Abstract
The development of new bio-friendly alternatives for wood conservation is of great interest and necessary for environmental protection. In this paper, the preparations based on the propolis extract and silicon compounds were used as green wood preservatives. The wood was treated with 15% propolis extract (EEP) and two propolis-silane preparations, namely, EEP-VTMOS/TEOS (EEP with vinyltrimethoxysilane and tetraethyl orthosilicate) and EEP-MPTMOS/TEOS (EEP with 3-(trimethoxysilyl) propyl methacrylate and tetraethyl orthosilicate). The aim of the research was to determine the properties of treated wood, which was characterized by Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), atomic absorption spectroscopy (AAS), X-ray fluorescence (XRF), and scanning electron microscopy (SEM). Moreover, the resistance against brown-rot fungus Coniophora puteana and the mechanical properties of treated wood were also determined. The analysis of phenolic compounds concentration in treated wood indicated that phenols were in greater extent leached from wood treated with the propolis extract than from wood impregnated with the propolis-silane preparations. The presence of silicon in treated wood both before and after leaching was confirmed by CP MAS NMR measurements. In turn, AAS and XRF analyses indicated that the degree of Si leaching from wood impregnated with EEP-VTMOS/TEOS was approximately two times lower than from EEP-MPTMOS/TEOS treated wood. The results of chemical analyses confirmed that the constituents of the propolis-silane preparations formed permanent bonds with wood. In turn, the results of the antifungal efficacy against C. puteana showed that the propolis extract and the propolis-silane preparations limited the fungus activity, even the wood was subjected to leaching procedure. The treated wood showed an increase in bending strength and a decrease in the modulus of elasticity compared to untreated wood. The obtained results indicate that the propolis-silane preparations can be promising green wood preservatives, harmless for the natural environment.
Keywords:
propolis; silicon compounds; Coniophora puteana; mechanical properties; natural preservatives; NMR; XRF; FAAS 1. Introduction
Wood, as a natural renewable resource, is susceptible to decay and biodegradation by fungi and, to a lesser extent, by bacteria. Therefore, the durability and service life of wooden products is increased by using various methods of wood protection, including thermal modification or treatment with numerous chemicals, such as silicon compounds, titanium dioxide nanoparticles, or silver nanoparticles [1,2,3,4,5]. Nowadays, wood protection employs also natural compounds with low or no toxicity to humans and the environment, including chitosan, natural oils or propolis [6,7,8,9]. Essential oils and their components (e.g., citral, thymol, eugenol, cinnamaldehyde and carvacrol) were also applied in wood protection, increasing the wood durability against molds and decay fungi [10,11,12,13]. The important source of natural wood preservatives is plant and wood extractives, which contain a mixture of many components with various biological activity. The extracts of Cupressus sempervirens and Thuja occidentalis demonstrated activity against wood decay fungi—Ganoderma lucidum and Hexagonia apiaria [14]. In turn, wood treated with Pinus rigida heartwood extract showed good inhibition to the growth of molds, such as Alteria alternata, Chaetomium globosum and Trichoderma viride [15]. The bioactive components of wood extracts, such as phenolic compounds, play an important role in the natural durability of the wood. Latorraca et al. [16] reported lower durability of juvenile heartwood of Robinia pseudoacacia to Coniophora puteana and Trametes versicolor, due to lower total concentrations of phenolic compounds and flavonoids than in the mature heartwood. According to Sirmah et al. [17], the natural durability of Prosopis juliflora wood can be attributed to flavonoid—mesquital. In turn, latifolin and 4-methoxydalbergione isolated from Dalbergia latifolia heartwood showed activity against T. versicolor [18].
Propolis is a resinous material collected by Apis mellifera from the buds of various trees and plant exudates [19,20]. The chemical composition of propolis is very complex, mainly due to the variability of plant sources growing around hives. More than 300 chemical constituents have been identified in propolis from different geographical regions [20]. The main class of chemical components present in propolis are phenolic compounds, including flavonoids, phenolic acids and their esters [21,22,23,24]. According to literature, the phenol fraction of propolis is indicated as the main fraction responsible for its biological activity [19,25]. Moreover, terpenes, minerals or amino acids have also been identified in propolis samples [20,26,27]. The extracts of propolis showed biological properties, including antioxidant, anticancer, antiviral, and antibacterial activity [20,21,23,24,28,29]. Propolis also exhibited antifungal activity against molds and yeasts [21,23,30,31]. In addition, the extract of Argentine propolis showed activity against xylophagous fungi isolated from decayed wood: Ganoderma applanatum, Lenzites elegans and Schizophyllum commune [32]. Due to biological properties, mainly antifungal activity, propolis has been applied in wood protection. The wood treated with the extract of Turkish propolis showed resistance against T. versicolor and Neolentinus lepideus, and treated with the extract of Polish propolis against C. puteana [9,33]. The wood treated with the extract of Spanish propolis limited decay of wood caused by T. versicolor [34]. The propolis extract was also a component of preparations applied to protect wood. The wood impregnated with the preparation consisted of chitosan, and the propolis extract showed higher resistance against T. versicolor compared to untreated wood samples [35]. In turn, the preparation based on propolis extract, caffeine, and silicon compounds inhibited the growth of C. puteana on treated wood samples [36].
The silicon compounds are used in various applications, such as buildings, paper, textiles, as well as wood impregnation [37]. The wood treated with silicon compounds is characterized by the improvement of hydrophobic and antifungal properties [3,38,39,40]. However, the silanes with amino groups showed higher antifungal effect than the silicon compounds without –NH2 groups [38]. Therefore, silicon compounds have been often used as a component of wood preservatives, whose purpose is to support the action of active substances or to prevent their leaching from the wood structure [36,41,42].
The aim of the research was to determine the properties of wood treated with bio-friendly preservatives based on the propolis extract and silicon compounds. The paper presents the results of chemical analysis of treated wood, including Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), atomic absorption spectroscopy (AAS), X-ray fluorescence (XRF) and scanning electron microscopy (SEM). The resistance of the treated wood against brown-rot fungus C. puteana was also assessed. Moreover, the mechanical properties, namely, bending strength and modulus of elasticity of treated wood, were determined.
2. Materials and Methods
2.1. Propolis-Silane Preparations
In this study, the 15% ethanolic extract of Polish propolis prepared in 70% ethanol and two propolis-silane preparations were used for wood impregnation. The first preparation (EEP-VTMOS/TEOS) consisted of 15% ethanolic extract of propolis (EEP) and silanes: vinyltrimethoxysilane (VTMOS) and tetraethyl orthosilicate (TEOS) at 5% concentration. EEP (15%), 3-(trimethoxysilyl) propyl methacrylate (MPTMOS) and tetraethyl orthosilicate (TEOS) at 5% concentration were the components of the second preparation (EEP-MPTMOS/TEOS). The silicon compounds (Figure 1) were purchased from Sigma Aldrich (Darmstadt, Germany) and the propolis extract from PROP-MAD (Poznań, Poland).
