Stand-Level Transpiration Increases after Eastern Redcedar (Juniperus virginiana L.) Encroachment into the Midstory of Oak Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
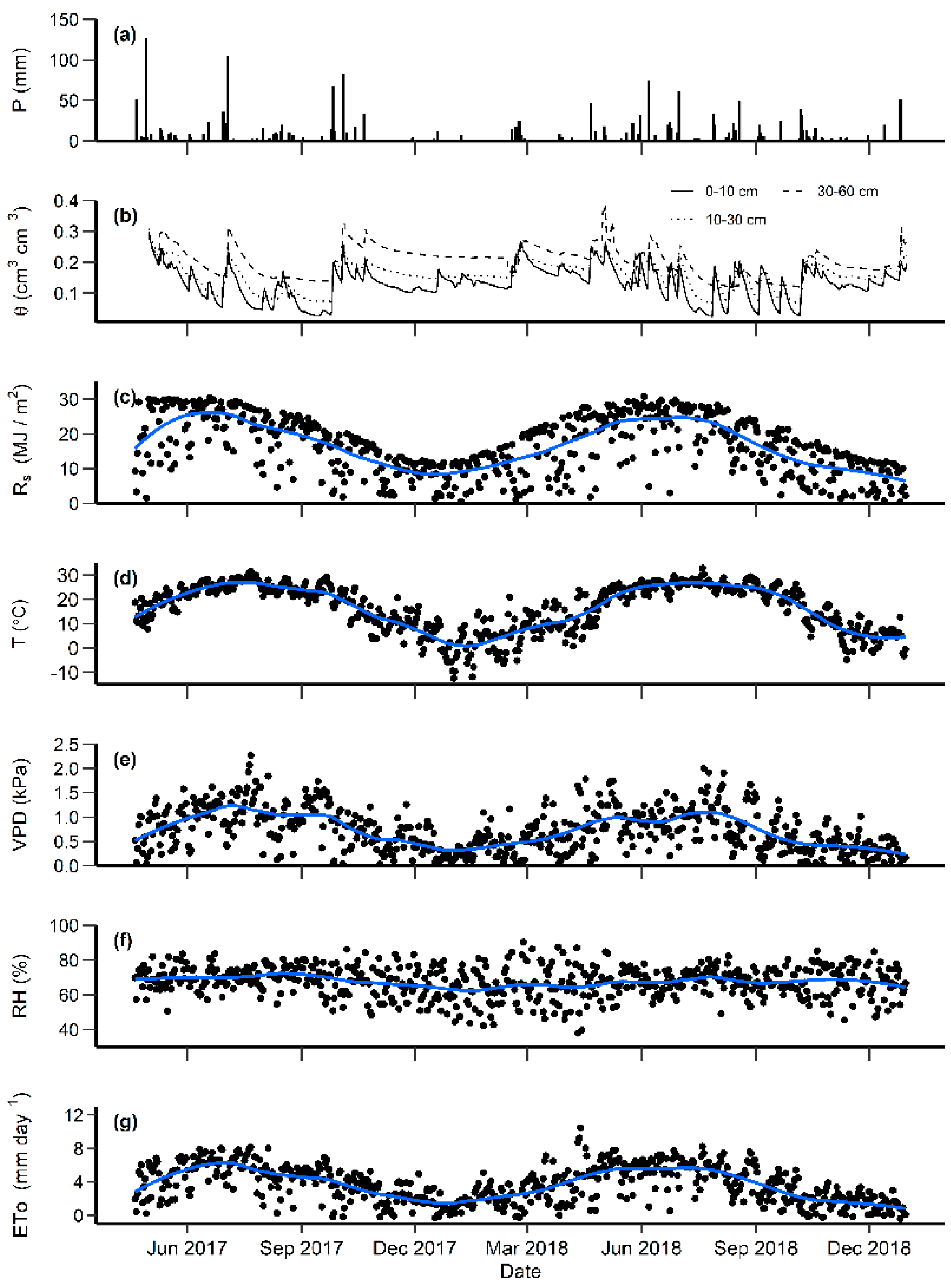
2.3. Environmental Variables
2.4. Thermal Dissipation Probes and Sapwood Estimation
2.5. Sap Flow Density to Whole-Tree and Whole-Stand Water Use
2.6. Data Analysis
3. Results
3.1. Environmental Conditions
3.2. Sap Flow Densities
3.3. Water Use and Its Relationship with DBH, Sapwood Area and Sd
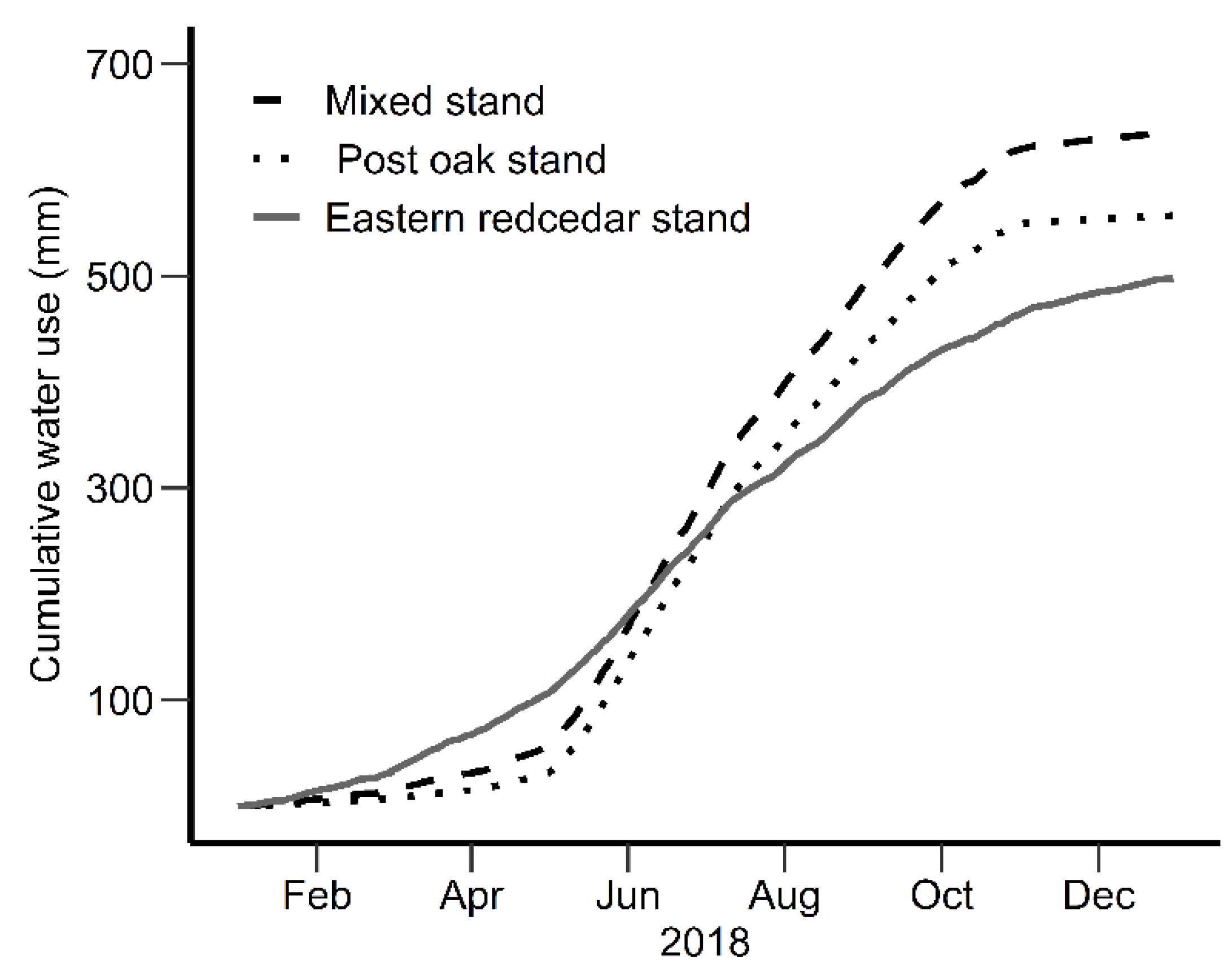
3.4. Stand-Level Water Use
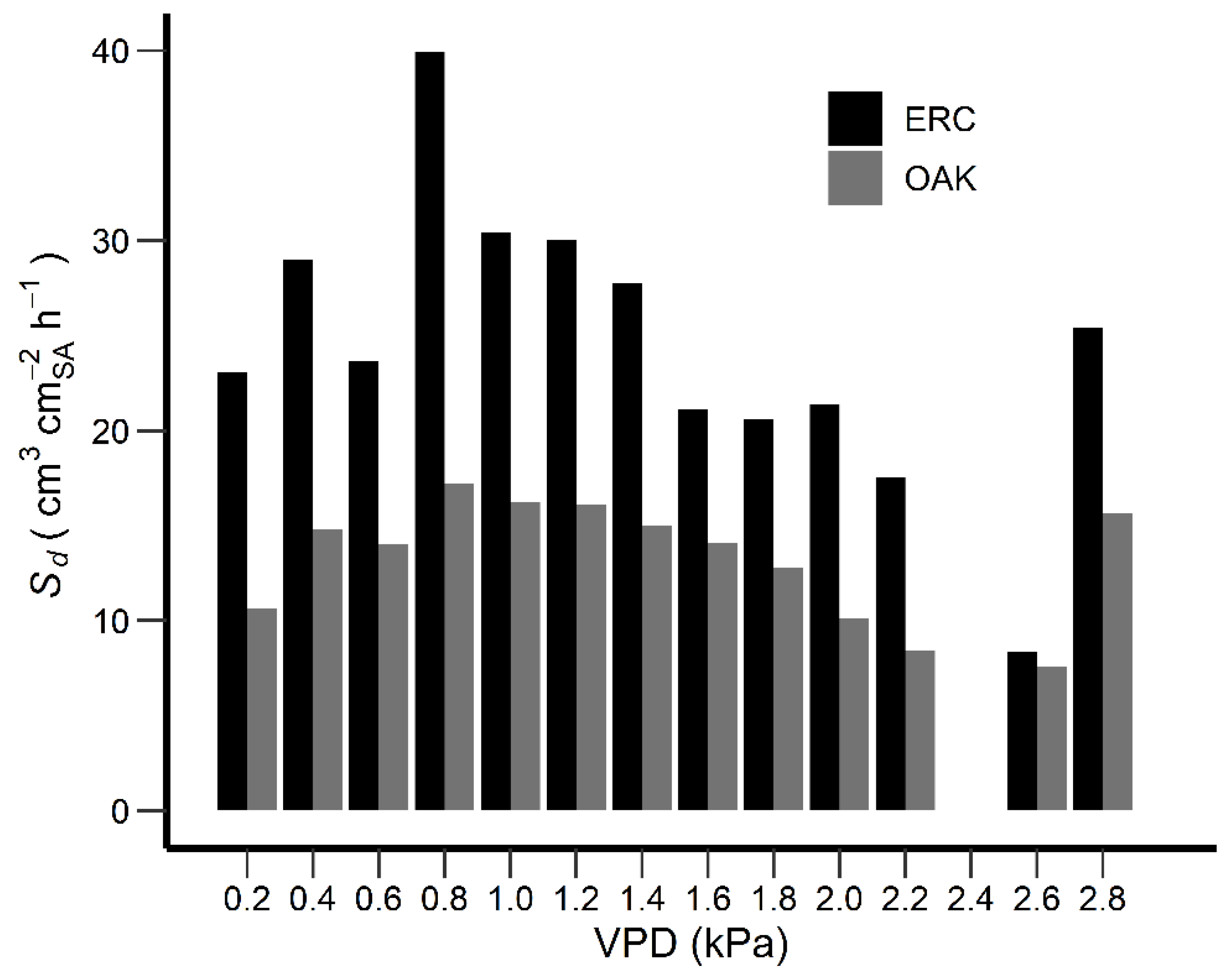
3.5. Sd day and Environmental Variables
4. Discussion
4.1. Sap Flow Density, Sd
4.2. Sd day and Environmental Variables
4.3. Water Use
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Twidwell, D.; Bielski, C.; Fogarty, D.; Mittelstet, A.; Starks, P.; Will, R.; Zhong, Y.; Acharya, B. Impact of eastern redcedar proliferation on water resources in the Great Plains USA—Current state of knowledge. Water 2018, 10, 1768. [Google Scholar] [CrossRef]
- Barger, N.N.; Archer, S.R.; Campbell, J.L.; Huang, C.y.; Morton, J.A.; Knapp, A.K. Woody plant proliferation in North American drylands: A synthesis of impacts on ecosystem carbon balance. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Therrell, M.; Stahle, D. A predictive model to locate ancient forests in the Cross Timbers of Osage County, Oklahoma. J. Biogeogr. 1998, 25, 847–854. [Google Scholar] [CrossRef]
- Anderson, R.C.; Fralish, J.S.; Baskin, J.M. Savannas, Barrens, and Rock Outcrop Plant Communities of North America; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- DeSantis, R.D.; Hallgren, S.W.; Lynch, T.B.; Burton, J.A.; Palmer, M.W. Long-term directional changes in upland Quercus forests throughout Oklahoma, USA. J. Veg. Sci. 2010, 21, 606–615. [Google Scholar] [CrossRef]
