Coarse Woody Debris’ Invertebrate Community is Affected Directly by Canopy Type and Indirectly by Thinning in Mixed Scots Pine—European Beech Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Samples Collection and Laboratory Work
2.4. Data Analysis
3. Results
3.1. Invertebrate Community Composition
3.2. Thinning Influence on Invertebrate Community
3.3. Canopy Type Influence on Invertebrate Community
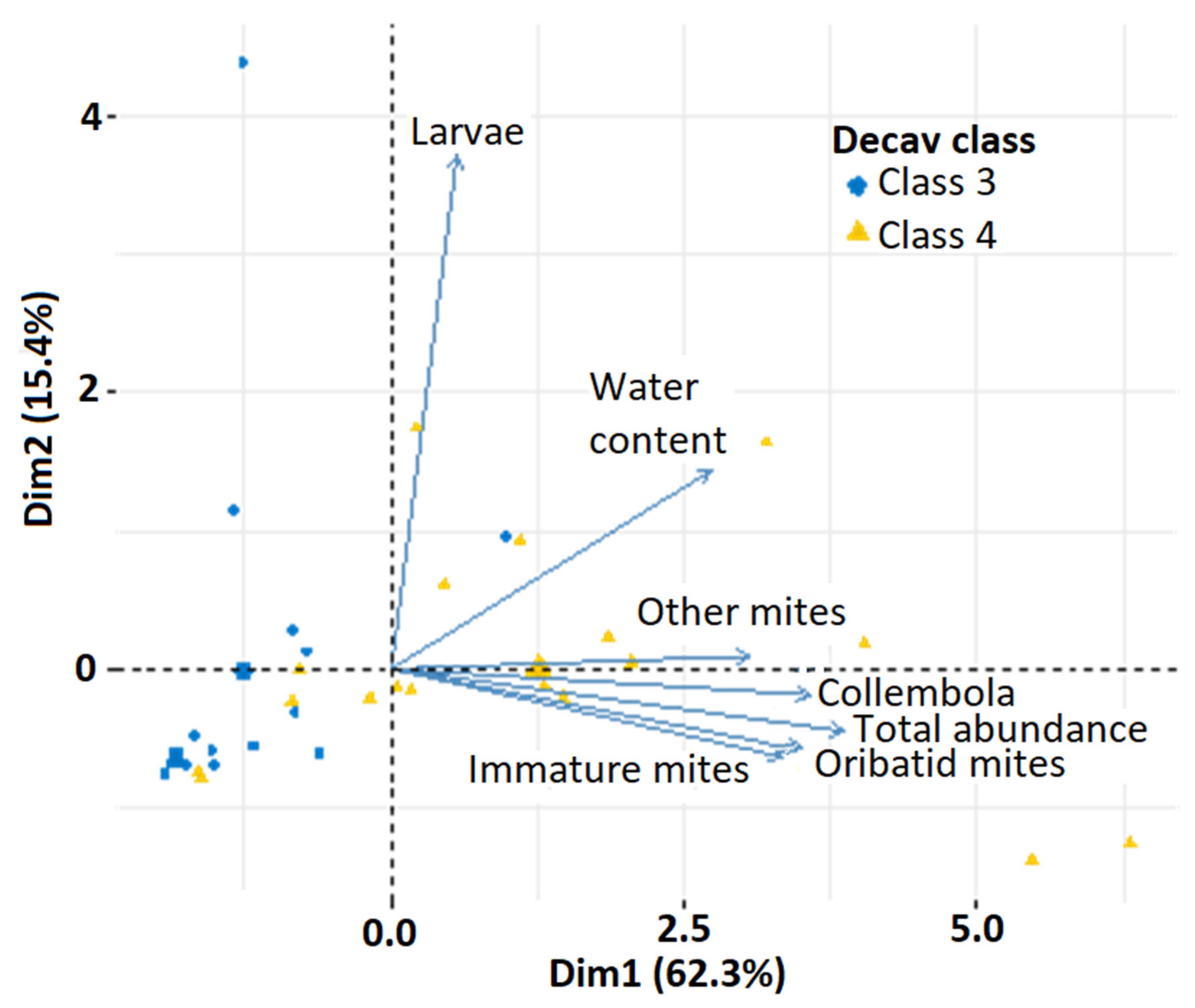
3.4. CWD Decay Class Influence on Invertebrate Community Composition
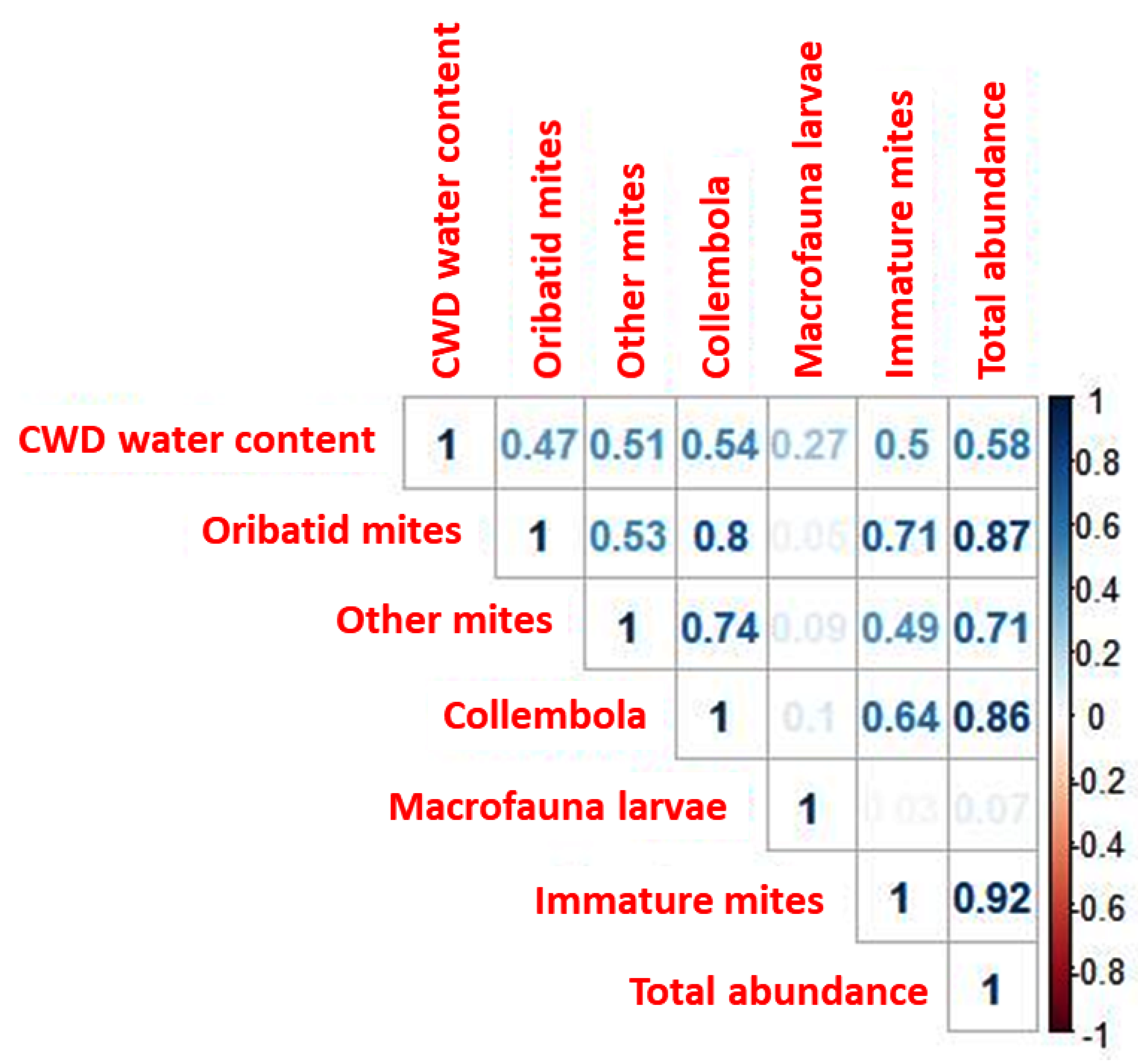
3.5. Correlations between Taxa, Total Abundance, and Water Content
4. Discussion
4.1. Invertebrate Community Composition
4.2. Thinning Influence on Invertebrate Community Composition
4.3. Canopy Type Influence on Invertebrate Community Composition
4.4. CWD Decay Class Influence on Invertebrate Community Composition
4.5. Interactions among Variables and Management Implications
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harmon, M.; Franklin, J.; Swanson, F.; Sollins, P.; Gregory, S.; Lattin, J.; Anderson, N.; Cline, S.; Aumen, N.; Sedell, J.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Harmon, M.E.; Fasth, B.G.; Yatskov, M.; Kastendick, D.; Rock, J.; Woodwall, C.W. Release of coarse woody detritus-related carbon: A synthesis across forest biomes. Carbon Balance Manag. 2020, 15, 1–21. [Google Scholar] [CrossRef]
- Frank, S.; Steyaert, S.; Swenson, J.; Storch, I.; Kindberg, J.; Barck, H.; Zedrosser, A. A ‘‘clearcut’’case? Brown bear selection of coarse woody debris and carpenter ants on clearcuts. For. Ecol. Manag. 2015, 348, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Sullivan, D.; Sullivan, J.-R. Mammalian responses to windrows of woody debris on clear-cuts: Abundance and diversity of forest-floor small mammals and presence of small mustelids. For. Ecol. Manag. 2017, 399, 143–154. [Google Scholar] [CrossRef]
- Riffel, S.; Verschuyl, J.; Miller, D.; Wigley, T. Biofuel harvests, coarse woody debris, and biodiversity—A meta-analysis. For. Ecol. Manag. 2011, 261, 878–887. [Google Scholar] [CrossRef]