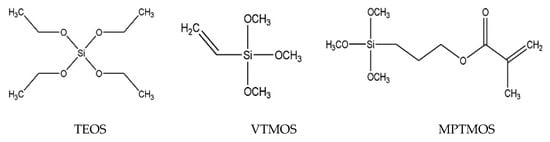
Figure 1.
The chemical structures of silicon compounds. TEOS, tetraethyl orthosilicate; VTMOS, vinyltrimethoxysilane; MPTMOS, 3-(trimethoxysilyl)propyl methacrylate.
2.2. Wood Treatment
The investigated material was Scots pine (Pinus sylvestris L.) sapwood without knots or other growth inhomogeneity. The wood samples with dimensions of 5(T) × 10(R) × 40(L) mm3 were used for biological and chemical analyses. The wood samples with dimensions of 5(T) × 10(R) × 150(L) mm3 were applied in the determination of mechanical parameters. Before impregnation process, the specimens were conditioned at 65 ± 5% relative humidity (RH) and 20 ± 2°C to attain equilibrium moisture content of approximately 12%. Next, all the wood samples were impregnated with EEP, EEP-VTMOS/TEOS and EEP-MPTMOS/TEOS using the vacuum method, according to EN 113: 1996 [43]. The samples underwent 15 min under vacuum conditions—0.8 kPa and 2 h under atmospheric pressure. After impregnation, all the samples were removed from the impregnating solutions and weighed to determine the uptake of the solutions. The wood sample retention (kg/m3) was calculated as the following equation:
where Ma—the wood mass before treatment (g); Mb—the wood mass after treatment (g); c—concentration of propolis extract or propolis-silane preparations constituents (%); v—the volume of the wood sample (cm3).
After impregnation, all the wood samples were cured for four weeks in room conditions (RH = 65 ± 5%; T = 20 ± 2 °C).
2.3. Accelerated Aging of Wood—Leaching Procedure
The aim of the artificial ageing (leaching in water) was to determine anti-leaching stability of the treatment preparations constituents from the treated wood. The leaching procedure of the treated wood samples for the outdoor application was performed according to EN 84:2000 [44]. The wood samples (used for biological test and chemical analyses) were impregnated with deionized water by vacuum method (20 min) and next soaked in deionized water for 14 days. The water was exchanged 10 times for all duration of leaching procedure.
2.4. Decay Resistance Test
The decay resistance of the control and treated wood samples with the propolis extract and the propolis-silane preparations took place before and after the leaching procedure wood samples against brown-rot fungus—Coniophora puteana (Schumacher ex Fries) Karsten BAM 112 (BAM Ebw. 15). This was carried out in accordance with the modified EN-113:1996 [43]. Scots pine (Pinus sylvestris L.) sapwood with dimensions of 5(T) × 10(R) × 40(L) mm3 was used in this study. The control and treated wood samples (five replicates of each impregnation variant, before and after leaching) were placed into Petri dishes and exposed to decay fungi at 22 ± 2 °C and relative humidity of 70 ± 5% for 8 weeks. One control sample and treated sample were placed in each Petri dish. After exposure to C. puteana, the fungus mycelium was removed from each wood sample, and the samples were weighed in order to determine the mass loss of each wood sample caused by fungi.
2.5. Mechanical Properties
Bending strength and modulus of elasticity were measured according to PN-77/D-04103 [45] and PN-63/D-0411 [46], respectively, using ZWICK ZO50TH wood testing machine (Zwick/Roell, Ulm, Germany). Determination of mechanical parameters was carried out on wood samples with dimensions of 5(T) × 10(R) × 150(L) mm3. This size of the samples was chosen to ensure that even penetration of treatment preparations had occurred throughout the wood sample. The distance between supports during the experiment was 120 mm. The load was applied in the midway of the sample, in the tangential direction. Before measurements, all wood samples were conditioned at 20 ± 2 °C and relative humidity of 52 ± 2% to constant weight. In accordance with ISO 13061-2 [47], the density of the conditioned samples was determined by a stereometric method, using an analytical balance accurate to 0.001 g (Sartorius GmbH, Göttingen, Germany) to measure the mass of samples and a digital caliper with an accuracy of up to 0.01 mm to determine their dimensions. The density was calculated as the ratio of the mass to the volume. Wood moisture content (MC) was determined by a gravimetric method, according to ISO 13061-1 [48]. All determination was carried out in ten replicates.
2.6. Chemical Characterization of Wood
For chemical analyses, the control and treated wood samples with dimensions of 5(T) × 10(R) × 40(L) mm3 were used. In chemical analyses (except for SEM measurements), the treated wood after the leaching procedure (according to EN 84:2000) was also used to determine the durability of the bond between treatment preparations constituents and wood. Six samples from each treatment variant (before and after leaching procedure) were used in XRF analysis. Then, the six samples of each treatment variant were ground on a laboratory mill (Ika Werke, Staufen, Germany) and in the form of sawdust were used in the FTIR, AAS, NMR, and phenolic content analyses.
2.6.1. Fourier Transform Infrared Spectroscopy (FTIR)
The ground untreated and treated wood samples were mixed with KBr (Sigma Aldrich, Darmstadt, Germany) at a 1/200 mg ratio, and in the form of a pellet were analyzed using the Nicolete iS5 spectrophotometer with Fourier transform (Thermo Fisher Scientific, Waltham, MA, USA). The spectra of wood samples were registered, at a range of 500–4000 cm−1, at a resolution of 4 cm−1, registering 32 scans.
2.6.2. Phenolic Concentration Analysis
The ground treated wood samples (0.2 g) before and after leaching in water were extracted with 10 mL methanol (Avantor Performance Materials, Gliwice, Poland) for 24 h at a room temperature using a PSU-10 orbital shaker (SIA Biosan, Riga, Latvia). After extraction, the samples were filtered, and supernatants were used to determine the total phenolic content using Folin-Ciocalteu reagent, according to the method described by Singleton et al. [49]. The supernatants (0.1 mL) were placed in a test tube, followed by the addition of 0.25 mL Folin-Ciocalteu reagent (Sigma Aldrich, Darmstadt, Germany) and 2.65 mL deionized water. After 5 min, 3 mL of a 10% sodium carbonate solution (Avantor Performance Materials, Gliwice, Poland) were added to each tube. The solutions were shaken and incubated for 40 min at room temperature. After this time, the absorbance was measured at λ = 760 nm using a UV-VIS spectrophotometer Carry-300BI (Agilent Technologies, Santa Clara, CA, USA). The results were expressed as mg of gallic acid equivalents/mL of solutions. All determinations were carried out in triplicate.
