Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India
Abstract
1. Introduction
2. Materials and Methods
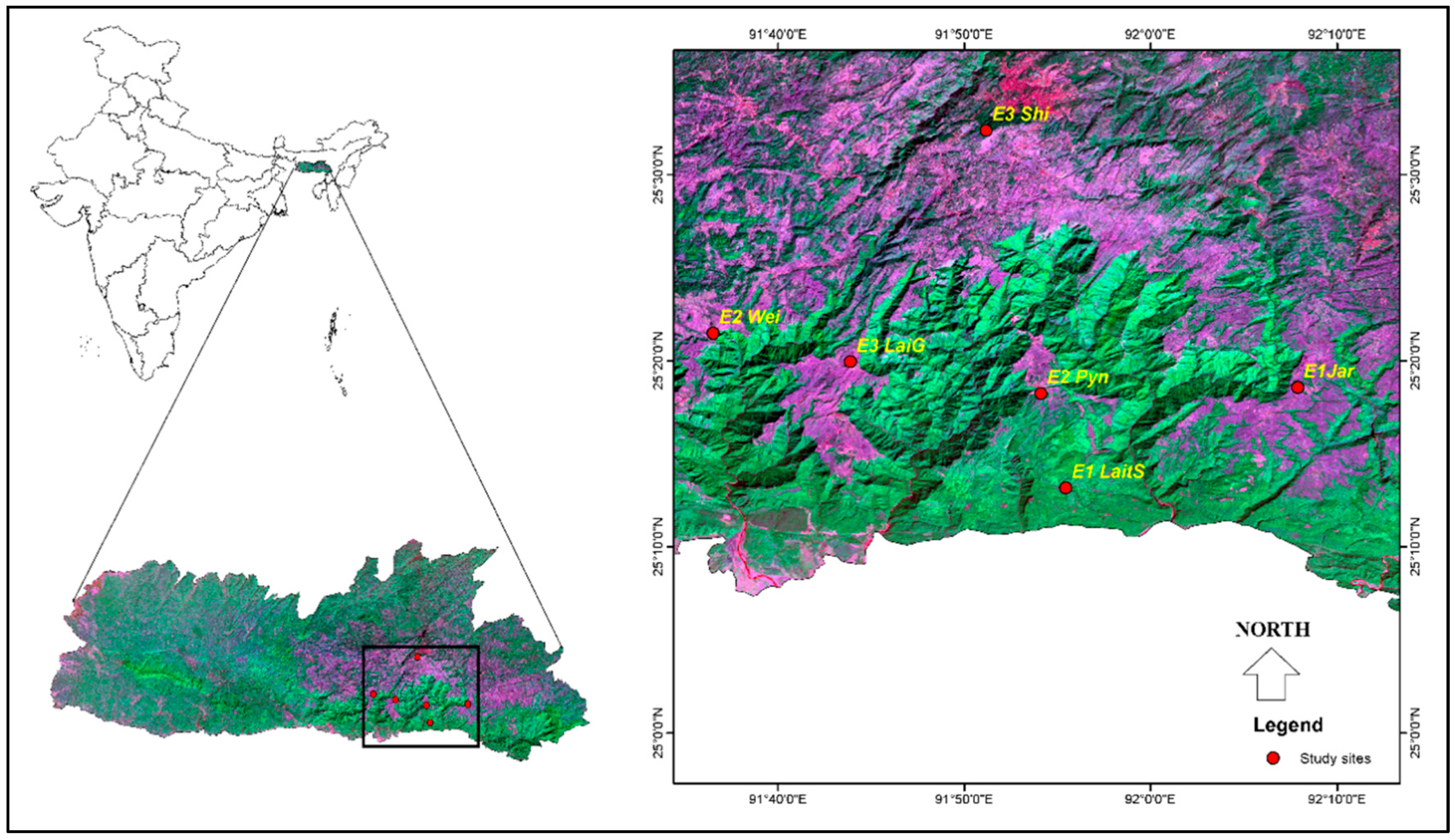
2.1. Study Area Description
2.2. Sampling Design
2.3. Field Data Collection
Species Structure, Composition, Anthropogenic Disturbance, and Soils
2.4. Compositional and Structural Diversity Assessment
2.4.1. Importance Value Index (IVI)
2.4.2. Diversity Indices
2.5. Stem Sprouts and Sprouts Turnover Rate
2.6. Data Analysis
2.6.1. Population Structure and Regeneration Potential
3. Results
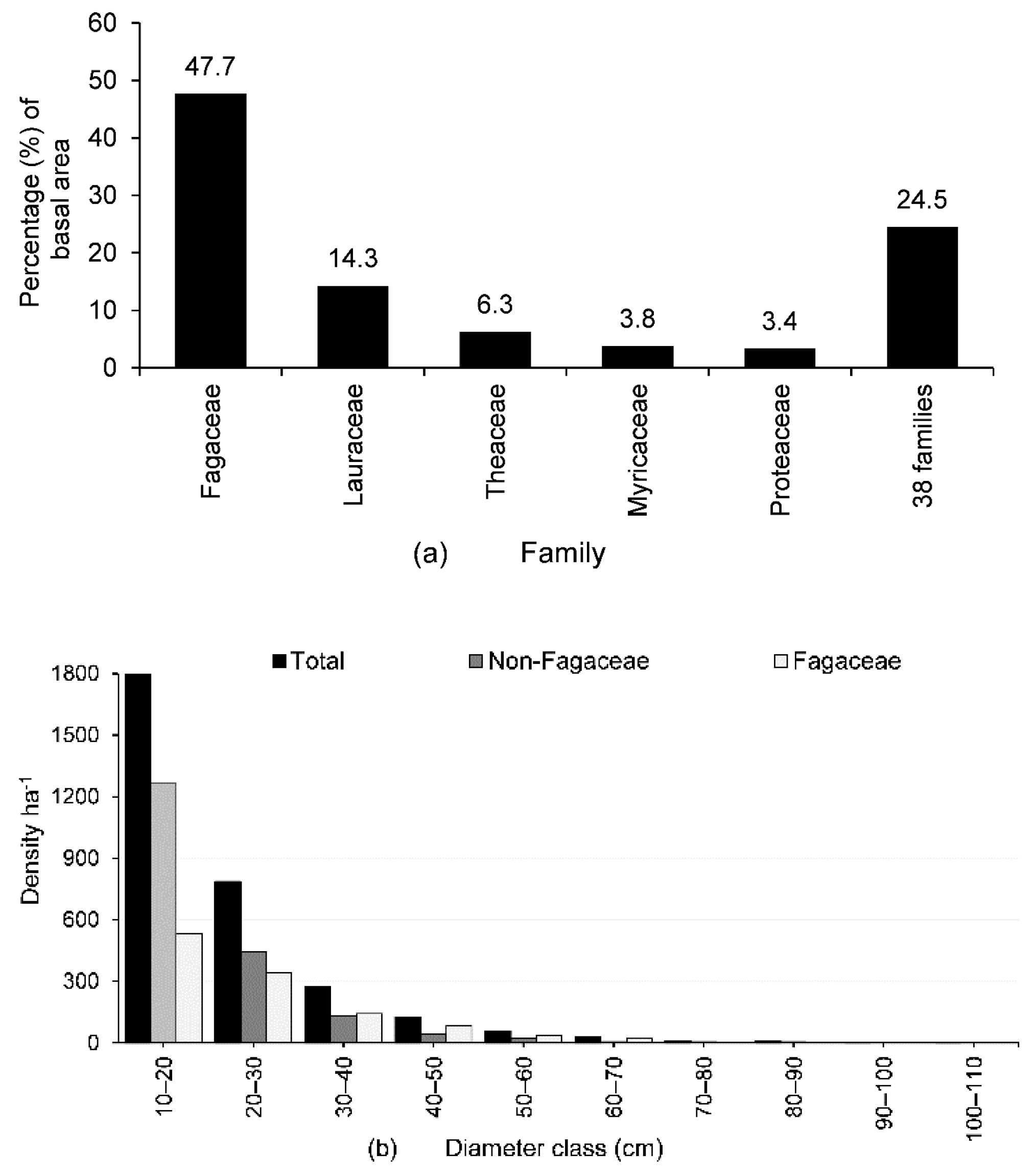
3.1. Species Diversity and Composition
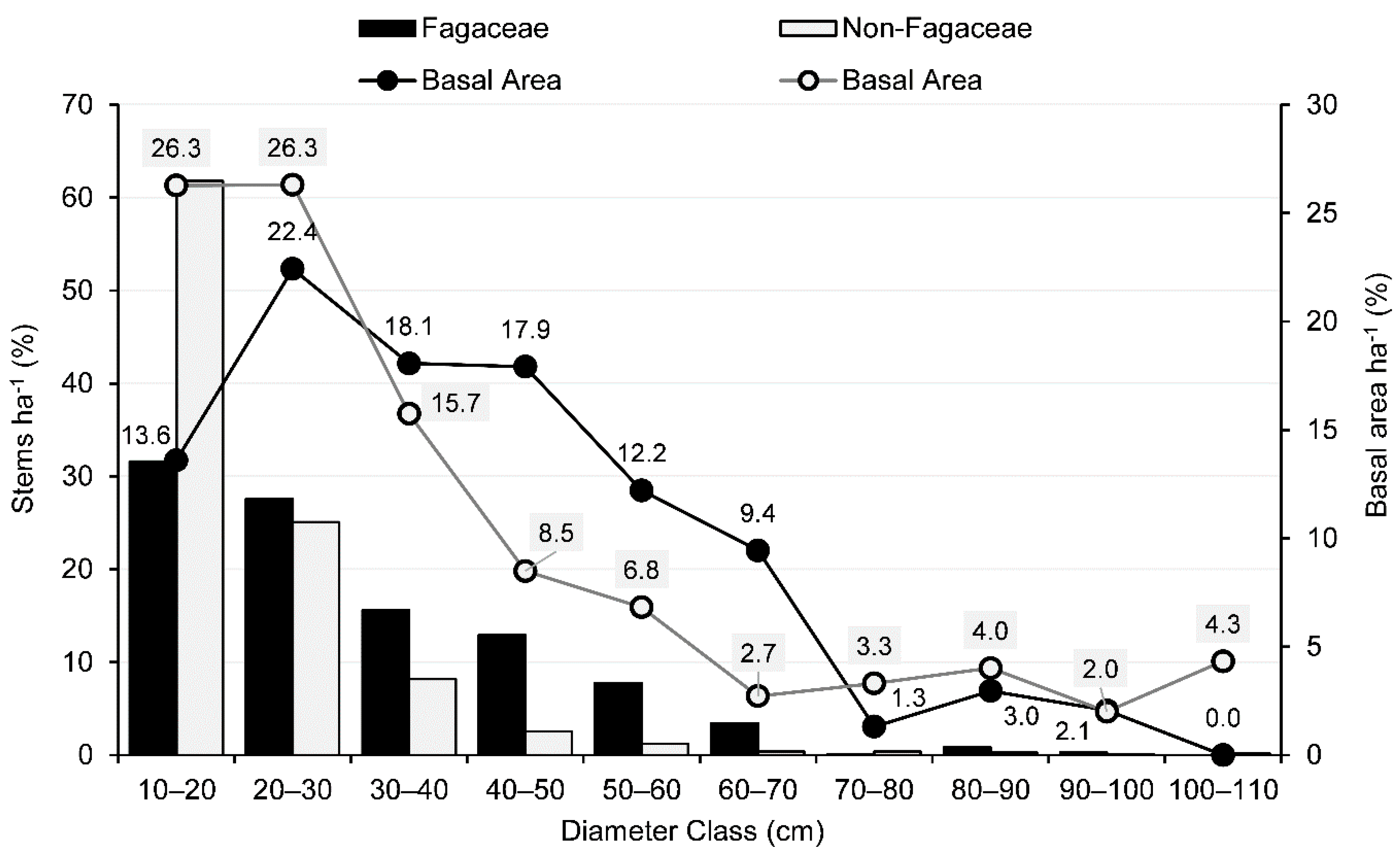
3.2. IVI, Basal Area, Tree Density, and Population Structure
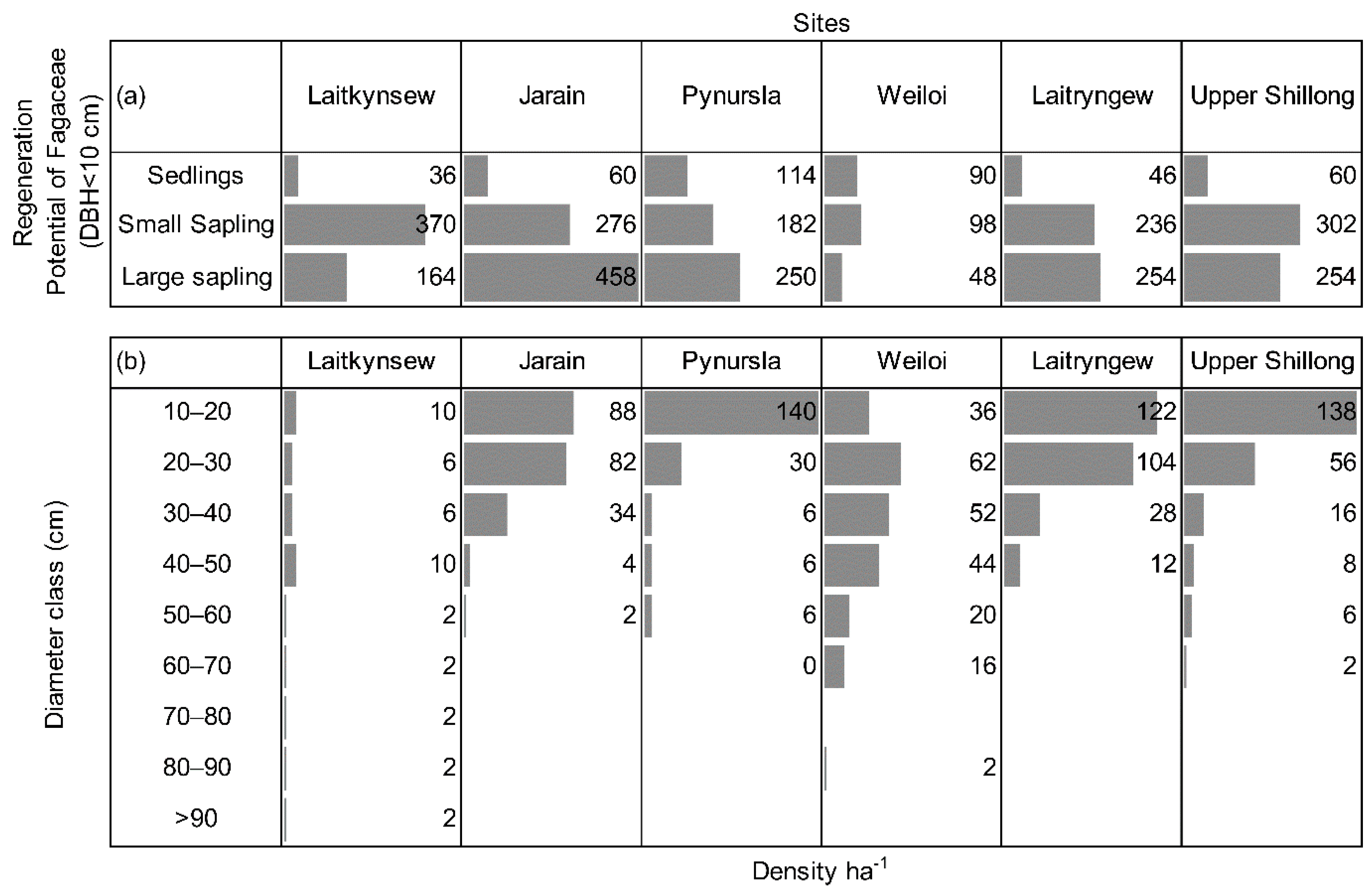
3.3. Diversity, Regeneration Potential, and Overstory Recruitment of Fagaceae Species
3.4. Adaptation Strategy to Disturbance Stress by Fagaceae Species
3.5. Influence of Biotic and Abiotic Factors on the Regeneration of Fagaceae Tree Species
4. Discussion
4.1. Community Characteristics
4.2. Regeneration Potential
4.3. Response of Fagaceae to Disturbance
4.4. Influence of Biotic and Abiotic Variables
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name | Laitkynsew | Jarain | Pynursla | Weiloi | Laitryngew | Upper Shillong | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | IVI | BA/ ha | D/ha | IVI | BA/ ha | D/ha | IVI | BA/ ha | D/ha | IVI | BA/ ha | D/ha | IVI | BA/ ha | D/ha | IVI | BA/ ha | D/ ha | |
| Gynocardia odorata R.Br. | Achariaceae | 2.6 | 0.05 | 2 | |||||||||||||||
| Brucea javanica (L.) Merr. | Anacardiaceae | 3.2 | 0.02 | 2 | |||||||||||||||
| Toxicodendron hookeri (K.C. Sahni & Bahadur) C. Y. Wu & T.L. Ming | Anacardiaceae | 3.3 | 0.04 | 2 | |||||||||||||||
| Rhus succedanea L. | Anacardiaceae | 12.4 | 1.0 | 24 | 3.3 | 0.04 | 2 | ||||||||||||
| Miliusa roxburghiana Hook.f. & Thomson | Annonaceae | 2.7 | 0.08 | 2 | |||||||||||||||
| Ilex embelioides Hook.f. | Aquifoliaceae | 2.5 | 0.1 | 2 | 8.8 | 0.5 | 32 | ||||||||||||
| Ilex excelsa (Wall.) Voigt | Aquifoliaceae | 2.8 | 0.2 | 2 | 2.7 | 0.0 | 4 | ||||||||||||
| Ilex khasiana Purkay. | Aquifoliaceae | 8.6 | 0.23 | 10 | |||||||||||||||
| Ilex venulosa Hook.f. | Aquifoliaceae | 21.9 | 1.0 | 74 | 2.3 | 0.1 | 2 | ||||||||||||
| Macropanax dispermus (Blume) Kuntze | Araliaceae | 2.6 | 0.04 | 2 | |||||||||||||||
| Merrilliopanax alpinus (C.B.Clarke) C.B.Shang | Araliaceae | 6.7 | 0.42 | 6 | |||||||||||||||
| Pentapanax sp. | Araliaceae | 2.0 | 0.0 | 2 | 3.9 | 0.1 | 4 | ||||||||||||
| Schefflera hypoleuca (Kurz) Harms | Araliaceae | 5.2 | 0.2 | 6 | 7.2 | 0.3 | 12 | 8.0 | 0.0 | 2 | 7.3 | 0.4 | 22 | ||||||
| Schefflera sp. | Araliaceae | 10.4 | 1.33 | 16 | |||||||||||||||