- DeSantis, R.D.; Hallgren, S.W.; Stahle, D.W. Drought and fire suppression lead to rapid forest composition change in a forest-prairie ecotone. For. Ecol. Manag. 2011, 261, 1833–1840. [Google Scholar] [CrossRef]
- Hoff, D.L.; Will, R.E.; Zou, C.B.; Lillie, N.D. Encroachment dynamics of Juniperus virginiana L. and mesic hardwood species into Cross Timbers forests of North-Central Oklahoma, USA. Forests 2018, 9, 75. [Google Scholar] [CrossRef]
- Hoff, D.L.; Will, R.E.; Zou, C.B.; Weir, J.R.; Gregory, M.S.; Lillie, N.D. Estimating increased fuel loading within the Cross Timbers forest matrix of Oklahoma, USA due to an encroaching conifer, Juniperus virginiana, using leaf-off satellite imagery. For. Ecol. Manag. 2018, 409, 215–224. [Google Scholar] [CrossRef]
- Nunes Biral, V.C.; Will, R.E.; Zou, C.B. Establishment of Quercus marilandica Muenchh. and Juniperus virginiana L. in the tallgrass prairie of Oklahoma, USA increases litter inputs and soil organic carbon. Forests 2019, 10, 329. [Google Scholar] [CrossRef]
- Joshi, O.; Will, R.E.; Zou, C.B.; Kharel, G.J.S. Sustaining Cross-Timbers forest resources: Current knowledge and future research needs. Sustainability 2019, 11, 4703. [Google Scholar] [CrossRef]
- Zou, C.B.; Turton, D.J.; Will, R.E.; Engle, D.M.; Fuhlendorf, S.D. Alteration of hydrological processes and streamflow with juniper (Juniperus virginiana) encroachment in a mesic grassland catchment. Hydrol. Process. 2014, 28, 6173–6182. [Google Scholar] [CrossRef]
- Qiao, L.; Zou, C.B.; Stebler, E.; Will, R.E. Woody plant encroachment reduces annual runoff and shifts runoff mechanisms in the tallgrass prairie, USA. Water Resour. Res. 2017, 53, 4838–4849. [Google Scholar] [CrossRef]
- Acharya, B.S.; Halihan, T.; Zou, C.B.; Will, R.E. Vegetation controls on the spatio-temporal heterogeneity of deep moisture in the unsaturated zone: A hydrogeophysical evaluation. Sci. Rep. 2017, 7, 1499. [Google Scholar] [CrossRef] [PubMed]