2.6.3. 29Si Nuclear Magnetic Resonance (NMR)
The solid-state cross-polarization magic angle spinning (CP MAS) NMR experiments were performed on a BRUKER Avance III 400 spectrometer (Billerica, MA, USA) operating at 400.13 for 1H and 79.495 MHz for 29Si and equipped with a MAS probe head using 4-mm ZrO2 rotors. A sample of Q8M8 was used for setting the Hartmann–Hahn condition and as a chemical shift reference (δ = 0.00 ppm). The spectra of treated wood samples were recorded with a MAS frequency of 8000 Hz and proton 90° pulse of 6.0 μs in length and a contact time of 5 ms. The repetition delay was 4 s, and the spectral width was 48.0 kHz. The FIDs (free induction decay) were accumulated with a time domain size of 2 K data points with SPINAL16 decoupling sequence during the acquisition time.
2.6.4. X-Ray Fluorescence (XRF)
The samples of treated wood with dimensions of 5(T) × 10(R) × 40(L) mm3 were used in this study. The surfaces of treated wood were analyzed using X-ray fluorescence spectrometer Bruker Tracer III-SD (Billerica, MA, USA). Each sample was scanned in five points using a collimator with a 5 × 5 mm2 screen. The time of one measurement was 30 s. Quantitative values of silicon on the treated wood surface were determined using the MajMudRock calibration method.
2.6.5. Flame Atomic Absorption Spectrometry (FAAS)
The ground treated wood samples (0.5 g) were mineralized with 8 mL of nitric acid (Sigma Aldrich, Darmstadt, Germany) in the mineralization system (CEM Corporations, Matthews, NC, USA). The digested solutions were filtered and diluted to 50 mL with deionized water. The concentration of silicon in the samples was determined by a flame atomic absorption spectrometry (FAAS) using an AA280FS spectrometer (Agilent Technologies, Santa Clara, CA, USA). The correctness of the method was verified by analysis of the certified reference material NCS DC 73350—leaves of poplar (NACIS, Shanghai, China). The results were expressed as the average values in triplicate measurements.
2.6.6. Scanning Electron Microscopy (SEM)
The surface morphologies of treated wood samples were examined by a Zeiss EVO 40 scanning electron microscope (Carl Zeiss AG, Oberkochen, Germany), which used an electron acceleration voltage of 10 keV. Before microscope analysis, small wood samples (10 mm square) were trimmed from treated wood and next coated with a layer of gold using a Balzers SCD00 sputter coater (BalTec Maschinenbau AG, Pfäffikon, Switzerland).
2.7. Statistical Analysis
The results were analyzed using a one-way analysis of variance (ANOVA) applying Tukey’s Honest Significant Differences (THSD) Test. Statistical significance was defined as p < 0.05. All the statistical analyses were performed using the TIBCO Software Inc. Statistica version 13.1 (Palo Alto, CA, USA).
3. Results and Discussion
3.1. Chemical Characterization of Treated Wood
In the first stage of the research, the chemical interaction between the components of the propolis-silane preparations and wood was determined using instrumental methods. The wood was treated with 15% extract of Polish propolis and two propolis-silane preparations, namely, EEP-VTMOS/TEOS and EEP-MPTMOS/TEOS. The concentration of the propolis extract was chosen based on our previous studies, which indicated that Scots pine wood impregnated with ethanolic propolis extract above 12% concentration limited fungal decay [33].
3.1.1. FTIR Characterization
Figure 2 presents the FTIR spectrum of the propolis extract, and Table 1 shows the most important bands of this spectrum, characterized according to literature data [50,51,52,53].
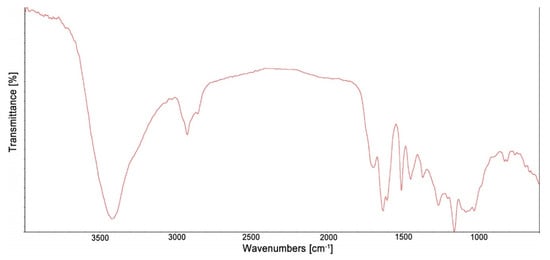
Figure 2.
The FTIR spectrum of the propolis extract.

Table 1.
The characteristic absorption bands of the propolis extract.
In the spectrum of the propolis extract, the bands assigned to the vibrations of C=O, C=C, N–H and C–H bonds derived from phenolic compounds present in propolis were observed. The bands in the spectrum were observed at: 2926 and 2849 cm−1, which are connected with C–H stretching vibrations and confirm the presence of long-chain alkyl compounds in the propolis extract. The observed stretching and bending bands at 1636, 1514 and 1450 cm−1 correspond mainly to aromatic rings of phenolic compounds specific for propolis extracts. In addition, the wide band with a maximum at 3420 cm−1 described to the stretching vibrations of O–H band also confirms the presence of phenolic compounds in the extract.
In Figure 3, the spectra of wood treated with the propolis extract before and after leaching with water are shown.
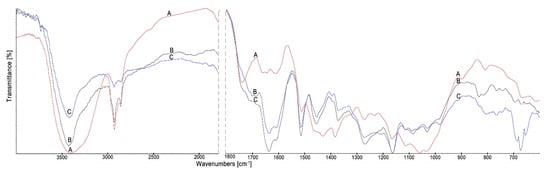
Figure 3.
The FTIR spectra of Scots pine wood (A), wood treated with the propolis extract (B) and wood treated with the propolis extract after leaching (C).
The wide band of O–H stretching vibration at 3445 cm−1 observed in the spectrum of untreated wood narrowed in the spectra of treated wood, which may indicate that the hydroxyl group of the wood formed hydrogen bonds with propolis constituents. In the spectra of treated wood, there is the observed loss of band at 1735 cm−1, assigned to C=O stretching vibration of carboxyl and acetyl groups in wood, which is visible in the spectrum of untreated wood. In the spectra of propolis treated wood appeared the new band at 1637 cm−1 associated with C=O, C=C and N–H vibrations from propolis constituents, namely, flavonoids and amino acids. The band at 1456 cm−1 for skeletal C=C aromatic rings of flavonoids was observed in the spectra of treated wood. The bands at 1085 and 1035 cm−1 responsible for C-C, C-OH and C-O-C vibrations originating from flavonoids and alcohols in propolis were observed in the spectra of treated wood both before and after leaching. The changes in the FTIR spectra of untreated and propolis treated wood indicated that constituents of the propolis extract formed chemical bonds with the wood components. However, the changes in the intensity of the bands mainly at 2850, 2920 and in the range 850–600 cm−1 in the spectra of treated wood before and after leaching suggest that water may leach part of the propolis components from the treated wood structure.