| Betula alnoides Buch.-Ham. ex D.Don | Betulaceae | 4.0 | 0.09 | 4 | |||||||||||||||
| Carpinus viminea Wall. ex Lindl. | Betulaceae | 3.8 | 0.2 | 4 | |||||||||||||||
| Euonymus sp1. | Celastraceae | 2.5 | 0.02 | 2 | |||||||||||||||
| Euonymus sp2. | Celastraceae | 2.4 | 0.0 | 2 | |||||||||||||||
| Microtropis discolor (Wall.) Arn. | Celastraceae | 2.0 | 0.0 | 2 | |||||||||||||||
| Calophyllum polyanthum Wall. ex Planch. & Triana | Clusiaceae | 21.1 | 2.78 | 32 | 7.9 | 0.3 | 18 | ||||||||||||
| Garcinia cowa Roxb. ex Choisy | Clusiaceae | 2.0 | 0.1 | 2 | |||||||||||||||
| Daphniphyllum himalayense (Benth.) Müll. Arg. | Daphniphyllaceae | 2.5 | 0.1 | 2 | 3.2 | 0.02 | 2 | ||||||||||||
| Diospyros sp. | Ebenaceae | 1.9 | 0.0 | 2 | |||||||||||||||
| Elaeocarpus sp1. | Elaeocarpaceae | 20.0 | 1.6 | 48 | |||||||||||||||
| Elaeocarpus acuminatus Wall. ex Mast. | Elaeocarpaceae | 4.5 | 0.2 | 6 | |||||||||||||||
| Elaeocarpus bracteatus Kurz | Elaeocarpaceae | 3.3 | 0.04 | 2 | |||||||||||||||
| Elaeocarpus floribundus Blume | Elaeocarpaceae | 4.7 | 0.3 | 6 | |||||||||||||||
| Elaeocarpus lanceifolius Roxb. | Elaeocarpaceae | 15.2 | 1.4 | 56 | |||||||||||||||
| Elaeocarpus prunifolius Wall. ex Müll. Berol. | Elaeocarpaceae | 5.9 | 0.2 | 10 | |||||||||||||||
| Elaeocarpus sp2. | Elaeocarpaceae | 2.6 | 0.03 | 2 | |||||||||||||||
| Lyonia ovalifolia (Wall.) Drude | Ericaceae | 4.7 | 0.15 | 6 | |||||||||||||||
| Rhododendron arboreum Sm. | Ericaceae | 1.9 | 0.0 | 2 | 16.8 | 1.41 | 22 | ||||||||||||
| Croton oblongus Burm.f. | Euphorbiaceae | 3.8 | 0.2 | 6 | |||||||||||||||
| Macaranga peltata (Roxb.) Müll.Arg. | Euphorbiaceae | 3.5 | 0.16 | 4 | |||||||||||||||
| Ostodes paniculata Blume | Euphorbiaceae | 16.8 | 0.85 | 36 | |||||||||||||||
| Castanopsis indica (Roxb. ex Lindl.) A.DC. | Fagaceae | 25.1 | 5.20 | 22 | |||||||||||||||
| Castanopsis purpurella (Miq.) N.P.Balakr. | Fagaceae | 3.8 | 0.2 | 6 | 5.1 | 0.4 | 6 | ||||||||||||
| Castanopsis tribuloides (Sm.) A.DC. | Fagaceae | 27.3 | 2.8 | 58 | 6.7 | 0.7 | 6 | 8.8 | 0.9 | 20 | |||||||||
| Lithocarpus dealbatus (Hook.f. & Thomson ex Miq.) Rehder | Fagaceae | 12.4 | 1.16 | 18 | 43.3 | 4.4 | 100 | 51.9 | 4.4 | 132 | 18.6 | 0.4 | 8 | 62.9 | 8.8 | 210 | 95.5 | 9.08 | 220 |
| Lithocarpus elegans (Blume) Hatus. ex Soepadmo | Fagaceae | 5.5 | 0.3 | 6 | 16.1 | 0.9 | 42 | ||||||||||||
| Quercus glauca Thunb. | Fagaceae | 19.0 | 1.7 | 40 | 2.5 | 0.1 | 2 | 2.2 | 0.0 | 4 | |||||||||
| Quercus griffithii Hook.f. & Thomson ex Miq. | Fagaceae | 10.0 | 0.69 | 6 | |||||||||||||||
| Quercus lineata Blume | Fagaceae | 185.3 | 25.8 | 224 | 12.5 | 1.4 | 32 | ||||||||||||
| Quercus semiserrata Roxb. | Fagaceae | 4.2 | 0.61 | 2 | |||||||||||||||
| Exbucklandia populnea (R.Br. ex Griff.) R.W.Br. | Hamamelidaceae | 3.0 | 0.1 | 2 | 5.3 | 0.6 | 12 | ||||||||||||