- Hanula, J. Relationship of wood-feeding insects and coarse woody debris. In Biodiversity and Coarse Woody Debris in Southern Forests; USDA General Technical Report SE-94, Southeast; US Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 1996; pp. 55–81. [Google Scholar]
- Waddell, K. Sampling coarse woody debris for multiple attributes in extensive resource inventories. Ecol. Ind. 2002, 1, 139–153. [Google Scholar] [CrossRef]
- Savely, H. Ecological relations of certain animals in dead pine and oak logs. Ecol. Monogr. 1939, 9, 322–385. [Google Scholar] [CrossRef]
- Magnússon, R.I.; Tietema, A.; Cornelissen, J.H.C.; Hefting, M.M.; Kalbitz, K. Tamm Review: Sequestration of carbon from coarse woody debris in forest soils. For. Ecol. Manag. 2016, 377, 1–15. [Google Scholar] [CrossRef]
- Fager, E. The community of invertebrates in decaying oak wood. J. Anim. Ecol. 1968, 7, 121–142. [Google Scholar] [CrossRef]
- Vanderwel, M.; Malcolm, J.; Smith, S.; Islam, N. Insect community composition and trophic guild structure in decaying logs from eastern canadian pine-dominated forests. For. Ecol. Manag. 2006, 225, 190–199. [Google Scholar] [CrossRef]
- Radtke, P.J.; Amateis, R.L.; Prisley, S.P.; Copenheaver, C.A.; Pittman, J.R.; Burkhart, H.E. Modeling production and decay of coarse woody debris in loblolly pine plantations. For. Ecol. Manag. 2009, 257, 790–799. [Google Scholar] [CrossRef]
- Fujimori, T. Ecological and Silvicultural Strategies for Sustainable Forest Management; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Primicia, I.; Camarero, J.J.; Imbert, J.B.; Castillo, F.J. Effects of thinning and canopy type on growth dynamics of Pinus sylvestris: Inter-annual variations and intra-annual interactions with microclimate. Eur. J. For. Res 2013, 132, 121–135. [Google Scholar] [CrossRef]
- González de Andrés, E. Interactions between climate and nutrient cycles on forest response to global change: The role of mixed forests. Forests 2019, 10, 609. [Google Scholar] [CrossRef]