The spectra of wood treated with the propolis-silane preparations, namely, EEP-VTMOS/TEOS and EEP-MPTMOS/TEOS, are presented in Figure 4 and Figure 5, respectively.
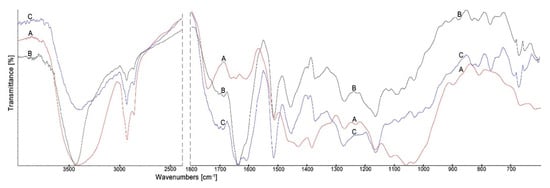
Figure 4.
The FTIR spectra of Scots pine wood (A), wood treated with EEP-VTMOS/TEOS (B) and wood treated with EEP-VTMOS/TEOS after leaching (C).
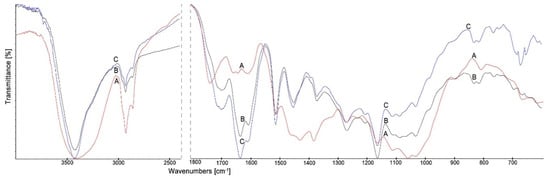
Figure 5.
The FTIR spectra of Scots pine wood (A), wood treated with EEP-MPTMOS/TEOS (B) and wood treated with EEP-MPTMOS/TEOS after leaching (C).
The most important changes in the spectra of wood treated with the propolis-silane preparations compared to the spectrum of untreated wood were as follow: narrowing of the wide band in the range 3200–3500 cm−1 for O–H stretching vibrations, losing of the band at 1735 cm−1, assigned to C=O stretching vibration of carboxyl and acetyl groups in wood, appearing the new band at 1640 cm−1 associated with C=O, C=C and N–H vibrations derived from propolis constituents, namely, flavonoids and amino acids and the new band at 1456 cm−1 for skeletal C=C aromatic rings of flavonoids. In addition, in the spectra of wood treated with EPP-VTMOS/TEOS, the bands at 1270 and 1160 cm−1 assigned to vibrations of SiOCH3 and SiCH3 bonds and the bands in the range of 1095–1085 cm−1 indicating vibrations of SiOCH3 group were observed. The bands at 833, 770 and 685 cm−1 attributed to the vibrations of Si-C and Si-O bands were also visible in the spectra of wood treated with EEP-VTMOS/TEOS both before and after leaching [52,54,55,56]. In turn, in the spectra of wood treated with EEP-MPTMOS/TEOS, new bands at: 833, 765, and 696 cm−1 attributed to vibrations of Si-C and Si-O bands were observed [52,54,55].
The changes observed in the spectra of wood treated with the propolis-silane preparations both before and after leaching with water indicated that the constituents of the preparations formed permanent bonds with the wood components.
3.1.2. Leaching of Treatment Preparations Constituents from Treated Wood
Table 2 presents the results of the leaching of phenolic compounds, which are the main bioactive components of propolis, from wood treated with the propolis extract and the propolis-silane preparations.

Table 2.
The content of total phenolic compounds (PC) in treated wood and the degree of PC leaching.
In order to determine the degree of PC leaching from treated wood, the total content of phenolic compounds in the treated wood before and after leaching procedure with water was analyzed. The degree of PC leaching from the treated wood was in the range of 3.7–18.1%, which indicate that phenolic compounds were scarcely leached from the wood structure. Akcay et al. [9] stated that propolis was not leached from wood treated with the extract of Turkish propolis. The authors did not detect phenolic compounds in the leachate after 16 h of leaching wood impregnated with the propolis extract [9]. In turn, the obtained results indicated that phenolic compounds were in greater extent leached from wood treated with the propolis extract than from wood treated with the propolis-silane preparations. The lowest degree of phenolic compounds leaching was observed for wood treated with EEP-VTMOS/TEOS, which was only 3.7%. The results show that used silicon compounds with hydrophobic properties as constituents of treatment preparations limited the leaching of phenolic compounds from impregnated wood. The silicon compounds have previously used in wood protection to reduce leaching of the bioactive component from the wood structure. Literature data indicated that silicon compounds were able to limit the leaching of boron and caffeine from treated wood [36,42].
In the next stage of the research process, NMR spectra of the treated wood were performed in order to determine the presence of silicon in the wood structure. The 29Si CP MAS NMR spectra of wood impregnated with the propolis-silane preparations before and after leaching procedure are presented in Figure 6.
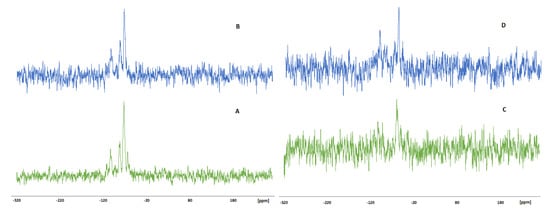
Figure 6.
The 29Si CP MAS NMR spectra of wood treated with EEP-VTMOS/TEOS (A), wood treated with EEP-VTMOS/TEOS after leaching (B), wood treated with EEP-MPTMOS/TEOS (C) and wood treated with EEP-MPTMOS/TEOS after leaching (D).
In the spectra of EEP-VTMOS/TEOS treated wood (Figure 6A), three well-defined signals were observed at −73 ppm, −81 ppm and −102 ppm, which were assigned to T2 structure, T3 structure and Q3 free silanols structure, respectively [56,57,58,59,60]. In the spectrum of treated wood after leaching (Figure 6B), three signals were also observed with the same shifts as in the spectrum of treated wood before leaching. However, the intensity of these signals was slightly lower compared to the intensity of the signals in the spectrum of unleached wood, suggesting that the Si concentration in the leached wood was lower than in wood before leaching. In the spectrum of wood treated with EEP-MPTMOS/TEOS (Figure 6C), two low-intensity signals were found at –60 ppm and –100 ppm, which were assigned to T2 and Q3 structures [57,58,59,60]. In the spectrum of treated wood after leaching procedure (Figure 6D), these signals were shifted and observed at −59 ppm and −103 ppm. The low signal-to-noise ration observed in the spectra of wood treated with EEP-MPTMOS/TEOS indicates a relatively small number of silicon atoms in the wood structure.
