Relationship between Soil Fungi and Seedling Density in the Vicinity of Adult Conspecifics in an Arid Desert Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Plot and Sample Collection
2.3. Sequencing of Soil Fungal DNA
2.4. Statistical Analysis
3. Results
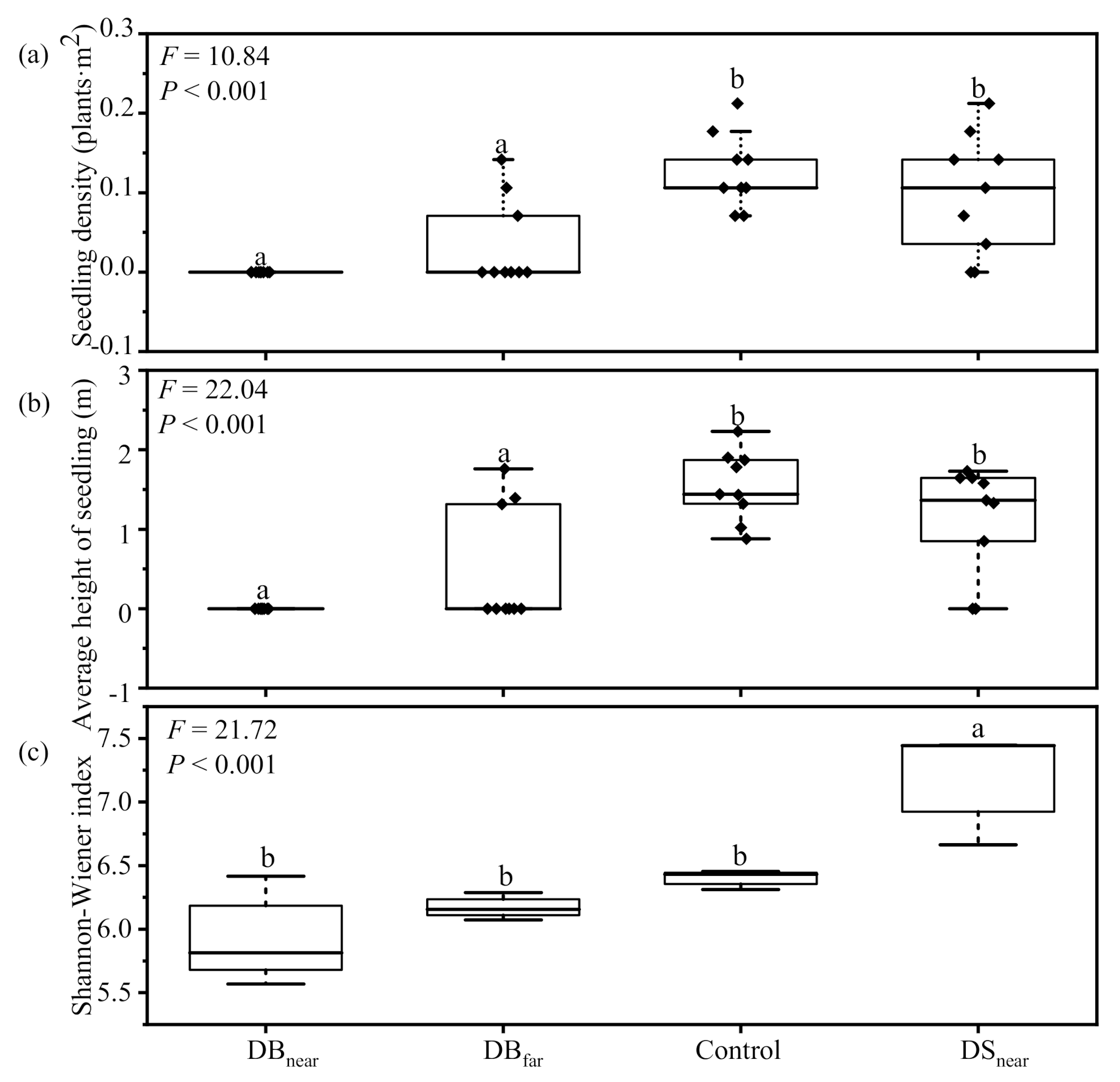
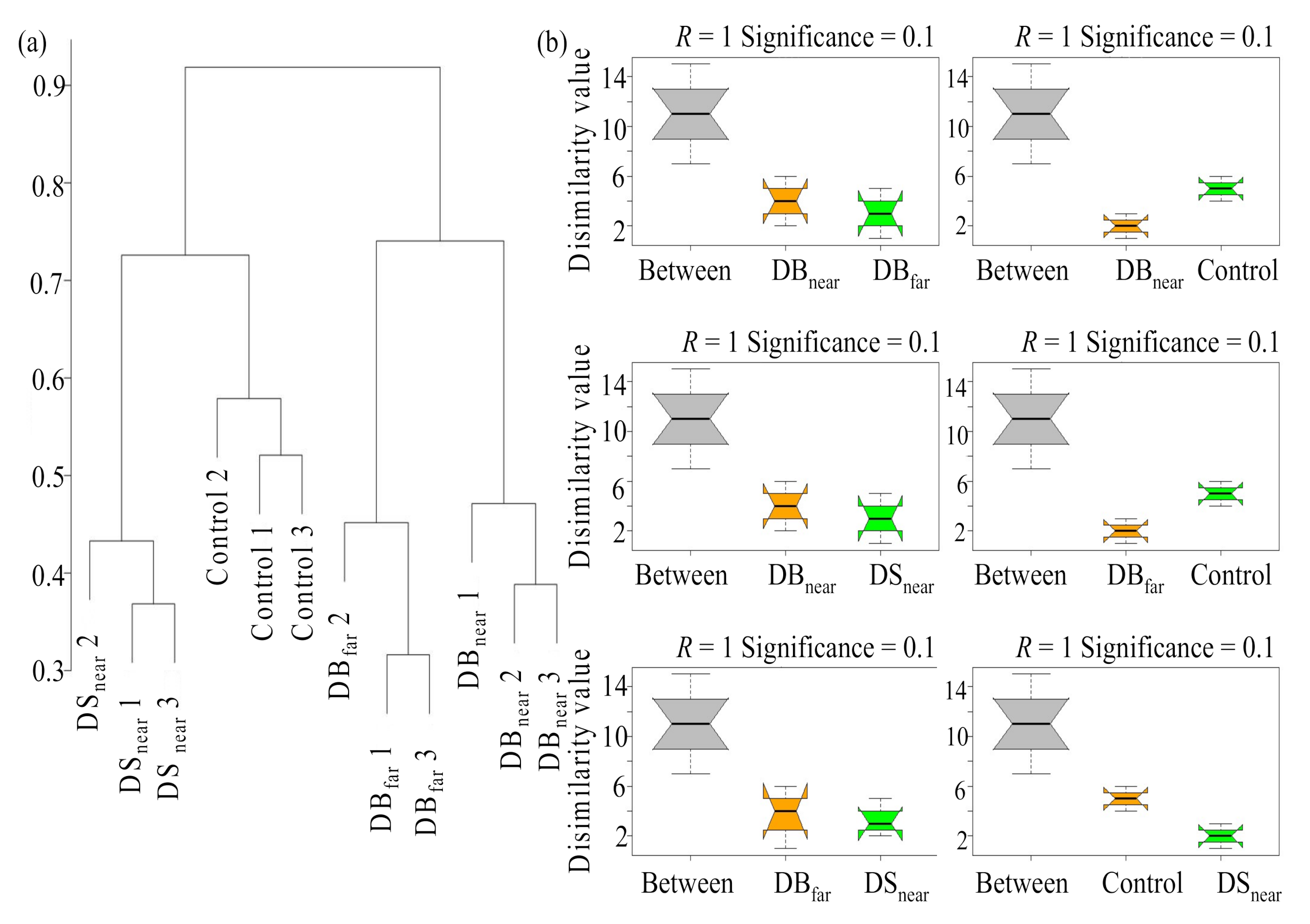
3.1. Differences in Seedling Density, Height, and Fungal Community Composition among Plot Types
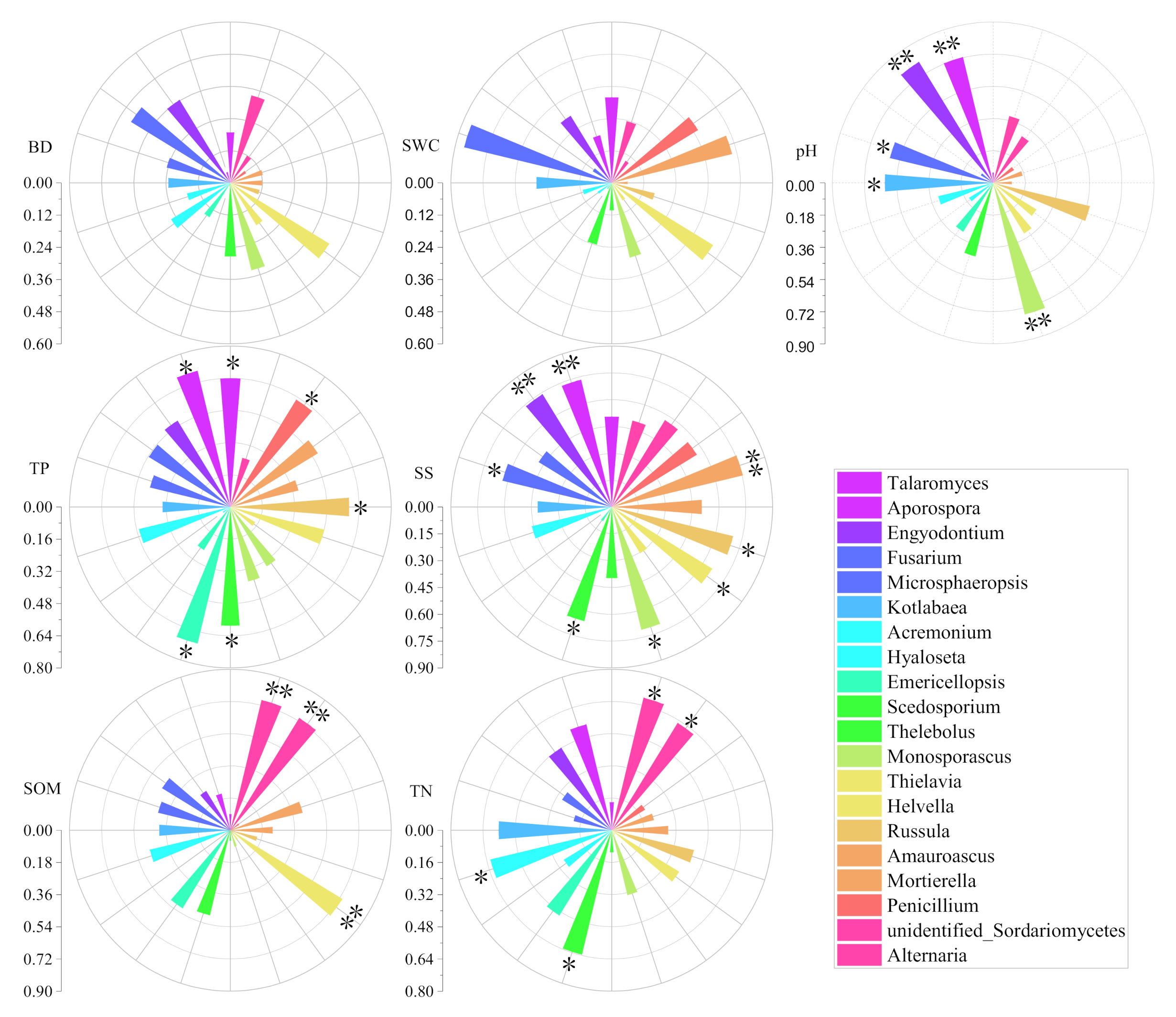
3.2. Influences of Soil Environmental Factors on Soil Fungi and Seedling Density
3.3. Contributions of the Soil Environmental Factors and Soil Fungi to the Change in Seedling Density among the Four Plot Types
4. Discussion
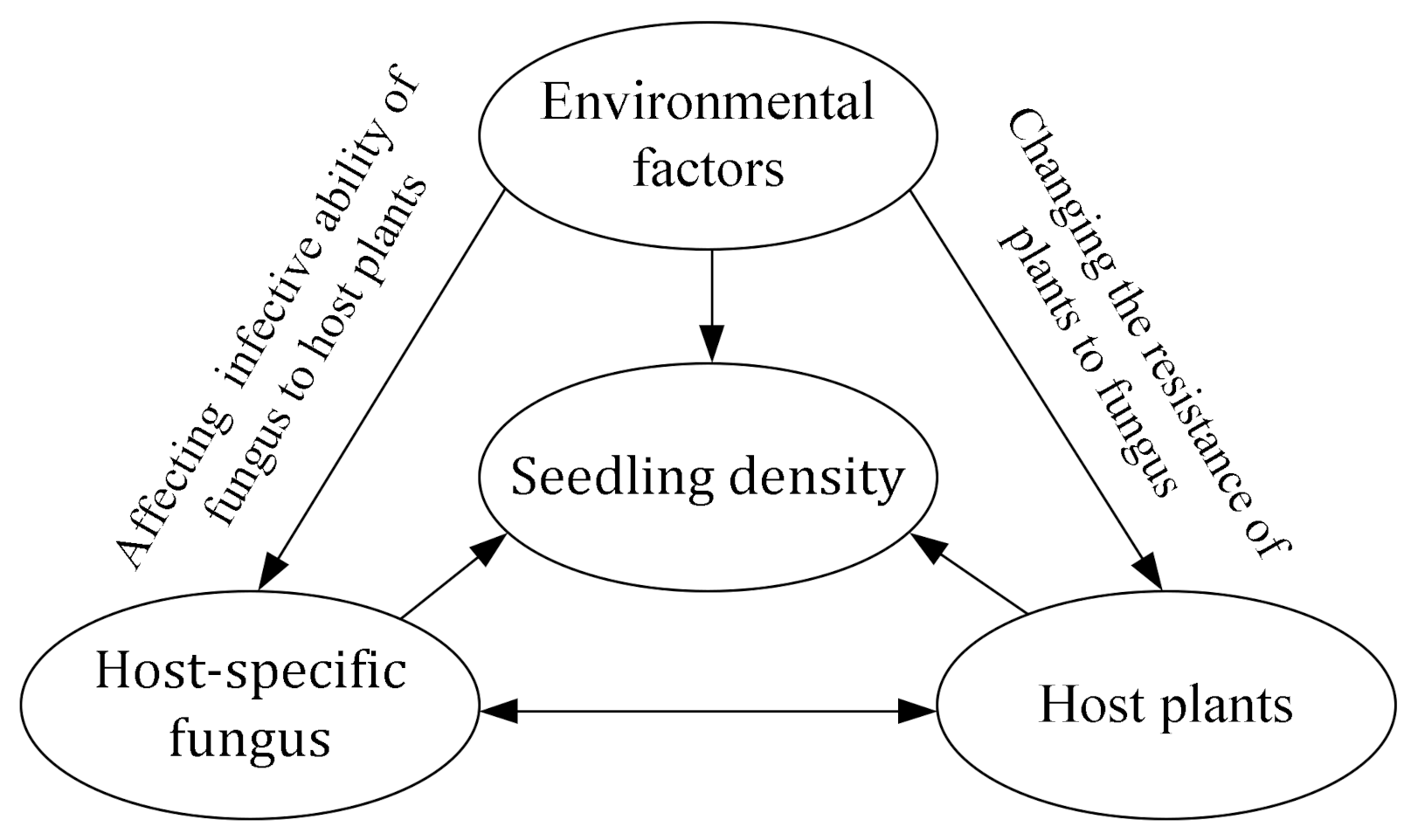
4.1. The Influence of Soil Fungi on Seedling Density near Adults of Conspecifics in Arid Desert Forest
4.2. The Influencing Mechanisms of Soil Fungi on Seedling Density near Adult Conspecifics in Arid Desert Forest
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Junmeng, L.; Johnson, D.J.; Xiujuan, Q.; Zhijun, L.; Qinggang, W.; Mingxi, J. Density dependence and habitat preference shape seedling survival in a subtropical forest in central China. J. Plant Ecol. 2015, 8, 568–577. [Google Scholar]
- Bell, T.; Freckleton, R.P.; Lewis, O.T. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol. Lett. 2006, 9, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, S.; Chesson, P.; He, F. The effect of soil-borne pathogens depends on the abundance of host tree species. Nat. Commun. 2015, 6, 10017. [Google Scholar] [CrossRef]