- Huxman, T.E.; Wilcox, B.P.; Breshears, D.D.; Scott, R.L.; Snyder, K.A.; Small, E.E.; Hultine, K.; Pockman, W.T.; Jackson, R.B. Ecohydrological implications of woody plant encroachment. Ecology 2005, 86, 308–319. [Google Scholar] [CrossRef]
- Caterina, G.L.; Will, R.E.; Turton, D.J.; Wilson, D.S.; Zou, C.B. Water use of Juniperus virginiana trees encroached into mesic prairies in Oklahoma, USA. Ecohydrology 2014, 7, 1124–1134. [Google Scholar] [CrossRef]
- Bahari, Z.A.; Pallardy, S.G.; Parker, W.C. Photosynthesis, water relations, and drought adaptation in six woody species of oak-hickory forests in central Missouri. For. Sci. (USA) 1985. [Google Scholar] [CrossRef]
- Maherali, H.; Moura, C.F.; Caldeira, M.C.; Willson, C.J.; Jackson, R.B. Functional coordination between leaf gas exchange and vulnerability to xylem cavitation in temperate forest trees. Plant Cell Environ. 2006, 29, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Awada, T.; El-Hage, R.; Geha, M.; Wedin, D.A.; Huddle, J.A.; Zhou, X.; Msanne, J.; Sudmeyer, R.A.; Martin, D.L.; Brandle, J.R. Intra-annual variability and environmental controls over transpiration in a 58-year-old even-aged stand of invasive woody Juniperus virginiana L. in the Nebraska Sandhills, USA. Ecohydrology 2013, 6, 731–740. [Google Scholar] [CrossRef]
- Starks, P.J.; Venuto, B.C.; Dugas, W.A.; Kiniry, J. Measurements of canopy interception and transpiration of eastern redcedar grown in open environments. Environ. Nat. Resour. Res. 2014, 4, 103. [Google Scholar] [CrossRef]
- Cooper, C.E.; Aparecido, L.M.T.; Muir, J.P.; Morgan, C.L.S.; Heilman, J.L.; Moore, G.W. Transpiration in recovering mixed loblolly pine and oak stands following wildfire in the Lost Pines region of Texas. Ecohydrology 2018, 12, e2052. [Google Scholar] [CrossRef]