In order to determine the degree of silicon compounds leaching from treated wood, the silicon concentration determination in the samples was performed using atomic absorption spectroscopy (AAS) and X-ray fluorescence spectroscopy (XRF). The Si concentration results in the wood samples presented in Table 3 indicated that the wood treated with EEP-VTMOS/TEOS contained higher silicon concentration than wood treated with EEP-MPTMOS/TEOS which is in agreement with the results of NMR measurements. The degree of Si leaching from wood impregnated with EEP-VTMOS/TEOS was more than two times lower than the degree of Si leaching from wood treated with EEP-MPTMOS/TEOS. The degree of Si leaching from wood treated with both preparations was lower than reported in the literature. The degree of Si leaching from pine wood treated with [3-(2-aminoethylamino)propyl]-trimethoxysilane was 53%, and the degree of Si leaching from wood treated with the propolis-caffeine-silane preparation was 24% [36,59]. In turn, the degree of Si leaching from wood treated with preparation consisted of the propolis extract and silanes (methyltrimethoxysilane and 3-(trimethoxysilyl)propyl methacrylate) was 10%, which indicate that the VTMOS-TEOS and MPTMOS-TEOS silanes used in this study formed permanent bonds with wood [61].

Table 3.
The silicon concentration in treated wood and the degree of Si leaching determined by atomic absorption spectroscopy (AAS).
Due to the fact that the analysis of Si concentration in wood by atomic absorption spectroscopy is a destructive method, in research the Si concentration on the wood surface was also determined by X-ray fluorescence diffraction, which is a non-destructive method. The XRF method was previously used in the analysis of different element concentration in the wood [62,63,64]. The silicon concentration in treated wood and the degree of Si leaching determined by XRF are shown in Table 4.

Table 4.
The silicon concentration in treated wood and the degree of Si leaching determined by X-ray fluorescence spectroscopy (XRF).
Comparing the results of Si concentration in impregnated wood obtained by atomic absorption spectroscopy (AAS) and X-ray fluorescence spectroscopy (XRF), it is noticeable that the results obtained by the XRF method are two orders of magnitude smaller than the AAS results, which is associated with the measurement method. The Si concentration in wood samples determined by AAS is associated with the analysis of the element concentration in the entire sample volume. In turn, the determination of Si concentration in wood samples using XRF took place only on the wood surface. Moreover, the Si concentration results presented in Table 4 indicated that surface of wood treated with EEP-MPTMOS/TEOS characterized by higher concentration of this element compared to wood treated with EEP-VTMOS/TEOS. On the other hand, the results of the AAS analysis (Table 3) indicated that wood treated with EEP-VTMOS/TEOS contained higher Si concentration than wood impregnated with EEP-MPTMOS/TEOS, which may be associated with longer chain lengths of MPTMOS, which is a component of EEP-MPTMOS/TEOS preparation. The MPTMOS could have been deposited on the wood surface because the silicon compounds with long chains penetrated the cell wall worse than silanes with shorter chains [39]. It should be noted, however, that the degree of Si leaching obtained based on the XRF results is comparable to the degree of Si leaching obtained from the AAS results, which indicate that the non-destructive XRF method can be used to determine various elements that are components of wood preservatives in treated wood.
3.2. The Resistance of Treated Wood Against C. puteana
The results of the antifungal efficacy against C. puteana, expressed as average mass loss of wood samples treated with the propolis extract and the propolis-silane preparations, are presented in Table 5.

Table 5.
Retention and mass loss of treated wood after exposure to C. puteana.
The results of the biological test show that the action of the fungus caused the mass loss of treated wood in the range of 2.9% to 4.8%, and untreated control wood samples in the range of 44.7% to 49.2%. The protective activity of the propolis extract and the propolis-silane preparations can be seen compared to the mass loss of treated and untreated wood samples. The mass loss values of each treated wood were statistically similar, except for the value of propolis treated wood after leaching, which was statistically higher than the others. In the case of wood treated with the propolis extract, an increase in the mass loss of wood after the leaching procedure was noticeable, which was associated with partial leaching of phenolic compounds from the wood structure (Table 2). In turn, the mass losses of wood treated with the propolis-silane preparations before and after leaching procedure were statistically similar, which indicates that the components of impregnating preparations not leached from the wood structure and effectively protected it against the degradation action of C. puteana. The wood treated with EEP-MPTMOS/TEOS exhibited the lowest value of mass loss both before and after leaching with water.
The literature data indicated that the extract of Turkish propolis at a 7% concentration protected wood against T. versicolor and N. lepideus, while the extract of Polish propolis at a concentration above 12% protected wood against fungal decay caused by C. puteana [9,33]. The mass loss of pine wood treated with the extract of Polish propolis at a concentration of 12% and 18.9% after exposure to C. puteana was 3.3% and 2.3%, respectively [33]. In turn, the wood treated with soda-based propolis solution after leaching procedure did not show resistance against C. puteana [65]. The mass losses of wood treated with the propolis-silane preparations both before and after leaching procedure were similar, indicating that the constituents of these preparations did not leach from the wood structure, and the wood after leaching with water still showed resistance against the destructive action of fungus. The previous work of the authors indicated that wood treated with the propolis extract with silanes (methyltrimethoxysilane and 3-(trimethoxysilyl)propyl methacrylate) exhibited resistance against C. puteana both before and after leaching procedure [61]. The preparation consisted of the propolis extract, caffeine, and silanes (methyltrimethoxysilane and octyltriethoxysilane) inhibited the growth of C. puteana on wood samples—even the wood was subjected to leaching procedure [36].
3.3. Bending Strength and Modulus of Elasticity of Treated Wood
In accordance with the fact that the impregnation of wood with chemical preservatives may have an effect on its mechanical properties in Table 6, there are presented the results of bending strength and modulus of elasticity determined for wood treated with the propolis extract and the propolis-silane preparations.

Table 6.
Moisture content, density, bending strength and modulus of elasticity of treated wood.
The wood treated with the propolis extract and the propolis-silane preparations was characterized by a 3–4% increase in the equilibrium moisture content compared to untreated samples, which was associated with higher hydrophobic properties of treated wood, which was previously described by the authors [66]. The modulus of elasticity determined for treated wood was statistically lower compared to the modulus elasticity of untreated control samples. The modulus of elasticity determined for wood protected with the propolis extract and the propolis-silane preparations was about 7% lower than for unprotected wood. In turn, the bending strength values of wood impregnated with the propolis extract and the propolis-silane preparations were statistically higher than the value for untreated wood samples. The value of bending strength determined for EEP treated wood was about 4% and wood impregnated with EEP-MPTMOS/TEOS and treated with EEP-VTMOS/TEOS was about 6% higher than for untreated, control samples. The increase in the bending strength of treated wood compared to untreated samples may be associated with the deposition of impregnating agents, and thus, with an increase in the density of treated wood. Moreover, according to the SEM imagines presented in Figure 7, the silicon compounds filled the cell lumen. In turn, the SEM imagine of wood treated with the propolis extract showed that the wood cells were separated from each other, which can be associated with the action of aqueous ethanol used as a propolis solvent. Literature data indicated that ethanol-water mixtures have an influence on the wood structure causing dissolving a main part of lignin in compound middle lamellae (CML) and releasing individual cells at sectioning [67,68]. However, the ethanolic solution does not affect on the cell wall S2 layer, which is one of the main factors determining the strength properties of wood [69].