- Connell, J.H.; Tracey, J.G.; Webb, L.J. Compensatory Recruitment, Growth, and Mortality as Factors Maintaining Rain Forest Tree Diversity. Ecol. Monogr. 1984, 54, 141–164. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Xie, Z.P.; Staehelin, C. Analysis of a negative plant–soil feedback in a subtropical monsoon forest. J. Ecol. 2012, 100, 1019–1028. [Google Scholar] [CrossRef]
- Jia, S.; Wang, X.; Yuan, Z.; Lin, F.; Bagchi, R. Tree species traits affect which natural enemies drive the Janzen-Connell effect in a temperate forest. Nat. Commun. 2020, 11, 286. [Google Scholar] [CrossRef]
- Bagchi, R.; Gallery, R.E.; Gripenberg, S.; Gurr, S.J.; Narayan, L.; Addis, C.E.; Freckleton, R.P.; Lewis, O.T. Pathogens and insect herbivores drive rainforest plant diversity and composition. Nature 2014, 506, 85–88. [Google Scholar] [CrossRef]
- Packer, A.; Clay, K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 2000, 404, 278–281. [Google Scholar] [CrossRef]
- Chanthorn, W.; Caughlin, T.; Dechkla, S.; Brockelman, W.Y. The Relative Importance of Fungal Infection, Conspecific Density and Environmental Heterogeneity for Seedling Survival in a Dominant Tropical Tree. Biotropica 2013, 45, 587–593. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Power, A.G. Release of invasive plants from fungal and viral pathogens. Nature 2003, 421, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Agrios, G.N. Plant Pathology, 5th ed; Elsevier Academic Press: Cambridge, MA, USA, 2005; pp. 26–27. [Google Scholar]
- Hirano, S.S.; Upper, C.D. Population biology and epidemiology of Pseudomonas syringae. Annu. Rev. Phytopathol. 1990, 28, 155–177. [Google Scholar] [CrossRef]
- Antonovics, J.; Boots, M.; Ebert, D.; Koskella, B.; Poss, M. The origin of specificity by means of natural selection: Evolved and nonhost resistance in host-pathogen interactions. Evolution 2013, 67, 1–9. [Google Scholar] [CrossRef]