| Itea macrophylla Wall. | Iteaceae | 8.2 | 0.30 | 12 | |||||||||||||||
| Engelhardtia spicata Lechen ex Blume | Juglandaceae | 3.4 | 0.1 | 6 | 1.8 | 0.0 | 2 | 3.5 | 0.08 | 2 | |||||||||
| Callicarpa arborea Roxb. | Lamiaceae | 2.8 | 0.10 | 2 | |||||||||||||||
| Alseodaphne khasyana (Meisn.) Kosterm. | Lauraceae | 8.5 | 0.62 | 10 | |||||||||||||||
| Beilschmiedia assamica Meisn. | Lauraceae | 11.4 | 0.8 | 18 | |||||||||||||||
| Cinnamomum cassia (L.) J.Presl | Lauraceae | 2.3 | 0.0 | 2 | 26.9 | 2.5 | 118 | 3.3 | 0.04 | 2 | |||||||||
| Cinnamomum glanduliferum (Wall.) Meisn. | Lauraceae | 9.3 | 0.37 | 10 | |||||||||||||||
| Cinnamomum sp. | Lauraceae | 10.8 | 0.7 | 22 | |||||||||||||||
| Cinnamomum tamala (Buch.-Ham.) T.Nees & Eberm. | Lauraceae | 14.6 | 1.52 | 22 | 11.3 | 0.7 | 20 | ||||||||||||
| Cinnamomum verum J.Presl | Lauraceae | 1.8 | 0.0 | 2 | |||||||||||||||
| Iteadaphne caudata (Nees) H.W. Li | Lauraceae | 3.2 | 0.02 | 2 | |||||||||||||||
| Lindera caudata (Nees) Hook. f. | Lauraceae | 3.7 | 0.04 | 4 | |||||||||||||||
| Lindera latifolia Hook. f. | Lauraceae | 11.3 | 2.25 | 4 | 1.9 | 0.0 | 2 | ||||||||||||
| Lindera sp. | Lauraceae | 1.9 | 0.0 | 2 | |||||||||||||||
| Lindera nacusua (D. Don) Merr. | Lauraceae | 2.5 | 0.1 | 2 | |||||||||||||||
| Litsea sp1. | Lauraceae | 2.4 | 0.1 | 2 | |||||||||||||||
| Litsea sp2. | Lauraceae | 2.2 | 0.1 | 2 | |||||||||||||||
| Litsea sp3. | Lauraceae | 2.0 | 0.0 | 2 | |||||||||||||||
| Litsea elongata (Nees) Hook. f. | Lauraceae | 2.3 | 0.1 | 2 | 8.0 | 0.5 | 10 | 6.0 | 0.3 | 14 | |||||||||
| Litsea sp4. | Lauraceae | 2.0 | 0.1 | 2 | |||||||||||||||
| Litsea sp5. | Lauraceae | 2.8 | 0.11 | 2 | |||||||||||||||
| Machilus duthiei King | Lauraceae | 2.8 | 0.10 | 2 | |||||||||||||||
| Neolitsea umbrosa (Nees) Gamble | Lauraceae | 2.7 | 0.1 | 4 | |||||||||||||||
| Ocotea lancifolia (Schott) Mez | Lauraceae | 5.2 | 0.3 | 8 | |||||||||||||||
| Persea sp3 | Lauraceae | 3.6 | 0.1 | 6 | |||||||||||||||
| Persea odoratissima (Nees) Kosterm. | Lauraceae | 27.8 | 5.96 | 24 | 2.2 | 0.1 | 2 | 18.4 | 1.1 | 50 | 22.4 | 1.9 | 102 | 3.3 | 0.04 | 2 | |||
| Persea sp1. | Lauraceae | 8.0 | 0.0 | 2 | |||||||||||||||
| Persea sp2. | Lauraceae | 3.9 | 0.08 | 4 | |||||||||||||||
| Albizia sp. | Leguminosae | 1.9 | 0.0 | 2 | |||||||||||||||
| Pongamia pinnata (L.) Pierre | Leguminosae | 2.9 | 0.15 | 2 | |||||||||||||||
| Magnolia sp4. | Magnoliaceae | 7.0 | 0.3 | 10 | |||||||||||||||
| Magnolia sp3. | Magnoliaceae | 2.0 | 0.1 | 2 | |||||||||||||||
| Magnolia insignis Wall. | Magnoliaceae | 3.1 | 0.1 | 6 | 15.6 | 1.3 | 62 | ||||||||||||
| Magnolia punduana (Hook.f. & Thomson) Figlar | Magnoliaceae | 6.7 | 0.8 | 6 | |||||||||||||||
| Magnolia sp2. | Magnoliaceae | 3.1 | 0.1 | 6 | |||||||||||||||