- Pretzsch, H.; Del Río, M.; Ammer, C.; Avdagic, A.; Barbeito, I.; Bielak, K.; Brazaitis, G.; Coll, L.; Dirnberger, G.; Drössler, L.; et al. Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe. Eur. J. For. Res. 2015, 134, 927–947. [Google Scholar] [CrossRef]
- Coleman, D.C.; Whitman, W.B. Linking species richness, biodiversity and ecosystem function in soils systems. Pedobiologia 2005, 49, 479–497. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Influence of site characteristics and thinning intensity on litterfall production in two Pinus sylvestris L. forests in the Western Pyrenees. For. Ecol. Manag. 2006, 237, 342–352. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Nutrient return via litter fall in two contrasting Pinus sylvestris forests in the pyrenees under different thinning intensities. For. Ecol. Manag. 2008, 256, 1840–1852. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Thinning affects Pinus sylvestris needle decomposition rates and chemistry differently depending on site conditions. Biogeochemistry 2011, 106, 397–414. [Google Scholar] [CrossRef]
- Martínez, C. Influencia de las Claras Forestales en la Producción de Restos Leñosos en Bosques de pino Silvestre del Pirineo Navarro. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2015. [Google Scholar]
- Herrera-Alvarez, X. Influencia de las Claras Forestales en los Restos Leñosos de Pino Silvestre en un Bosque Mixto en Aspurz: Un Enfoque e Volumen, Biomasa y Hábitat de Invertebrados. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2015. [Google Scholar]
- Fravoline, G.; Tognetti, R.; Lombardi, F.; Egli, M.; Ascher-Jenull, J.; Arfaioli, P.; Bardellin, T.; Cherubini, P.; Marchetti, M. Quantifying decay progression of deadwood in Mediterranean mountain forests. For. Ecol. Manag. 2018, 408, 228–237. [Google Scholar] [CrossRef]
- Gonsalves, L.; Law, B.; Brassil, T.; Waters, C.; Toole, I.; Tap, P. Ecological outcomes for multiple taxa from silvicultural thinning of regrowth forest. For. Ecol. Manag. 2018, 425, 177–188. [Google Scholar] [CrossRef]