- Liang, M. Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens. Ecology 2015, 96, 562–574. [Google Scholar] [CrossRef]
- Sun, J.Q.; Liu, R.J.; Li, M. Advances in the study of increasing plant stress resistance and mechanisms by arbuscular mycorrhizal fungi. Plant Phys. J. 2012, 48, 845–852. [Google Scholar]
- Zhu, K.; Woodall, C.W.; Monteiro, J.V.D.; Clark, J.S. Prevalence and strength of density-dependent tree recruitment. Ecology 2015, 96, 2319–2327. [Google Scholar] [CrossRef]
- Liu, Y.; He, F. Incorporating the disease triangle framework for testing the effect of soil-borne pathogens on tree species diversity. Funct. Ecol. 2019, 33, 1211–1222. [Google Scholar] [CrossRef]
- Salazar, P.C.; Navarro-Cerrillo, R.M.; Grados, N.; Cruz, G.; Barrón, V.; Villar, R. Tree size and leaf traits determine the fertility island effect in Prosopis pallida dryland forest in Northern Peru. Plant Soil 2019, 437, 117–135. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, Y.; Xu, D.; Xu, X.; Eldridge, D.J. The fertile island effect collapses under extreme overgrazing: Evidence from a shrub-encroached grassland. Plant Soil 2020, 448, 201–212. [Google Scholar] [CrossRef]
- Mangan, S.A.; Schnitzer, S.A.; Herre, E.A.; Mack, K.M.L. Negative plant–soil feedback predicts tree-species relative abundance in a tropical forest. Nature 2010, 466, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, P.; Mehrabi, Z.; Bezemer, T.M.; De Deyn, G.B.; Kulmatiski, A.; Drigo, B.; Veen, G.F.; Van Der Heijden, M.; Kardol, P. Plant–Soil Feedback: Bridging Natural and Agricultural Sciences. Trends Ecol. Evol. 2017, 33, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.C.; Meixnera, T.; Hogan, J.F. The role of flood size and duration on streamflow and riparian groundwater composition in a semi-arid basin. J. Hydrol. 2013, 488, 126–135. [Google Scholar] [CrossRef]