| Magnolia sp1. | Magnoliaceae | 2.3 | 0.1 | 2 | |||||||||||||||
| Manglietia caveana Hook.f. & Thomson | Magnoliaceae | 2.6 | 0.05 | 2 | |||||||||||||||
| Sterculia roxburghiana Wall. | Malvaceae | 7.4 | 1.73 | 2 | |||||||||||||||
| Ficus auriculata Lour. | Moraceae | 2.2 | 0.0 | 2 | |||||||||||||||
| Ficus curtipes Corner | Moraceae | 3.9 | 0.52 | 2 | |||||||||||||||
| Ficus neriifolia Sm. | Moraceae | 6.1 | 0.3 | 14 | 3.2 | 0.02 | 2 | ||||||||||||
| Ficus nervosa B.Heyne ex Roth | Moraceae | 4.9 | 0.2 | 8 | |||||||||||||||
| Myrica esculenta Buch.-Ham. ex D. Don | Myricaceae | 3.0 | 0.2 | 2 | 8.8 | 0.5 | 12 | 21.1 | 0.9 | 10 | 8.1 | 0.7 | 18 | 25.9 | 3.18 | 24 | |||
| Knema sp. | Myristicaceae | 2.7 | 0.1 | 4 | |||||||||||||||
| Syzygium sp1. | Myrtaceae | 2.0 | 0.0 | 2 | |||||||||||||||
| Syzygium tetragonum (Wight) Wall. ex Walp. | Myrtaceae | 5.1 | 0.1 | 8 | 5.0 | 0.1 | 6 | 1.8 | 0.0 | 2 | |||||||||
| Olea salicifolia Wall. ex G.Don | Oleaceae | 3.3 | 0.2 | 4 | |||||||||||||||
| Eurya acuminata DC. | Pentaphylacaceae | 6.5 | 0.06 | 4 | |||||||||||||||
| Eurya cerasifolia (D.Don) Kobuski | Pentaphylacaceae | 1.9 | 0.0 | 2 | |||||||||||||||
| Eurya japonica Thunb. | Pentaphylacaceae | 2.3 | 0.0 | 2 | 7.9 | 0.0 | 2 | 2.2 | 0.1 | 4 | |||||||||
| Glochidion acuminatum Müll.Arg. | Phyllanthaceae | 2.8 | 0.1 | 8 | |||||||||||||||
| Glochidion sp. | Phyllanthaceae | 3.2 | 0.1 | 6 | |||||||||||||||
| Pinus kesiya Royle ex Gordon | Pinaceae | 9.0 | 0.3 | 2 | 31.9 | 2.35 | 72 | ||||||||||||
| Ardisia sp. | Primulaceae | 2.3 | 0.0 | 2 | |||||||||||||||
| Myrsine semiserrata Wall. | Primulaceae | 2.1 | 0.1 | 2 | 5.9 | 0.1 | 10 | ||||||||||||
| Helicia nilagirica Bedd. | Proteaceae | 12.5 | 0.8 | 30 | 23.8 | 1.3 | 76 | 4.5 | 0.1 | 8 | |||||||||
| Helicia robusta (Roxb.) R.Br. ex Blume | Proteaceae | 20.0 | 2.79 | 28 | |||||||||||||||
| Docynia indica (Wall.) Decne. | Rosaceae | 1.9 | 0.0 | 2 | |||||||||||||||
| Eriobotrya sp. | Rosaceae | 2.1 | 0.1 | 2 | |||||||||||||||
| Photinia integrifolia Lindl. | Rosaceae | 2.9 | 0.1 | 2 | |||||||||||||||
| Prunus cerasoides Buch.-Ham. ex D.Don | Rosaceae | 3.2 | 0.02 | 2 | |||||||||||||||
| Prunus phaeosticta (Hance) Maxim. | Rosaceae | 1.9 | 0.0 | 2 | |||||||||||||||
| Hyptianthera stricta (Roxb. ex Schult.) Wight & Arn. | Rubiaceae | 2.1 | 0.1 | 2 | |||||||||||||||
| Meyna spinosa Roxb. ex Link | Rubiaceae | 2.8 | 0.11 | 2 | |||||||||||||||
| Psychotria sp. | Rubiaceae | 4.1 | 0.1 | 4 | |||||||||||||||
| Psychotria symplocifolia Kurz | Rubiaceae | 2.1 | 0.1 | 2 | |||||||||||||||
| Wendlandia sp2. | Rubiaceae | 1.9 | 0.02 | 2 | |||||||||||||||
| UNK 11 | Rubiaceae | 8.9 | 1.37 | 4 | |||||||||||||||
| UNK 12 | Rubiaceae | 2.0 | 0.03 | 2 | |||||||||||||||
| Randia sp. | Rubiaceae | 5.5 | 0.20 | 4 | |||||||||||||||