- Schowalter, T.; Zhang, Y.; Rykken, J. Litter invertebrate responses to variable density thinning in western Washington forest. Ecol. Appl. 2003, 13, 1204–1211. [Google Scholar] [CrossRef]
- Sipos, J.; Hédl, R.; Hula, V.; Chudomelova, M.; Kosulic, O.; Niedobova, J.; Riedl, V. Patterns of functional diversity of two trophic groups after canopy thinning in an abandoned coppice. Folia Geobotanica 2017, 52, 45–58. [Google Scholar] [CrossRef]
- Kosulic, O.; Michalko, R.; Hula, V. Impact of canopy openness on spider communities: Implications for conservation management of formerly coppiced oak forests. PLoS ONE 2016, 11, eo148585. [Google Scholar] [CrossRef] [PubMed]
- Perry, K.; Herms, D. Response of the forest floor invertebrate community to canopy gap formation caused by early stages of emerald ash borer-induced ash mortality. For. Ecol. Manag. 2016, 375, 259–267. [Google Scholar] [CrossRef]
- Jabat, U. Influencia de la Intensidad de Clara, el Tipo de Dosel Arbóreo y las Hozaduras de Jabalí, en la Descomposición de Hojarasca y Colonización por Mesofauna de un Bosque Mixto del Pirineo Navarro. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2006. [Google Scholar]
- Marturet, S. Influencia de la Intensidad de Clara y Tipo de Dosel Arbóreo Sobre la Descomposición y Respiración del Suelo en un Bosque Mixto del Pirineo Navarro. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2016. [Google Scholar]
- González de Andrés, E.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.H.; Castillo, F.J. Increased complementarity in water-limited environments in scots pine and European beech mixtures under climate change. Ecohydrology 2017, 10, e1810. [Google Scholar] [CrossRef]
- Euforgen. Distribution Map of Scots Pine (Pinus sylvestris L.). Available online: http://www.euforgen.org/species/pinus-sylvestris/ (accessed on 12 August 2020).
- Government of Navarre. Meteorología y Climatología de Navarra. Available online: http://meteo.navarra.es/climatologia/selfichaclima.cfm?IDEstacion=178&tipo=MAN (accessed on 12 August 2020).