- El-Keblawy, A. Impact of Climate Change on Biodiversity Loss and Extinction of Endemic plants of Arid Land Mountains. J Biodivers Endanger Species 2014, 2, 2–20. [Google Scholar] [CrossRef]
- Yang, X.-D.; Wang, J.; Xu, M.-S.; Ali, A.; Xu, Y.; Lamb, D.; Duan, L.-C.; Yan, K.-H.; Yang, S.-T.; Bhadauria, T. Effects of the ephemeral stream on plant species diversity and distribution in an alluvial fan of arid desert region: An application of a low altitude UAV. PLoS ONE 2019, 14, e0212057. [Google Scholar] [CrossRef]
- Bestelmeyer, B.T.; Ward, J.P.; Havstad, K.M. Soil-Geomorphic Heterogeneity Governs Patchy Vegetation Dynamics at an Arid Ecotone. Ecology 2006, 87, 963–973. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, C.; Shi, F.; Schneider, M.; Li, Y. Impact of groundwater depth and soil salinity on riparian plant diversity and distribution in an arid area of China. Sci. Rep. 2020, 10, 7272. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. A critical examination of a rapid method for determining organic carbon in soils-effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Cha, T. Soil Physicochemical Analysis; Forestry Publishing: Beijing, China, 2017. [Google Scholar]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Knelman, J.E.; Jones, J.M.; Beals, S.C.; Bowman, W.D.; Nemergut, D.R. Soil bacterial community structure remains stable over a 5-year chronosequence of insect-induced tree mortality. Front. Microbiol. 