| Tarennoidea wallichii (Hook.f.) Tirveng. & Sastre | Rubiaceae | 2.5 | 0.1 | 6 | |||||||||||||||
| Wendlandia sp. | Rubiaceae | 2.6 | 0.05 | 2 | |||||||||||||||
| Acronychia pedunculata (L.) Miq. | Rutaceae | 8.0 | 0.4 | 12 | |||||||||||||||
| Casearia glomerata Roxb. | Salicaceae | 6.0 | 0.15 | 6 | 7.0 | 0.2 | 12 | ||||||||||||
| Acer laevigatum Wall. | Sapindaceae | 6.4 | 0.3 | 10 | 4.2 | 0.1 | 6 | 3.3 | 0.04 | 2 | |||||||||
| Aesculus assamica Griff. | Sapindaceae | 9.0 | 1.20 | 6 | |||||||||||||||
| Sarcosperma griffithii Hook.f. ex C.B.Clarke | Sapotaceae | 6.0 | 0.16 | 6 | 7.2 | 0.34 | 14 | 8.2 | 0.3 | 16 | |||||||||
| Illicium griffithii Hook.f. & Thomson | Schisandraceae | 1.9 | 0.0 | 2 | |||||||||||||||
| Styrax hookeri C.B. Clarke | Styracaceae | 3.2 | 0.1 | 4 | |||||||||||||||
| Styrax sp1. | Styracaceae | 5.3 | 0.2 | 8 | |||||||||||||||
| Styrax serrulatus Roxb. | Styracaceae | 3.6 | 0.18 | 4 | 2.0 | 0.03 | 2 | 8.1 | 0.4 | 12 | |||||||||
| Symplocos sp. | Symplocaceae | 1.8 | 0.0 | 2 | |||||||||||||||
| Symplocos paniculata (Thunb.) Miq. | Symplocaceae | 3.2 | 0.2 | 8 | 7.1 | 0.10 | 6 | ||||||||||||
| Symplocos cochinchinensis var. laurina (Retz.) Noot. | Symplocaceae | 5.1 | 0.13 | 8 | 7.9 | 0.0 | 2 | 5.6 | 0.2 | 14 | 3.4 | 0.05 | 2 | ||||||
| Symplocos sp1. | Symplocaceae | 1.8 | 0.0 | 2 | |||||||||||||||
| Camellia caudata Wall. | Theaceae | 11.3 | 0.1 | 10 | |||||||||||||||
| Schima wallichii Choisy | Theaceae | 28.1 | 4.18 | 42 | 6.1 | 0.40 | 14 | 5.3 | 0.2 | 12 | 22.9 | 1.0 | 14 | 10.7 | 0.9 | 34 | 29.1 | 2.30 | 60 |
| UNK 1 | UNK 1 | 2.60 | 0.04 | 2 | |||||||||||||||
| UNK 10 | UNK 10 | 2.0 | 0.06 | 2 | |||||||||||||||
| UNK 2 | UNK 2 | 2.64 | 0.05 | 2 | |||||||||||||||
| UNK 3 | UNK 3 | 3.0 | 0.23 | 2 | |||||||||||||||
| UNK 4 | UNK 4 | 2.1 | 0.06 | 2 | |||||||||||||||
| UNK 5 | UNK 5 | 2.2 | 0.07 | 2 | |||||||||||||||
| UNK 6 | UNK 6 | 3.2 | 0.21 | 4 | |||||||||||||||
| UNK 7 | UNK 7 | 2.2 | 0.08 | 2 | |||||||||||||||
| UNK 8 | UNK 8 | 2.3 | 0.08 | 2 | |||||||||||||||
| UNK 9 | UNK 9 | 2.4 | 0.04 | 2 | |||||||||||||||
| Leea alata Edgew. | Vitaceae | 2.57 | 0.03 | 2 | |||||||||||||||
| Grand Total | 300 | 35 | 338 | 300 | 21 | 524 | 300 | 16 | 608 | 300 | 29 | 276 | 300 | 25 | 868 | 300 | 21 | 478 | |
References
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth ’ s ecosystems. Appl. Ecol. 2015, 1–9. [Google Scholar]
- Pereira, H.M.; Leadley, P.W.; Proenca, V.; Alkemade, R.; Scharlemann, J.P.W.; Fernandez-Manjarres, J.F.; Araujo, M.B.; Balvanera, P.; Biggs, R.; Cheung, W.W.L.; et al. Scenarios for Global Biodiversity in the 21st Century. Science 2010, 330, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L. Second Growth; University of Chicago Press: Chicago, IL, USA, 2014; ISBN 9780226118079. [Google Scholar]