- Blanco, J.A. La Práctica de las Claras Forestales y su Influencia en el Ciclo Interno de Nutrientes en dos Bosques de Pino Silvestre de los Pirineos Navarros. Ph.D. Thesis, Public University of Navarre, Pamplona, Spain, 2004. [Google Scholar]
- Primicia, I. Influence of Thinning and Canopy Type on the Internal Nutrient Cycling and the Secondary Growth of Pinus Sylvestris l. in a Mixed Forest in the Pyrenees. Ph.D. Thesis, Public University of Navarre, Pamplona, Spain, 2012. [Google Scholar]
- Papadakis, J. Climates of the World, Their Classification, Similitudes, Differences, and Geographic Distribution; Self-Edited: Buenos Aires, Argentina, 1970. [Google Scholar]
- Blanco, J.A.; Zavala, M.A.; Imbert, J.B.; Castillo, F.J. Sustainability of forest management practices: Evaluation through a simulation model of nutrient cycling. For. Ecol. Manag. 2005, 213, 209–228. [Google Scholar] [CrossRef]
- Candel-Pérez, C.; Lo, Y.H.; Blanco, J.A.; Chiu, C.M.; Camarero, J.J.; de Andrés, E.G.; Imbert, J.B.; Castillo, F.J. Drought-induced changes in wood density are not prevented by thinning in scots pine stands. Forests 2018, 9, 4. [Google Scholar] [CrossRef]
- Garcia Sancet, M.A. Influencia de los Parámetros de Dosel y Lumínicos Sobre la Regeneración de Pinus sylvestris L. en un Bosque Mixto Con Tres Intensidades de Clara. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2017. [Google Scholar]
- Cardil, A.; Imbert, J.B.; Camarero, J.J.; Primicia, I.; Castillo, F.J. Temporal interactions among throughfall, type of canopy and thinning drive radial growth in an Iberian mixed pine-beech forest. Agric. For. Meteor. 2018, 252, 63–74. [Google Scholar] [CrossRef]
- Barrientos, J. Bases Para un Curso Práctico de Entomología; Asociación Española de Entomología: Barcelona, Spain, 1988. [Google Scholar]
- Swift, M.; Heal, O.; Anderson, J. Decomposition in Terrestrial Ecosystems; Blackwell Scientific: Oxford, UK, 1979. [Google Scholar]
- Groombridge, B. Soil Microfauna. In Global Biodiversity: Status of the Earth’s Living Resources; World Conservation Monitoring Centre, Ed.; Chapman & Hall: London, UK, 1992; pp. 103–115. [Google Scholar]
- Arzuaga Iribarren, M. Influencia de la Intensidad de Clara, el Tipo de Dosel Arbóreo y Las Hozaduras de Jabalí en Las Variables Fisico-Químicas y los Microartrópodos del Suelo de un Bosque Mixto. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2005. [Google Scholar]
- Garai Iturri, I. Influencia del Aclareo en la Colonización y Descomposición de Hojarasca de Pinus sylvestris L. por Microartropodos en dos Bosques del Pirineo Navarro. Master’s Thesis, Public University of Navarre, Pamplona, Spain, 2004. [Google Scholar]
- Salazar, M.; Accattoli, C.; Martínez, P.; Schack, J. Nuevas citas de ácaros oribátidos (Acari: Oribatid) para la argentina. Rev. Soc. Entomol. Argent. 2006, 65, 19–22. [Google Scholar]
- Hibbert, A. Importance of Fallen Coarse Woody Debris to the Diversity of Saproxylic Diptera in the Boreal Mixedwood Forests of Eastern North America. Master’s Thesis, Université du Québec à Montréal, Montreal, QC, Canada, 2001. [Google Scholar]
- Moldenke, A. Natural Resources Conservation Service Soils. Natural Resources Conservation Service, United States Department of Agriculture. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/soils/health/biology/?cid=nrcs142p2_05386 (accessed on 12 August 2020).
- Lizotte, R. Desert Museum Arizona, Sonora. Available online: https://www.desertmuseum.org/books/nhsd_centipede.php (accessed on 12 August 2020).