2014, 5, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Ramage, B.S.; Johnson, D.J.; Gonzalez-Akre, E.; Mcshea, W.J.; Anderson-Teixeira, K.J.; Bourg, N.A.; Clay, K. Sapling growth rates reveal conspecific negative density dependence in a temperate forest. Ecol. Evol. 2017, 7, 7661–7671. [Google Scholar] [CrossRef] [PubMed]
- Paine, C.E.T.; Norden, N.; Chave, J.; Forget, P.-M.; Fortunel, C.; Dexter, K.G.; Baraloto, C. Phylogenetic density dependence and environmental filtering predict seedling mortality in a tropical forest. Ecol. Lett. 2012, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Comita, L.S.; Muller-Landau, H.C.; Aguilar, S.; Hubbell, S.P. Asymmetric Density Dependence Shapes Species Abundances in a Tropical Tree Community. Science 2010, 329, 330–332. [Google Scholar] [CrossRef]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the Janzen–Connell hypothesis: A meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Kitajima, K. Experimental Studies of Seedling Recruitment from Contrasting Seed Distributions. Ecology 1992, 73, 1270–1284. [Google Scholar] [CrossRef]
- Wu, J.; Swenson, N.G.; Brown, C.; Zhang, C.; Yang, J.; Ci, X.; Li, J.; Sha, L.; Cao, M.; Lin, L. How does habitat filtering affect the detection of conspecific and phylogenetic density dependence? Ecology 2016, 97, 1182–1193. [Google Scholar] [CrossRef]
- Cascio, C.; Schaub, M.; Novak, K.; Desotgiu, R.; Bussotti, F.; Strasser, R.J. Foliar responses to ozone of Fagus sylvatica L. seedlings grown in shaded and in full sunlight conditions. Environ. Exp. Bot. 2010, 68, 188–197. [Google Scholar] [CrossRef]
- Sher, Z.; Hussain, F.; Ahmad, B.; Wahab, M. Allelopathic potential of Populus euphratica olivier. Pak. J. Bot. 2011, 43, 1899–1903. [Google Scholar]