- Heilman, J.L.; Litvak, M.E.; McInnes, K.J.; Kjelgaard, J.F.; Kamps, R.H.; Schwinning, S. Water-storage capacity controls energy partitioning and water use in karst ecosystems on the Edwards Plateau, Texas. Ecohydrology 2014, 7, 127–138. [Google Scholar] [CrossRef]
- Grossiord, C.; Gessler, A.; Granier, A.; Berger, S.; Bréchet, C.; Hentschel, R.; Hommel, R.; Scherer-Lorenzen, M.; Bonal, D. Impact of interspecific interactions on the soil water uptake depth in a young temperate mixed species plantation. J. Hydrol. 2014, 519, 3511–3519. [Google Scholar] [CrossRef]
- del Castillo, J.; Comas, C.; Voltas, J.; Ferrio, J.P. Dynamics of competition over water in a mixed oak-pine Mediterranean forest: Spatio-temporal and physiological components. For. Ecol. Manag. 2016, 382, 214–224. [Google Scholar] [CrossRef]
- Novick, K.A.; Ficklin, D.L.; Stoy, P.C.; Williams, C.A.; Bohrer, G.; Oishi, A.C.; Papuga, S.A.; Blanken, P.D.; Noormets, A.; Sulman, B.N.; et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Chang. 2016, 6, 1023–1027. [Google Scholar] [CrossRef]
- Asbjornsen, H.; Mora, G.; Helmers, M.J. Variation in water uptake dynamics among contrasting agricultural and native plant communities in the Midwestern US. Agric. Ecosyst. Environ. 2007, 121, 343–356. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration—Guidelines for computing crop water requirements - FAO Irrigation and drainage paper 56. FAO Rome 1998, 300, D05109. [Google Scholar]
- Sellers, P.; Randall, D.; Collatz, G.; Berry, J.; Field, C.; Dazlich, D.; Zhang, C.; Collelo, G.; Bounoua, L. A revised land surface parameterization (SiB2) for atmospheric GCMs. Part I: Model formulation. J. Clim. 1996, 9, 676–705. [Google Scholar] [CrossRef]