- Foley, J.A. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Calmon, M.; Brancalion, P.H.S.; Paese, A.; Aronson, J.; Castro, P.; da Silva, S.C.; Rodrigues, R.R. Emerging Threats and Opportunities for Large-Scale Ecological Restoration in the Atlantic Forest of Brazil. Restor. Ecol. 2011, 19, 154–158. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond Deforestation: Restoring Forests and Ecosystem Services on Degraded Lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Crouzeilles, R.; Ferreira, M.S.; Chazdon, R.L.; Lindenmayer, D.B.; Sansevero, J.B.B.; Monteiro, L.; Iribarrem, A.; Latawiec, A.E.; Strassburg, B.B.N. Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci. Adv. 2017, 3, e1701345. [Google Scholar] [CrossRef]
- Mansourian, S.; Stanturf, J.A.; Derkyi, M.A.A.; Engel, V.L. Forest Landscape Restoration: increasing the positive impacts of forest restoration or simply the area under tree cover? Restor. Ecol. 2017, 25, 178–183. [Google Scholar] [CrossRef]
- Suding, K.; Higgs, E.; Palmer, M.; Callicott, J.B.; Anderson, C.B.; Baker, M.; Gutrich, J.J.; Hondula, K.L.; LaFevor, M.C.; Larson, B.M.H.; et al. Committing to ecological restoration. Science 2015, 348, 638–640. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Guariguata, M.R. Natural regeneration as a tool for large-scale forest restoration in the tropics: prospects and challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Barr, C.M.; Sayer, J.A. The political economy of reforestation and forest restoration in Asia-Pacific: Critical issues for REDD+. Biol. Conserv. 2012, 154, 9–19. [Google Scholar] [CrossRef]
- Tesfaye, G.; Teketay, D.; Fetene, M.; Beck, E. Regeneration of seven indigenous tree species in a dry Afromontane forest, southern Ethiopia. Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 135–143. [Google Scholar] [CrossRef]
- Rahman, M.; Nishat, A.; Vacik, H. Anthropogenic disturbances and plant diversity of the Madhupur Sal forests ( Shorea robusta C.F. Gaertn) of Bangladesh. Int. J. Biodivers. Sci. Manag. 2009, 5, 162–173. [Google Scholar] [CrossRef]
- Dey, D.C. Sustaining Oak Forests in Eastern North America: Regeneration and Recruitment, the Pillars of Sustainability. For. Sci. 2014, 60, 926–942. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Kammesheidt, L. The role of tree sprouts in the restorations of stand structure and species diversity in tropical moist forest after slash-and-burn agriculture in Eastern Paraguay. Plant Ecol. 1998, 139, 155–165. [Google Scholar] [CrossRef]
- Khan, M.L.; Menon, S.; Bawa, K.S. Effectiveness of the protected area network in biodiversity conservation: a case-study of Meghalaya state. Biodivers. Conserv. 1997, 6, 853–868. [Google Scholar] [CrossRef]