- Kardol, P.; De Deyn, G.B.; Laliberté, E.; Mariotte, P.; Hawkes, C.V.; van der Putten, W. Biotic plant-soil feedbacks across temporal scales. J. Ecol. 2013, 101, 309–315. [Google Scholar] [CrossRef]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Soltani, A.; Gholipoor, M.; Zeinali, E. Seed reserve utilization and seedling growth of wheat as affected by drought and salinity. Environ. Exp. Bot. 2006, 55, 195–200. [Google Scholar] [CrossRef]
- Barlow, K.M.; Mortensen, D.A.; Drohan, P.J. Soil pH influences patterns of plant community composition after restoration with native-based seed mixes. Restor. Ecol. 2020, 28, 869–879. [Google Scholar] [CrossRef]
- Guan, P.; Yang, J.; Yang, Y.; Wang, W.; Wu, D. Land conversion from cropland to grassland alleviates climate warming effects on nutrient limitation: Evidence from soil enzymatic activity and stoichiometry. Glob. Ecol. Conserv. 2020, 24, e01328. [Google Scholar] [CrossRef]
- Johnson, B.G.; Verburg, P.S.J.; Arnone, J.A. Plant species effects on soil nutrients and chemistry in arid ecological zones. Oecologia 2016, 182, 1–19. [Google Scholar] [CrossRef]
- Gehring, C.A.; Sthultz, C.M.; Flores-Rentería, L.; Whipple, A.V.; Whitham, T.G. Tree genetics defines fungal partner communities that may confer drought tolerance. Proc. Nat. Acad. Sci. USA 2017, 114, 11169–11174. [Google Scholar] [CrossRef]
- Bettucci, L.; Saravay, M. Endophytic fungi of Eucalyptus globulus: A preliminary study. Mycol. Res. 1993, 97, 679–682. [Google Scholar] [CrossRef]
- Razaghi, P.; Zafari, D. First report of Microsphaeropsis Olivacea causing brown spine rot on alhagi maurorum in iran. J. Plant Pathol. 2016, 98, 369–377. [Google Scholar]