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Cambios en el sotobosque de pinares en los pirineos occidentales bajo distintas intensidades de clara. In Proceedings of the 3rd Spanish forest Congress, Sierra Nevada 2001, Granada, Spain, 25–29 September 2001; Volume 1, pp. 340–345. [Google Scholar]
- Imbert, J.B.; Blanco, J.A.; Valladares, F.; Castillo, F.J. Influence of thinning on plant species richness and diversity, and solar radiation indices in two contrasting Iberian Pinus sylvestris L. forests during a five year period. In Proceedings of the First Diversitas Open Science Conference “Integrating Biodiversity Science for Human Well-Being”, Oaxaca, Mexico, 9–12 November 2005. [Google Scholar]
- González, G.; Seastedt, T. Soil fauna and plant litter decomposition in tropical and subalpine forest. Ecology 2001, 82, 955–964. [Google Scholar] [CrossRef]
- Valladares, F. La disponibilidad de luz bajo el dosel de los bosques y matorrales ibéricos estimada mediante fotografía hemisférica. Ecología 2006, 20, 11–30. [Google Scholar]
- Andre, H.; Voegtlin, D. Some observations on the biology of Camisia carrolli (Acari:Oribatid). Acarologia 1981, 23, 81–89. [Google Scholar]
- Castro, A.; Wise, D.H. Influence of fallen coarse woody debris on the diversity and community structure of forest-floor spiders (Arachnida: Araneae). For. Ecol. Manag. 2010, 260, 2088–2101. [Google Scholar] [CrossRef]
- De Andrés, E.G.; Blanco, J.A.; Imbert, J.B.; Guan, B.T.; Lo, Y.H.; Castillo, F.J. ENSO and NAO affect long-term leaf litter dynamics and stoichiometry of Scots pine and European beech mixedwoods. Glob. Chang. Biol. 2019, 25, 3070–3090. [Google Scholar] [CrossRef] [PubMed]
- Kahl, T.; Arnstadt, T.; Baber, K.; Bässler, C.; Bauhus, J.; Borken, W.; Martin, M.G. Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities. For. Ecol. Manag. 2017, 391, 86–95. [Google Scholar] [CrossRef]
- Kahl, T.; Baber, K.; Otto, P.; Wirth, C.; Bauhus, J. Drivers of CO2 emission rates from dead wood logs of 13 tree species in the initial decomposition phase. Forests 2015, 6, 2484–2504. [Google Scholar] [CrossRef]
- Blanco, J.A.; Page-Dumroese, D.S.; Jurgensen, M.F.; Curran, M.P.; Tirocke, J.M.; Walitalo, J. Modelling the management of forest ecosystems: Importance of wood decomposition. Nat. Resour. Modell. 2018, 31, e12173. [Google Scholar] [CrossRef]

| Variable | Value |
|---|---|
| Forest type | Mediterranean-montane |
| Latitude | 42°42′31″ N |
| Length | 1°8′40″ W |
| Altitude (meters) | 625 |
| Slope (%) | 7 |
| Soil type | Haplic alisol |
| Mean Temperature (°C) | 12.0 |
| Mean Temperature media May–October (°C) | 16.8 |
| Annual Mean Precipitation (mm) | 912 |
| Annual Mean Precipitation May–October (mm) | 402 |
| Climate type [36] | Cold Mediterranean climate |
| Understory dominant species | Pteridium aquilinum (L.) Kuhn, Rubus ulmifolius Schott |
| Variable | Canopy Type | Source | |||||
|---|---|---|---|---|---|---|---|
| Thinning Intensity (Pine Basal Area Removed) | 0% | 20% | 40% | ||||
| Pine density (stems ha−1) | 1456 ± 156 | 1125 ± 83 | 1078 ± 1 47 | [31,38] | |||
| Beech density (stems ha−1) | 246 ± 26 | 190 ± 14 | 182 ± 25 | [31,38] | |||