- Williams, I.N.; Lu, Y.; Kueppers, L.M.; Riley, W.J.; Biraud, S.C.; Bagley, J.E.; Torn, M.S. Land-atmosphere coupling and climate prediction over the US Southern Great Plains. J. Geophys. Res. Atmos. 2016, 121, 12125–12144. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C.; Hassol, S.J. Global Climate Change Impacts in the United States; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013-The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Siqueira, M.; Katul, G.; Porporato, A. Onset of water stress, hysteresis in plant conductance, and hydraulic lift: Scaling soil water dynamics from millimeters to meters. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef]
- Ferguson, C.R.; Wood, E.F.; Vinukollu, R.K. A global intercomparison of modeled and observed land–atmosphere coupling. J. Hydrometeorol. 2012, 13, 749–784. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Hanson, P.J.; Todd, D.E. Transpiration from a multi-species deciduous forest as estimated by xylem sap flow techniques. For. Ecol. Manag. 2001, 143, 205–213. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Zhuang, J.Y.; Han, C.; Zhai, L.; Zhao, W.R.; Zhang, J.C. The relationship between sap flow density and environmental factors in the Yangtze River delta region of China. Forests 2017, 8, 74. [Google Scholar] [CrossRef]
- Kyung, M. A computational Bayesian method for estimating the number of knots in regression splines. Bayesian Anal. 2011, 6, 793–828. [Google Scholar] [CrossRef]
- Sahari, J.; Sapuan, S.; Zainudin, E.; Maleque, M. Thermo-mechanical behaviors of thermoplastic starch derived from sugar palm tree (Arenga pinnata). Carbohydr. Polym. 2013, 92, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Chang. Boil. 2018, 24, e171–e182. [Google Scholar] [CrossRef]
- Daou, L.; Shipley, B. The measurement and quantification of generalized gradients of soil fertility relevant to plant community ecology. Ecology 2019, 100, e02549. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Boil. 2019, 25, 1078–1088. [Google Scholar] [CrossRef]
- Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 10 March 2020).