- Harvey, R.J.; Shishkoff, N.; Pecchia, J.A.; Davis, D.D. Impact of Ammonia During Composting on Calonectria pseudonaviculata and C. henricotiae, Causal Agents of Boxwood Blight. Compost. Sci. Util. 2019, 27, 116–123. [Google Scholar] [CrossRef]
- Guarro, J.; Gams, W.; Pujol, I.; Gené, J. Acremonium Species: New Emerging Fungal Opportunists—In Vitro Antifungal Susceptibilities and Review. Clin. Infect. Dis. 1997, 25, 1222–1229. [Google Scholar] [CrossRef]
- Harun, A.; Blyth, C.C.; Gilgado, F.; Middleton, P.; Chen, C.A.; Meyer, W. Development and Validation of a Multiplex PCR for Detection of Scedosporium spp. in Respiratory Tract Specimens from Patients with Cystic Fibrosis. J. Clin. Microbiol. 2011, 49, 1508–1512. [Google Scholar] [PubMed]
- Miller, S.L.; Buyck, B. Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol. Res. 2002, 106, 259–276. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Chen, Z. Secondary Metabolites Produced by the Deep-Sea-Derived Fungus Engyodontium album. Chem. Nat. Compd. 2017, 53, 224–226. [Google Scholar] [CrossRef]
- Brum, M.C.P.; Araújo, W.L.; Maki, C.S.; Azevedo, J.L. Endophytic fungi from Vitis labrusca L. (‘Niagara Rosada’) and its potential for the biological control of Fusarium oxysporum. Genet. Mol. Res. 2012, 11, 4187–4197. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, F.; De Kroon, J.C.J.M.; Berendse, F.; Prins, H.H.T. The influence of savanna trees on nutrient, water and light availability and the understorey vegetation. Plant Ecol. 2004, 170, 93–105. [Google Scholar] [CrossRef]



| Soil Environmental Factors | DBnear | DBfar | Control | DSnear | F | p-Values |
|---|---|---|---|---|---|---|
| Soil water content (%) | 17.0 ± 1.32 a | 15.9 ± 2.07 b | 15.0 ± 0.61 b | 14.7 ± 0.40 b | 6.83 | <0.05 |