- Saxena, A.K.; Singh, J.S. Tree population structure of certain Himalayan forest associations and implications concerning their future composition. Vegetatio 1984, 58, 61–69. [Google Scholar] [CrossRef]
- Saikia, P.; Khan, M. Population structure and regeneration status of Aquilaria malaccensis Lam. in homegardens of Upper Assam, northeast India. Trop. Ecol. 2013, 54, 1–13. [Google Scholar]
- Khan, M.L.; Tripathi, R.S. Tree regeneration in a disturbed sub-tropical wet hill forest of north-east India: Effect of stump diameter and height on sprouting of Four tree species. For. Ecol. Manag. 1986, 17, 199–209. [Google Scholar] [CrossRef]
- Soepadmo, E. Fagaceae. ‘Flora Malesiana Vol. 7’. Ser. 1.; van Steenis, C.G.G.J., Ed.; Wolters-Noordhoff Publisher: Groningen, The Netherlands, 1972; Volume 7, pp. 265–403. [Google Scholar]
- Van Benthem, F.; Clarke, G.C.S.; Punt, W. Fagaceae. Rev. Palaeobot. Palynol. 1984, 42, 87–110. [Google Scholar] [CrossRef]
- Manos, P.S.; Stanford, A.M. The Historical Biogeography of Fagaceae: Tracking the Tertiary History of Temperate and Subtropical Forests of the Northern Hemisphere. Int. J. Plant Sci. 2001, 162, S77–S93. [Google Scholar] [CrossRef]
- Kremer, A.; Abbott, A.G.; Carlson, J.E.; Manos, P.S.; Plomion, C.; Sisco, P.; Staton, M.E.; Ueno, S.; Vendramin, G.G. Genomics of Fagaceae. Tree Genet. Genomes 2012, 8, 583–610. [Google Scholar] [CrossRef]
- Aoki, K.; Ueno, S.; Kamijo, T.; Setoguchi, H.; Murakami, N.; Kato, M.; Tsumura, Y. Genetic Differentiation and Genetic Diversity of Castanopsis (Fagaceae), the Dominant Tree Species in Japanese Broadleaved Evergreen Forests, Revealed by Analysis of EST-Associated Microsatellites. PLoS One 2014, 9, e87429. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.H.; Brendel, O.; Deng, M.; Hipp, A.L.; Kremer, A.; Kua, C.-S.; Plomion, C.; Romero-Severson, J.; Sork, V.L. Gaining a global perspective on Fagaceae genomic diversification and adaptation. New Phytol. 2018, 218, 894–897. [Google Scholar] [CrossRef]
- Johnson, W.C.; Webb, T. The Role of Blue Jays (Cyanocitta cristata L.) in the Postglacial Dispersal of Fagaceous Trees in Eastern North America. J. Biogeogr. 1989, 16, 561. [Google Scholar] [CrossRef]
- Cavender-Bares, J. Diversity, distribution and ecosystem services of the North American oaks. Int. Oaks 2016, 27, 37–48. [Google Scholar]
- Kappelle, M. Neotropical Montane Oak Forests: Overview and Outlook. In Ecology and Conservation of Neotropical Montane Oak Forests; Springer: Berlin/Heidelberg, Germany, 2004; Volume 185, pp. 449–467. [Google Scholar]
- Li, Q.; Ma, K. Factors affecting establishment of Quercus liaotungensis Koidz. under mature mixed oak forest overstory and in shrubland. For. Ecol. Manage. 2003, 176, 133–146. [Google Scholar] [CrossRef]