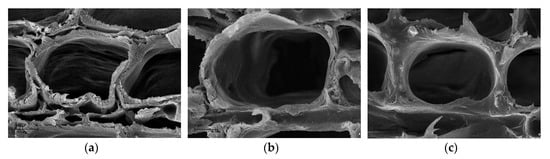
Figure 7.
The SEM imagines of wood treated with EEP (a), wood treated with EEP-VTMOS/TEOS (b), and wood treated with EEP-MPTMOS/TEOS (c).
The impregnation of wood with chemical preservatives caused changes in the mechanical properties of wood [70,71,72]. The pine wood impregnated with ionic liquids showed a lower value of bending strength and modulus of elasticity compared to untreated wood [70]. The wood treated with chitosan solution exhibited an increase of the modulus of elasticity and no significant changes in the modulus of rupture compared to untreated wood [73]. The pine wood modified with methyl-etherified melamine formaldehyde resin, a low molecular weight phenol-formaldehyde resin, a higher molecular weight phenol-formaldehyde resin and dimethylol dihydroxyethyleneurea showed significantly lower bending strength values than control specimens [71]. In turn, the treatment with tetraethoxysilane showed no effect on the mechanical properties of pine wood compared to untreated samples [72]. The full explanation of the influence of impregnation with the propolis extract and the propolis-silane preparations on the mechanical properties of treated wood requires further investigations.
4. Conclusions
The paper presents the results of chemical, biological and mechanical characterization of wood treated with the propolis extract and the propolis-silane preparations. The results of FTIR analysis and determination of phenolic compounds concentration in wood treated with the propolis extract indicated that part of propolis constituents was leached by water from the wood structure. In turn, the changes in the FTIR spectra of wood impregnated with the propolis-silane preparations showed that the constituents of the preparations formed permanent bonds with the wood components. The analysis of phenolic compounds concentration in treated wood indicated that phenols were in greater extent leached from propolis treated wood than from wood impregnated with the propolis-silane preparations. The lowest degree of phenols leaching was observed for wood treated with EEP-VTMOS/TEOS, which was only 3.7%. The results show that used silicon compounds with hydrophobic properties as a component of treatment preparations limited the leaching of phenolic compounds from impregnated wood. The presence of silicon in wood treated with the propolis-silane preparations both before and after leaching was confirmed by 29Si CP MAS NMR measurements. In turn, AAS and XRF analyses indicated that the degree of Si leaching from wood impregnated with EEP-VTMOS/TEOS was approximately two times lower than from wood treated with EEP-MPTMOS/TEOS. The results of chemical analyses confirmed that the constituents of the propolis-silane preparations formed permanent bonds with wood. The results of the antifungal efficacy against C. puteana show that the propolis extract and the propolis-silane preparations limited the fungus activity, even the wood was subjected to leaching procedure. The most effective protection against tested fungus was observed for wood treated with EEP-MPTMOS/TEOS. The mass loss of EEP-MPTMOS/TEOS treated wood before and after leaching was 2.9% and 3.2%, respectively. However, the mass loss values of each treated wood were statistically similar, except for the value of propolis treated wood after leaching, which was statistically higher than the others. The protective activity of the propolis extract was lower when the wood was subjected to leaching, which was associated with partial leaching of phenolic compounds from the wood structure. The treated wood showed an increase in bending strength and a decrease in the modulus of elasticity compared to untreated wood.
In summary, based on the obtained results, it can be concluded that the propolis-silane preparations can be promising green wood preservatives, harmless for the natural environment. The bio-friendly preservatives can be used for the treatment of wood both in indoor and outdoor applications. However, the further investigation on the effect of the propolis-silane preparations and their single components (propolis extract, silicon compounds and the solvent) on the mechanical properties of treated wood should be performed in the future.
Author Contributions
Conceptualization. M.W. and I.R.; investigation. M.W., P.K.-S., and M.K.; writing—original draft preparation. M.W.; writing—review and editing. P.K.-S., M.K., E.R. and I.R.; supervision. I.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research was partially funded by National Science Centre, grant number 2019/03/X/NZ9/01800, and by a statutory activity from the Polish Ministry of Science and Higher Education (506.472.02.00). The article was co-financed within the Ministry of Science and Higher Education programme—“Regional Initiative Excellence” 2019–2022, project No. 005/RID/2018/19.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, A.; Pavla, R.; Sever, S.A.; Humar, M.; Pavlič, M.; Jan, T.; Petr, H.; Zigon, J.; Petric, M. Influence of surface modification of wood with octadecyltrichlorosilane on its dimensional stability and resistance against Coniophora puteana and molds. Cellulose 2016, 23, 3249–3263. [Google Scholar] [CrossRef]
- Can, A.; Palanti, S.; Sivrikaya, H.; Hazer, B.; Stefanı, F. Physical, biological and chemical characterisation of wood treated with silver nanoparticles. Cellulose 2019, 26, 5075–5084. [Google Scholar] [CrossRef]
- Panov, D.; Terziev, N. Study on some alkoxysilanes used for hydrophobation and protection of wood against decay. Int. Biodeterior. Biodegrad. 2009, 63, 456–461. [Google Scholar] [CrossRef]
- De Filpo, G.; Palermo, A.M.; Rachiele, F.; Nicoletta, F.P. Preventing fungal growth in wood by titanium dioxide nanoparticles. Int. Biodeterior. Biodegrad. 2013, 85, 217–222. [Google Scholar] [CrossRef]
- Ozgenc, O.; Durmaz, S.; Hakki Boyaci, I.; Eksi-Kocak, H. ATR-FTIR spectroscopic analysis of thermally modified wood degraded by rot fungi. Wood 2018, 61. [Google Scholar] [CrossRef]
- Eikenes, M.; Alfredsen, G.; Christensen, B.E.; Militz, H.; Solheim, H. Comparison of chitosans with different molecular weights as possible wood preservatives. J. Wood Sci. 2005, 51, 387–394. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2012, 46, 851–870. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Efficacy of linseed- and tung-oil-treated wood against wood-decay fungi and water uptake. Int. Biodeterior. Biodegrad. 2013, 85. [Google Scholar] [CrossRef]