| Soil salinity (g/kg) | 99.7 ± 7.80 a | 74.8 ± 13. 6 b | 48. 6 ± 3.79 c | 56.0 ± 5.92 c | 23.9 | <0.01 |
| Soil total phosphorus (g/kg) | 1.80 ± 0.44 a | 1.40 ± 0.16 ab | 1.29 ± 0.08 ab | 1.38 ± 0.17 b | 4.20 | <0.05 |
| Soil organic matter (g/kg) | 28.1 ± 17.2 a | 6.88 ± 3.64 b | 7.57 ± 4.30 b | 4.82 ± 3.15 b | 6.99 | <0.05 |
| Soil total nitrogen (g/kg) | 1.12 ± 0.73 a | 0.29 ± 0.07 b | 0.24 ± 0.13 b | 0.19 ± 0.08 b | 7.34 | <0.05 |
| Bulk density (g/dm3) | 1.02 ± 0.01 a | 1.03 ± 0.02 a | 1.12 ± 0.16 a | 1.30 a | 1.07 | >0.05 |
| pH | 8.42 ± 0.03 a | 8.43 ± 0.07 a | 8.26 ± 0.03 b | 8.37 ± 0.04 a | 2.32 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Yang, X.; Cao, Y.; Lv, G.; Li, Y.; Pan, Y.; Yan, K.; Liu, Y. Relationship between Soil Fungi and Seedling Density in the Vicinity of Adult Conspecifics in an Arid Desert Forest. Forests 2021, 12, 92. https://doi.org/10.3390/f12010092
Long Y, Yang X, Cao Y, Lv G, Li Y, Pan Y, Yan K, Liu Y. Relationship between Soil Fungi and Seedling Density in the Vicinity of Adult Conspecifics in an Arid Desert Forest. Forests. 2021; 12(1):92. https://doi.org/10.3390/f12010092
Chicago/Turabian StyleLong, Yanxin, Xiaodong Yang, Yuee Cao, Guanghui Lv, Yan Li, Yingji Pan, Kaihong Yan, and Yanju Liu. 2021. "Relationship between Soil Fungi and Seedling Density in the Vicinity of Adult Conspecifics in an Arid Desert Forest" Forests 12, no. 1: 92. https://doi.org/10.3390/f12010092
APA StyleLong, Y., Yang, X., Cao, Y., Lv, G., Li, Y., Pan, Y., Yan, K., & Liu, Y. (2021). Relationship between Soil Fungi and Seedling Density in the Vicinity of Adult Conspecifics in an Arid Desert Forest. Forests, 12(1), 92. https://doi.org/10.3390/f12010092