- Oklahoma Climatological Survey. Available online: http://climate.mesonet.org/county_climate/Products/County_Climatologies/county_climate_payne.pdf (accessed on 5 March 2020).
- Granier, A. Une nouvelle méthode pour la mesure du flux de sève brute dans le tronc des arbres. In Annales des Sciences Forestières; No. 2; EDP Sciences: Les Ulis, France, 1985; Volume 42, pp. 193–200. [Google Scholar]
- Renninger, H.J.; Schafer, K.V.R. Comparison of tissue heat balance-and thermal dissipation-derived sap flow measurements in ring-porous oaks and a pine. Front. Plant Sci. 2012, 3, 103. [Google Scholar] [CrossRef]
- Sun, H.; Aubrey, D.P.; Teskey, R.O. A simple calibration improved the accuracy of the thermal dissipation technique for sap flow measurements in juvenile trees of six species. Trees 2012, 26, 631–640. [Google Scholar] [CrossRef]
- Lloyd, J.; Grace, J.; Miranda, A.C.; Meir, P.; Wong, S.C.; Miranda, H.S.; Wright, I.R.; Gash, J.H.C.; McIntyre, J. A simple calibrated model of Amazon rainforest productivity based on leaf biochemical properties. Plant Cell Environ. 1995, 18, 1129–1145. [Google Scholar] [CrossRef]
- United States Drought Monitor—U.S. Department of Agriculture (USDA) and the National Oceanic and Atmospheric Association (NOAA). Available online: https://droughtmonitor.unl.edu/CurrentMap/StateDroughtMonitor.aspx (accessed on 15 July 2020).
- Torquato, P.R.; Zou, C.B.; Adhikari, A.; Adams, H.D.; Will, R.E. Drought tolerance and competition in eastern redcedar (Juniperus virginiana) encroachment of the oak-dominated Cross Timbers. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A.; Williams, M.; Do Vale, R.L.; Da Costa, A.L.; Meir, P. Evidence from Amazonian forests is consistent with isohydric control of leaf water potential. Plant Cell Environ. 2006, 29, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, T.; Teskey, R.; Duhme, F.; Richter, H. Temperate hardwood forests. Water Deficits Plant Growth 1981, 6, 153–208. [Google Scholar]
- Thorburn, P.J.; Hatton, T.J.; Walker, G.R. Combining measurements of transpiration and stable isotopes of water to determine groundwater discharge from forests. J. Hydrol. 1993, 150, 563–587. [Google Scholar] [CrossRef]
- Granier, A.; Loustau, D. Measuring and modelling the transpiration of a maritime pine canopy from sap-flow data. Agric. For. Meteorol. 1994, 71, 61–81. [Google Scholar] [CrossRef]
- Phillips, N.; Oren, R. Intra- and inter-annual variation in transpiration of a pine forest. Ecol. Appl. 2001, 11, 385–396. [Google Scholar] [CrossRef]
- Hernández-Santana, V.; David, T.S.; Martínez-Fernández, J. Environmental and plant-based controls of water use in a Mediterranean oak stand. For. Ecol. Manag. 2008, 255, 3707–3715. [Google Scholar] [CrossRef]