- Akcay, C.; Birinci, E.; Birinci, C.; Kolayli, S. Durability of wood treated with propolis. BioResources 2020, 15, 1547–1562. [Google Scholar]
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten essential oils for beech wood protection—Efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas Cienc. Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Mi, N.; Wang, Y.; Li, G.; Wang, L.; Xie, Y. Antifungal activity of monoterpenes against wood white-rot fungi. Int. Biodeterior. Biodegrad. 2016, 106, 157–160. [Google Scholar] [CrossRef]
- Cheng, S.S.; Liu, J.Y.; Chang, E.H.; Chang, S.T. Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi. Bioresour. Technol. 2008, 99, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, A.S.O.; Badawy, M.E.I.; Abdelgaleil, S.A.M. Antifungal activity of essential oils isolated from Egyptian plants against wood decay fungi. J. Wood Sci. 2013, 59, 499–505. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zidan, Y.E.; El Hadidi, N.M.N.; Mansour, M.M.A.; Abo Elgat, W.A.A. Evaluation of usage three natural extracts applied to three commercial wood species against five common molds. Int. Biodeterior. Biodegrad. 2016, 110, 206–226. [Google Scholar] [CrossRef]
- Latorraca, J.V.D.F.; Dünisch, O.; Koch, G. Chemical composition and natural durability of juvenile and mature heartwood of Robinia pseudoacacia L. An. Acad. Bras. Cienc. 2011, 83, 1059–1068. [Google Scholar] [CrossRef]
- Sirmah, P.; Dumarçay, S.; Masson, E.; Gérardin, P. Unusual amount of (-)-mesquitol from the heartwood of Prosopis juliflora. Nat. Prod. Res. 2009, 23, 183–189. [Google Scholar] [CrossRef]
- Sekine, N.; Ashitani, T.; Murayama, T.; Shibutani, S.; Hattori, S.; Takahashi, K. Bioactivity of latifolin and its derivatives against termites and fungi. J. Agric. Food Chem. 2009, 57, 5707–5712. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, 1–6. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evidence-based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- dos Santos, H.F.; Campos, J.F.; dos Santos, C.M.; Balestieri, J.B.P.; Silva, D.B.; Carollo, C.A.; Souza, K.D.P.; Estevinho, L.M.; dos Santos, E.L. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. Braz. J. Pharmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G.; Pan, S.Y. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011, 127, 210–215. [Google Scholar] [CrossRef]
- Inmaculada González-Martín, M.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Carmen Coello, M.; Riocerezo, C.P.; Moncada, G.W. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors (Switzerland) 2015, 15, 27854–27868. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pu, R.; Li, Y.; Wu, Z.; Li, C.; Miao, X.; Yang, W. Chemical compositions of propolis from China and the United States and their antimicrobial activities against Penicillium notatum. Molecules 2019, 24, 3576. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.L.; Alberto, M.R.; Zampini, I.C.; Cuello, A.S.; Maldonado, L.; Ríos, J.L.; Schmeda-Hirschmann, G.; Isla, M.I. Biological activities of polyphenols-enriched propolis from Argentina arid regions. Phytomedicine 2016, 23, 27–31. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 2014, 34, 16–23. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant compounds from propolis collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: An in vitro approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Soberón, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the northwest of Argentina as a source of antifungal principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Cofta, G.; Ratajczak, I. The possibility of propolis extract application in wood protection. Forests 2020, 11, 465. [Google Scholar] [CrossRef]
- Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. White-rot fungi control on Populus spp. wood by pressure treatments with silver nanoparticles, chitosan oligomers and propolis. Forests 2019, 10, 885. [Google Scholar] [CrossRef]
- Silva-Castro, I.; Casados-Sanz, M.; Alonso-Cortés, A.L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. Chitosan-based coatings to prevent the decay of Populus spp. wood caused by Trametes versicolor. Coatings 2018, 8, 415. [Google Scholar] [CrossRef]
- Ratajczak, I.; Woźniak, M.; Kwaśniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical characterization of wood treated with a formulation based on propolis, caffeine and organosilanes. Eur. J. Wood Wood Prod. 2018, 76, 775–781. [Google Scholar] [CrossRef]
- Donath, S.; Militz, H.; Mai, C. Creating water-repellent effects on wood by treatment with silanes. Holzforschung 2006, 60, 40–46. [Google Scholar] [CrossRef]
- Reinprecht, L.; Grznárik, T. Biological durability of Scots pine (Pinus sylvestris L.) sapwood modified with selected organo-silanes. Wood Res. 2015, 60, 687–696. [Google Scholar]
- Ghosh, S.C.; Militz, H.; Mai, C. Modification of Pinus sylvestris L. wood with quat- and amino-silicones of different chain lengths. Holzforschung 2013, 67, 421–427. [Google Scholar] [CrossRef]
- Weigenand, O.; Humar, M.; Daniel, G.; Militz, H.; Mai, C. Decay resistance of wood treated with amino-silicone compounds. Holzforschung 2008, 62, 112–118. [Google Scholar] [CrossRef]
- Reinprecht, L.; Vacek, V.; Grznárik, T. Enhanced fungal resistance of Scots pine (Pinus sylvestris L.) sapwood by treatment with methyltrimethoxysilane and benzalkoniumchloride. Eur. J. Wood Wood Prod. 2017, 75, 817–824. [Google Scholar] [CrossRef]
- Kartal, S.N.; Yoshimura, T.; Imamura, Y. Modification of wood with Si compounds to limit boron leaching from treated wood and to increase termite and decay resistance. Int. Biodeterior. Biodegrad. 2009, 63, 187–190. [Google Scholar] [CrossRef]
- CEN—EN 113—Wood Preservatives. Test Method for Determining the Protective Effectiveness Against Wood Destroying Basidiomycetes. Determination of the Toxic Values; European Committee for Standardization: Bruxelles, Belgium, 1996.
- CEN—EN 84—Wood Preservatives—Accelerated Ageing of Treated Wood Prior to Biological Testing—Leaching Procedure; European Committee for Standardization: Bruxelles, Belgium, 2000.
- PN-77/D-04103. Wood. Determination of static bending strength; Polish Committee for Standarization: Warsaw, Poland, 1977.
- PN-63/D-04117. Physical and Mechanical Properties of Wood. Determination of the Elasticity Coefficient for Static Bending; Polish Committee for Standarization: Warsaw, Poland, 1963.