| Other deciduous density (stems ha−1) | 399 + 43 | 308 ± 23 | 296 ± 40 | [31,38] | |||
| Pine basal area (m2 ha−1) | 40.9 ± 1.4 | 35.0 ± 2.4 | 30.4 ± 1.5 | [38] | |||
| Canopy Type | Pine | Mixed | Pine | Mixed | Pine | Mixed | |
| Leaf Area Index (m m−1) | 2.65 ± 0.12 | 2.86 ± 0.20 | 2.69 ± 0.10 | 2.68 ± 0.25 | 2.59 ± 0.16 | 2.62 ± 0.13 | [39] |
| Ground cover (%) | 0.67 ± 0.05 | 0.80 ± 0.04 | 0.60 ± 0.04 | 0.76 ± 0.06 | 0.55 ± 0.07 | 0.74 ± 0.06 | [39] |
| Canopy openness (%) | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 | 0.09 ± 0.01 | [39] |
| Annual throughfall (mm) | 898 ± 56 | 653 ± 43 | 904 ± 55 | 846 ± 50 | 918 ± 56 | 790 ± 42 | [40] |
| Taxonomic Group | N° of Individuals | Fraction (%) |
|---|---|---|
| All samples | 8348 | 100 |
| Mesofauna | 8077 | 96.75 |
| Cl. Arachnida | 6684 | 80.07 |
| Sb. cl. Acarina | ||
| Super O. Acariforme | 6684 | 80.07 |
| O. Oribatid | 1595 | 19.11 |
| Other mites | 1212 | 14.52 |
| Immature mites | 3877 | 46.44 |
| Cl. Entognatha | 1392 | 16.67 |
| Sb. cl. Collembola | ||
| O. Collembola | 1392 | 16.67 |
| Fil. Nematoda | 1 | 0.01 |
| Macrofauna | 271 | 3.25 |
| Cl. Insecta | 91 | 1.09 |
| Sb. cl. Pterygota | ||
| O. Diptera | 17 | 0.17 |
| O. Thysanoptera | 4 | 0.05 |
| O. Coleoptera | 44 | 0.53 |
| O. Hymenoptera | 25 | 0.3 |
| O. Hemiptera | 1 | 0.01 |
| Cl. Arachnida | 16 | 0.19 |
| O. Pseudoscorpionida | 1 | 0.01 |
| O. Araneae | 15 | 0.18 |
| Cl. Chilopoda | 16 | 0.19 |
| O. Geophilomorpha | 9 | 0.11 |
| O. Lithobiomorpha | 9 | 0.11 |
| Unidentified Chilopoda | 3 | 0.04 |
| Cl. Pauropoda | 6 | 0.07 |
| Cl. Clitellata | 3 | 0.04 |
| Sb. cl. Oligochaeta | 3 | 0.04 |
| Cl. Symphyla | 20 | 0.24 |
| Immature macrofauna stages (larvae) | 119 | 1.43 |
| Taxa | Thinning Intensity | Canopy Type | Decay Class | ||||
|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | Mixed | Pure Pine | Class 3 | Class 4 | |
| Oribatid mites | 0.846 ± 0.37 | 1.023 ± 0.43 | 0.601 ± 0.19 | 0.881 ± 0.26 | 0.765 ± 0.30 | 0.211 ± 0.05 | 1.435 ± 0.33 |
| Other mites | 0.797 ± 0.24 | 0.393 ± 0.17 | 0.676 ± 0.37 | 0.530 ± 0.15 | 0.714 ± 0.27 | 0.116 ± 0.04 | 1.128 ± 0.26 |
| Mites: Immature | 2.860 ± 1.10 | 1.896 ± 0.65 | 1.117 ± 0.51 | 1.683 ± 0.48 | 2.233 ± 0.85 | 0.651 ± 0.20 | 3.265 ± 0.84 |
| Macrofauna: larvae | 0.038 ± 0.01 | 0.064 ± 0.03 | 0.040 ± 0.01 | 0.038 ± 0.01 | 0.056 ± 0.02 | 0.044 ± 0.01 | 0.051 ± 0.01 |
| Collembola | 0.828 ± 0.30 | 0.686 ± 0.38 | 0.563 ± 0.26 | 0.759 ± 0.26 | 0.625 ± 0.25 | 0.154 ± 0.07 | 1.231 ± 0.31 |
| Diptera | 0.008 ± 0.00 | 0.006 ± 0.00 | 0.007 ± 0.00 | 0.010 ± 0.00 | 0.005 ± 0.00 | 0.003 ± 0.00 | 0.011 ± 0.00 |
| Geophilomorpha | 0.008 ± 0.01 | 0.005 ± 0.00 | 0.002 ± 0.00 | 0.008 ± 0.00 | 0.002 ± 0.00 | 0.000 ± 0.00 | 0.010 ± 0.00 |
| Hymenoptera | 0.018 ± 0.01 | 0.002 ± 0.00 | 0.009 ± 0.00 | 0.007 ± 0.00 | 0.013 ± 0.00 | 0.012 ± 0.01 | 0.008 ± 0.00 |
| Oligochaeta | 0.004 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.003 ± 0.00 | 0.000 ± 0.00 | 0.003 ± 0.00 |
| Symphyla | 0.011 ± 0.01 | 0.008 ± 0.00 | 0.007 ± 0.00 | 0.014 ± 0.00 | 0.004 ± 0.00 | 0.004 ± 0.00 | 0.014 ± 0.00 |
