behavior. 2009, 4, 1035. [Google Scholar] [CrossRef] [Green Version]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of Endogenous Phytohormones Over the Course of Norway Spruce Somatic Embryogenesis. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Roberts, D.R.; Sutton, B.C.S.; Flinn, B.S. Synchronous and high frequency germination of interior spruce SEs following partial drying at high relative humidity. Can. J. Bot. 1990, 68, 1086–1090. [Google Scholar] [CrossRef]
- Beardmore, T.; Charest, P.J. Black spruce somatic embryo germination and desiccation tolerance. I. Effects of abscisic acid, cold, and heat treatments on the germinability of mature black spruce SEs. Can. J. For. Res. 1995, 25, 1763–1772. [Google Scholar] [CrossRef]
- Jones, N.B.; van Staden, J. Improved somatic embryo production from embryogenic tissue of Pinus patula. Vitr. Cell. Dev. Biol. Plant 2001, 37, 543–549. [Google Scholar] [CrossRef]
- Bonga, J.M.; Klimaszewska, K.K.; von Aderkas, P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult. 2010, 100, 241–254. [Google Scholar] [CrossRef]
- Kharenko, O.A.; Zaharia, L.I.; Giblin, M.; Eki, V.; Taylor, D.C.; Palmer, C.D.; Abrams, S.R.; Loewen, M.C. Abscisic acid metabolism and lipid accumulation of a cell suspension culture of Lesquerella fendleri. Plant Cell Tissue Organ Cult. 2011, 105, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, M.; Valladares, S.; Larsson, E.; Clapham, D.; Arnold, S. Patterning during somatic embryogenesis in Scots pine in relation to polar auxin transport and programmed cell death. Plant Cell Tissue Organ Cult. 2012, 109, 391–400. [Google Scholar] [CrossRef]
- Liao, Y.K.; Juan, I. Improving the germination of SEs of Picea morrisonicola Hayata: Effects of cold storage and partial drying. J. For. Res. 2015, 20, 114–124. [Google Scholar] [CrossRef]
- Pullman, G.S.; Olson, K.; Fischer, T.; Egertsdotter, U.; Frampton, J.; Bucalo, K. Fraser fir somatic embryogenesis: High frequency initiation, maintenance, embryo development, germination and cryopreservation. New For. 2016, 47, 1–28. [Google Scholar] [CrossRef]

| Mother Tree No. | Number of Explants | Number of Callus | Number of ETs | Initiation Frequency (%) |
|---|---|---|---|---|
| 2 | 50 | 32 | 6 | 12.0 ± 13.0abc |
| 4 | 50 | 21 | 2 | 4.0 ± 5.5ab |
| 7 | 50 | 35 | 0 | 0a |
| 8 | 50 | 13 | 1 | 2.0 ± 4.5a |
| 10 | 50 | 40 | 10 | 20.0 ± 18.7bcd |
| 14 | 50 | 35 | 0 | 0a |
| 15 | 50 | 10 | 1 | 2.0 ± 4.5a |
| 18 | 50 | 29 | 8 | 16.0 ± 11.4abcd |
| 19 | 50 | 26 | 3 | 6.0 ± 9.0ab |
| 20 | 50 | 17 | 5 | 10.0 ± 7.1abc |
| 23 | 50 | 42 | 15 | 30.0 ± 14.1d |
| 25 | 50 | 38 | 15 | 30.0 ± 7.1d |
| 28 | 50 | 19 | 7 | 14.0 ± 5.5abc |
| 29 | 50 | 25 | 10 | 20.0 ± 10.0bcd |
| 31 | 50 | 26 | 2 | 4.0 ± 5.5ab |
| 32 | 50 | 40 | 8 | 16.0 ± 9.0abcd |
| 35 | 50 | 17 | 6 | 12.0 ± 16.4abc |
| 38 | 50 | 26 | 2 | 4.0 ± 9.0ab |
| 39 | 50 | 38 | 13 | 26.0 ± 15.2cd |
| 40 | 50 | 21 | 13 | 26.0 ± 13.9cd |
| Source of Variation | df | Mean Square | F Value | Sig. |
|---|---|---|---|---|
| sucrose | 2 | 6540.704 | 64.516 | 0.000 ** |
| phytagel | 2 | 24581.815 | 242.468 | 0.000 ** |
| PEG | 2 | 6572.259 | 64.827 | 0.000 ** |
| Error | 20 | 101.381 |
| Levels of Factors | Sucrose (g/L) | Mean Value of Somatic Embryos (g−1FW) | Sig. of Difference | Gellan Gum (g/L) | Mean Value of Somatic Embryos (g−1FW) | Sig. of Difference | PEG (g/L) | Mean Value of Somatic Embryos (g−1FW) | Sig. of Difference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 123.7778 | Aa | 3 | 95.0000 | Aa | 0 | 137.5556 | Aa |
| 2 | 40 | 167.4444 | Bb | 5 | 177.2222 | Bb | 50 | 140.7778 | Aa |
| 3 | 50 | 173.0000 | Bb | 7 | 192.0000 | Cc | 75 | 185.8889 | Bb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Chen, X.; Gao, Y.; Cui, Y.; Kong, L.; Zhao, J.; Zhang, J. Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr. Forests 2021, 12, 1335. https://doi.org/10.3390/f12101335
Jiang S, Chen X, Gao Y, Cui Y, Kong L, Zhao J, Zhang J. Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr. Forests. 2021; 12(10):1335. https://doi.org/10.3390/f12101335
Chicago/Turabian StyleJiang, Shuaifei, Xiaoyi Chen, Ying Gao, Ying Cui, Lisheng Kong, Jian Zhao, and Jinfeng Zhang. 2021. "Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr" Forests 12, no. 10: 1335. https://doi.org/10.3390/f12101335
APA StyleJiang, S., Chen, X., Gao, Y., Cui, Y., Kong, L., Zhao, J., & Zhang, J. (2021). Plant Regeneration via Somatic Embryogenesis in Larix principis-rupprechtii Mayr. Forests, 12(10), 1335. https://doi.org/10.3390/f12101335