- ISO. 13061-2:2014—Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 2: Determination of Density for Physical and Mechanical Tests; International Organization for Standarization: Geneva, Switzerland, 2014. [Google Scholar]
- ISO. 13061-1:2014—Physical and Mechanical Properties of Wood—Test Methods for Small Clear Wood Specimens—Part 1: Determination of Moisture Content for Physical and Mechanical Tests; International Organization for Standarization: Geneva, Switzerland, 2014. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidant substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and effective evaluation of the antioxidant capacity of propolis extracts using DPPH bleaching kinetic profiles, FT-IR and UV-vis spectroscopic data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Wu, Y.W.; Sun, S.Q.; Zhao, J.; Li, Y.; Zhou, Q. Rapid discrimination of extracts of Chinese propolis and poplar buds by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2008, 883–884, 48–54. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Szentner, K.; Kwaśniewska, P.; Mazela, B. Propolis and organosilanes in wood protection. Part I: FTIR analysis and biological tests. Ann. Warsaw Univ. Life Sci. SGGW For. Wood Technol. 2015, 91, 218–224. [Google Scholar]
- Peres, R.S.; Zmozinski, A.V.; Brust, F.R.; Macedo, A.J.; Armelin, E.; Alemán, C.; Ferreira, C.A. Multifunctional coatings based on silicone matrix and propolis extract. Prog. Org. Coat. 2018, 123, 223–231. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Militz, H.; Mai, C. The efficacy of commercial silicones against blue strain and mould fungi in wood. Eur. J. Wood Wood Prod. 2009, 67, 159–167. [Google Scholar] [CrossRef]
- Ratajczak, I.; Wichłacz-Szentner, K.; Mazela, B.; Hochmańska, P.; Rissmann, I. Silicon compounds as additives improving coating performance: Fixation of silicon compounds with cellulose. Eur. J. Wood Wood Prod. 2010, 68, 483–486. [Google Scholar] [CrossRef]
- Sèbe, G.; Tingaut, P.; Safou-Tchiama, R.; Pétraud, M.; Grelier, S.; De Jéso, B. Chemical reaction of maritime pine sapwood (Pinus pinaster Soland) with alkoxysilane molecules: A study of chemical pathways. Holzforschung 2004, 58, 511–518. [Google Scholar] [CrossRef]
- Van Opdenbosch, D.; Dorstein, J.; Klaithong, S.; Kornprobst, T.; Plank, J.; Hietala, S.; Zollfrank, C. Chemistry and water-repelling properties of phenyl-incorporating wood composites. Holzforschung 2013, 67, 931–940. [Google Scholar] [CrossRef]
- Baur, S.I.; Easteal, A.J. Improved photoprotection of wood by chemical modification with silanes: NMR and ESR studies. Polym. Adv. Technol. 2013, 24, 97–103. [Google Scholar] [CrossRef]
- Ratajczak, I.; Woźniak, M.; Szentner, K.; Babicka, M.; Jenczyk, J.; Mazela, B. Aminosilane binding to wood substance through an alkyd resin. J. Wood Chem. Technol. 2020, 40, 73–79. [Google Scholar] [CrossRef]
- Bauer, F.; Ernst, H.; Decker, U.; Findeisen, M.; Gläsel, H.J.; Langguth, H.; Hartmann, E.; Mehnert, R.; Peuker, C. Preparation of scratch and abrasion resistant polymeric nanocomposites by monomer grafting onto nanoparticles, 1: FTIR and multi-nuclear NMR spectroscopy to the characterization of methacryl grafting. Macromol. Chem. Phys. 2000, 201, 2654–2659. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Waśkiewicz, A.; Szentner, K.; Cofta, G.; Kwaśniewska-Sip, P. Investigation of the use of impregnating formulation with propolis extract and organosilanes in wood protection—Biological analyses. Ann. Warsaw Univ. Life Sci. SGGW. For. Wood Technol. 2016, 47, 43–47. [Google Scholar]
- Block, C.N.; Shibata, T.; Solo-Gabriele, H.M.; Townsend, T.G. Use of handheld X-ray fluorescence spectrometry units for identification of arsenic in treated wood. Environ. Pollut. 2007, 148, 627–633. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Influence of dipping time on uptake of preservative solution, adsorption, penetration and fixation of copper-ethanolamine based wood preservatives. Eur. J. Wood Wood Prod. 2009, 67, 265–270. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Szentner, K.; Rissmann, I.; Cofta, G. Investigation of the use of impregnating formulation with propolis extract and organosilanes in wood protection—Chemical analyses. Part I: FTIR and EA analyses. Ann. Warsaw Univ. Life Sci. SGGW. For. Wood Technol. 2016, 37, 32–37. [Google Scholar]
- Jones, D.; Howard, N.; Suttie, E. The Potential of Propolis and Other Naturally Occurring Products for Preventing Biological Decay; The International Research Group on Wood Protection: Bled, Slovenia, 2011; IRG/WP 11-30575. [Google Scholar]
- Woźniak, M.; Ratajczak, I.; Lis, B.; Krystofiak, T. Hydrophobic properties of wood treated with propolis-silane formulations. Wood Res. 2018, 63, 517–524. [Google Scholar]
- Meier, P.; Kaps, T.; Kallavus, U. Swelling of pinewood (Pinus sylvestris) in binary aqueous solutions of organic substances. Mater. Sci. 2005, 11, 140–145. [Google Scholar]
- Meier, P.; Kallavus, U.; Rohumaa, A.; Kaps, T. Multiple swelling of pinewood (Pinus sylvestris) in binary and ternary mixtures of ethanol, acetone and water. Mater. Sci. 2006, 12, 25–30. [Google Scholar]
- Meier, P.; Stöör, E.; Kaps, T.; Kallavus, U. Mechanical properties of pinewood (Pinus sylvestris) swollen in organic liquids. Proc. Est. Acad. Sci. 2006, 55, 125–133. [Google Scholar]
- Fojutowski, A.; Noskowiak, A.; Kot, M.; Kropacz, A.; Stangierska, A. The assessment of mechanical properties of wood treated with ionic liquids. Wood 2010, 184, 21–38. [Google Scholar]
- Bollmus, S.; Beeretz, C.; Militz, H. Tensile and impact bending properties of chemically modified Scots pine. Forests 2020, 11, 84. [Google Scholar] [CrossRef]
- Lopes, D.B.; Mai, C.; Militz, H. Mechanical properties of chemically modified Portuguese pinewood. Maderas Cienc. Tecnol. 2015, 17, 179–194. [Google Scholar] [CrossRef]
- Larnøy, E.; Dantz, S.; Eikenes, M.; Militz, H. Screening of properties of modified chitosan-treated wood. Wood Mater. Sci. Eng. 2006, 1, 59–68. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).