| Thysanoptera | 0.006 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.003 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.003 ± 0.00 |
| Araneae | 0.006 ± 0.00 | 0.002 ± 0.00 | 0.010 ± 0.01 | 0.011 ± 0.01 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.011 ± 0.01 |
| Pseudoscorpionidae | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 |
| Lithobiomorpha | 0.006 ± 0.01 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.005 ± 0.00 | 0.000 ± 0.00 | 0.005 ± 0.00 |
| Pauropoda | 0.012 ± 0.01 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.008 ± 0.01 | 0.000 ± 0.00 | 0.008 ± 0.01 |
| Undescribed Chilopoda | 0.004 ± 0.00 | 0.002 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.003 ± 0.00 | 0.000 ± 0.00 | 0.004 ± 0.00 |
| Coleoptera | 0.004 ± 0.00 | 0.040 ± 0.03 | 0.007 ± 0.00 | 0.011 ± 0.01 | 0.023 ± 0.02 | 0.013 ± 0.00 | 0.021 ± 0.02 |
| Nematoda | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 |
| Hemiptera | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.001 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 |
| Variable | Thinning Intensity | Canopy Type | CWD Decay Class | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | p | Mixed | Pure Pine | p | Class 3 | Class 4 | p | |
| Water content (%) | 56.10 ± 11.63 | 57.84 ± 20.20 | 45.54 ± 11.51 | 0.695 | 60.45 ± 8.75 | 60.20 ± 17.04 | 0.462 | 31.27 ± 8.33 | 89.47 ± 13.30 | 0.002 |
| Total abundance (individuals g−1) | 5.46 ± 6.52 | 4.14 ± 5.44 | 3.04 ± 3.93 | 0.671 | 3.97 ± 4.91 | 3.20 ± 4.61 | 0.155 | 1.32 ± 1.67 | 5.86 ± 5.63 | <0.001 |
| Richness (number of taxa) | 6.83 ± 2.37 | 6.33 ± 2.35 | 5.83 ± 1.99 | 0.571 | 7.08 ± 1.62 | 5.08 ± 2.19 | 0.031 | 5.42 ± 1.98 | 6.75 ± 2.78 | 0.008 |
| Shannon–Wiener Index | 1.20 ± 0.28 | 1.11 ± 0.29 | 1.17 ± 0.39 | 0.742 | 1.07 ± 0.35 | 1.18 ± 0.40 | 0.837 | 1.13 ± 0.34 | 1.14 ± 0.35 | 0.899 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Alvarez, X.; Blanco, J.A.; Imbert, J.B.; Alvarez, W.; Rivadeneira-Barba, G. Coarse Woody Debris’ Invertebrate Community is Affected Directly by Canopy Type and Indirectly by Thinning in Mixed Scots Pine—European Beech Forests. Forests 2020, 11, 975. https://doi.org/10.3390/f11090975
Herrera-Alvarez X, Blanco JA, Imbert JB, Alvarez W, Rivadeneira-Barba G. Coarse Woody Debris’ Invertebrate Community is Affected Directly by Canopy Type and Indirectly by Thinning in Mixed Scots Pine—European Beech Forests. Forests. 2020; 11(9):975. https://doi.org/10.3390/f11090975
Chicago/Turabian StyleHerrera-Alvarez, Ximena, Juan A. Blanco, J. Bosco Imbert, Willin Alvarez, and Gabriela Rivadeneira-Barba. 2020. "Coarse Woody Debris’ Invertebrate Community is Affected Directly by Canopy Type and Indirectly by Thinning in Mixed Scots Pine—European Beech Forests" Forests 11, no. 9: 975. https://doi.org/10.3390/f11090975
APA StyleHerrera-Alvarez, X., Blanco, J. A., Imbert, J. B., Alvarez, W., & Rivadeneira-Barba, G. (2020). Coarse Woody Debris’ Invertebrate Community is Affected Directly by Canopy Type and Indirectly by Thinning in Mixed Scots Pine—European Beech Forests. Forests, 11(9), 975. https://doi.org/10.3390/f11090975