- Hull, J.C.; Wood, S.G. Water relations of oak species on and adjacent to a Maryland serpentine soil. Am. Midl. Nat. 1984, 224–234. [Google Scholar] [CrossRef]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest–grassland ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef]
- Eggemeyer, K.D.; Awada, T.; Harvey, F.E.; Wedin, D.A.; Zhou, X.; Zanner, C.W. Seasonal changes in depth of water uptake for encroaching trees Juniperus virginiana and Pinus ponderosa and two dominant C4 grasses in a semiarid grassland. Tree Physiol. 2009, 29, 157–169. [Google Scholar] [CrossRef]
- Abrams, M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Abrams, M.D. Distribution, historical development and ecophysiological attributes of oak species in the eastern United States. Ann. Sci. For. 1996, 53, 487–512. [Google Scholar] [CrossRef]
- Zapater, M.; Hossann, C.; Bréda, N.; Bréchet, C.; Bonal, D.; Granier, A. Evidence of hydraulic lift in a young beech and oak mixed forest using 18O soil water labelling. Trees 2011, 25, 885. [Google Scholar] [CrossRef]
- Zou, C.B.; Caterina, G.L.; Will, R.E.; Stebler, E.; Turton, D. Canopy interception for a tallgrass prairie under Juniper Encroachment. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]






| Tree ID | Stand | Species | DBH (cm) | Height (m) | Bark Thickness (cm) | Sapwood Width (cm) | SA (cm2) | Age |
|---|---|---|---|---|---|---|---|---|
| 1 | OAK | Post oak | 31 | 16 | 2.2 | 2.8 | 215 | 94 |
| 2 | OAK | Post oak | 22 | 14 | 1.9 | 2.8 | 137 | 85 |
| 3 | OAK | Post oak | 12 | 10 | 1.1 | 1.9 | 50 | 102 |
| 4 | OAK | Post oak | 19 | 9 | 0.8 | 2.6 | 123 | † |
| 5 | OAK | Post oak | 12 | 9 | 0.8 | 1.9 | 48 | 96 |
| 6 | OAK | Post oak | 21 | 12 | 1.2 | 1.4 | 80 | 112 |
| 7 | OAK | Post oak | 15 | 9 | 0.7 | 1.7 | 64 | 115 |
| 8 | MIX | Post oak | 11 | 7 | 1.1 | 2.5 | 51 | 98 |
| 9 | MIX | Redcedar | 17 | 9 | 0.5 | 3.0 | 132 | 50 |
| 10 | MIX | Post oak | 25 | 12 | 1.1 | 4.3 | 253 | 90 |
| 11 | MIX | Post oak | 7 | 5 | 0.7 | 2.5 | 19 | 56 |
| 12 | MIX | Redcedar | 14 | 8 | 0.4 | 1.9 | 68 | 39 |
| 13 | MIX | Redcedar | 8 | 5 | 0.3 | 1.1 | 21 | 37 |
| 14 | MIX | Redcedar | 17 | 8 | 0.5 | 2.0 | 92 | 47 |
| 15 | MIX | Post oak | 14 | 5 | 0.9 | 1.7 | 56 | 85 |
| 16 | MIX | Redcedar | 22 | 8 | 0.5 | 1.3 | 79 | 69 |
| 17 | MIX | Post oak | 33 | 13 | 2.0 | 3.2 | 269 | 120 |
| 18 | ERC | Redcedar | 18 | 7 | 0.7 | 1.8 | 85 | 64 |
| 19 | ERC | Redcedar | 33 | 12 | 0.6 | 1.8 | 168 | 61 |
| 20 | ERC | Redcedar | 10 | 8 | 0.2 | 2.1 | 55 | 36 |
| 21 | ERC | Redcedar | 9 | 8 | 0.2 | 1.3 | 31 | 35 |
| 22 | ERC | Redcedar | 19 | 9 | 0.7 | 2.0 | 100 | 36 |
| 23 | ERC | Redcedar | 15 | 8 | 0.7 | 1.9 | 71 | 36 |
| 24 | ERC | Redcedar | 8 | 9 | 0.2 | 1.9 | 36 | 37 |
| Stand | Species | SA (cm2) | SA Trees with TDP (cm2) | Total SA (cm2) | SA per ha (m2 ha−1) |
|---|---|---|---|---|---|
| OAK | Oak † | 1878 | 718 | 2066 | 7.87 |
| Redcedar | 188 | 0.00 | |||
| MIX | Oak † | 1272 | 648 | 2072 | 7.89 |
| Redcedar | 800 | 392 | |||
| ERC | Oak † | 392 | 0.00 | 1851 | 7.05 |
| Redcedar | 1459 | 377 |
| Tree ID | Stand | Species | Average (± SD, L day−1) | Maximum (L day−1) | Minimum (L day−1) |
|---|---|---|---|---|---|
| 1 | OAK | Post oak | 26.4 (±13.6) | 66.6 | 1.0 |
| 2 | OAK | Post oak | 18.3 (±7.8) | 39.5 | 0.8 |
| 3 | OAK | Post oak | 0.6 (±0.5) | 2.2 | 0.1 |
| 4 | OAK | Post oak | 7.4 (±3.0) | 16.2 | 0.3 |
| 5 | OAK | Post oak | 1.0 (±0.7) | 3.8 | 0.1 |
| 6 | OAK | Post oak | 4.6 (±1.4) | 6.7 | 0.2 |
| 7 | OAK | Post oak | 1.4 (±0.7) | 2.8 | 0.1 |
| 8 | MIX | Post oak | 2.3 (±0.8) | 5.3 | 0.1 |
| 9 | MIX | Redcedar | 29.8 (±20.3) | 96.1 | 1.8 |
| 10 | MIX | Post oak | 37.4 (±13.8) | 73.1 | 3.8 |
| 11 | MIX | Post oak | 1.8 (±0.6) | 2.8 | 0.1 |
| 12 | MIX | Redcedar | 5.3 (±4.2) | 20.4 | 0.2 |
| 13 | MIX | Redcedar | 4.4 (±2.9) | 14.1 | 0.1 |
| 14 | MIX | Redcedar | 5.9 (±5.6) | 27.5 | 0.2 |
| 15 | MIX | Post oak | 2.1 (±0.6) | 4.4 | 0.2 |
| 16 | MIX | Redcedar | 2.2 (±1.4) | 7.2 | 0.2 |
| 17 | MIX | Post oak | 56.5 (±19.1) | 99.3 | 1.8 |
| 18 | ERC | Redcedar | 4.2 (±3.6) | 17.9 | 0.3 |
| 19 | ERC | Redcedar | 14 (±11.0) | 49.1 | 1.5 |
| 20 | ERC | Redcedar | 4.4 (±2.8) | 11.9 | 0.4 |
| 21 | ERC | Redcedar | 6.9 (±4.9) | 29.9 | 0.6 |
| 22 | ERC | Redcedar | 9.0 (±8.5) | 38.4 | 1.0 |
| 23 | ERC | Redcedar | 3.6 (±2.8) | 13.9 | 0.2 |
| 24 | ERC | Redcedar | 6.1 (±4.4) | 22.5 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torquato, P.R.; Will, R.E.; Zhang, B.; Zou, C.B. Stand-Level Transpiration Increases after Eastern Redcedar (Juniperus virginiana L.) Encroachment into the Midstory of Oak Forests. Forests 2020, 11, 901. https://doi.org/10.3390/f11090901
Torquato PR, Will RE, Zhang B, Zou CB. Stand-Level Transpiration Increases after Eastern Redcedar (Juniperus virginiana L.) Encroachment into the Midstory of Oak Forests. Forests. 2020; 11(9):901. https://doi.org/10.3390/f11090901
Chicago/Turabian StyleTorquato, Patricia R., Rodney E. Will, Bo Zhang, and Chris B. Zou. 2020. "Stand-Level Transpiration Increases after Eastern Redcedar (Juniperus virginiana L.) Encroachment into the Midstory of Oak Forests" Forests 11, no. 9: 901. https://doi.org/10.3390/f11090901
APA StyleTorquato, P. R., Will, R. E., Zhang, B., & Zou, C. B. (2020). Stand-Level Transpiration Increases after Eastern Redcedar (Juniperus virginiana L.) Encroachment into the Midstory of Oak Forests. Forests, 11(9), 901. https://doi.org/10.3390/f11090901