- Pulido, F.J.; Díaz, M. Regeneration of a Mediterranean oak: A whole-cycle approach. Écoscience 2005, 12, 92–102. [Google Scholar] [CrossRef]
- Johnson, P.S.; Shifley, S.R.; Rogers, R.; Dey, D.C.; Kabrick, J.M. The Ecology and Silviculture of Oaks; Cabi: London, UK, 2019; ISBN 1780647085. [Google Scholar]
- Singh, S.P.; Rawat, Y.S.; Garkoti, S.C. Failure of brown oak (Quercus semecarpifolia) to regenerate in central Himalaya: A case of environmental semisurprise. Curr. Sci. 1997, 73, 371–374. [Google Scholar]
- Singh, B.; Singh, B. Fagaceae contribution to floral wealth of Himalaya: Checklist on diversity and distribution in North-eastern states of India. Curr. Life Sci. 2016, 2, 72–78. [Google Scholar]
- Troup, R.S. The Silviculture of Indian Trees; Oxford at the Clarendon Press: Oxford, UK, 1921; Volume III. [Google Scholar]
- Stephenson, S.L.; Saxena, A.K. A Comparative Study of Oak-Dominated Forests in the Mid-Appalachians of the Eastern United States and the Kumaun Himalaya of Northern India. Bull. Torrey Bot. Club 1994, 121, 369. [Google Scholar] [CrossRef]
- Rao, P.; Barik, S.K.; Pandey, H.N.; Tripathi, R.S. Community Composition and Tree Population Structure in a Sub-Tropical Broad-Leaved Forest along a Disturbance Gradient. Vegetatio 1990, 88, 151–162. [Google Scholar] [CrossRef]
- Jamir, S.A.; Pandey, H.N. Vascular plant diversity in the sacred groves of Jaintia Hills in northeast India. Biodivers. Conserv. 2003, 12, 1497–1510. [Google Scholar] [CrossRef]
- Mishra, B.P.; Tripathi, O.P.; Laloo, R.C. Community characteristics of a climax subtropical humid forest of Meghalaya and population structure of ten important tree species. Trop. Ecol. 2005, 46, 241–251. [Google Scholar]
- Tripathi, O.P.; Tripathi, R.S. Community composition, structure and management of subtropical vegetation of forests in Meghalaya State, northeast India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2010, 6, 157–163. [Google Scholar] [CrossRef]
- Upadhaya, K. Structure and Floristic Composition of Subtropical Broad-Leaved Humid Forest of Cherapunjee in Meghalaya, Northeast India. J. Biodivers. Manag. For. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Khan, M.L.; Rai, J.P.N.; Tripathi, R.S. Regeneration and survival of tree seedlings and sprouts in tropical deciduous and sub-tropical forests of Meghalaya, India. For. Ecol. Manag. 1986, 14, 293–304. [Google Scholar] [CrossRef]
- Barik, S.K.; Pandey, H.N.; Tripathi, R.S.; Rao, P. Microenvironmental Variability and Species-Diversity in Treefall Gaps in a Subtropical Broadleaved Forest. Vegetatio 1992, 103, 31–40. [Google Scholar]
- Barik, S.K.; Tripathi, R.S.; Pandey, H.N.; Rao, P. Tree Regeneration in a Subtropical Humid Forest: Effect of Cultural Disturbance on Seed Production, Dispersal and Germination. J. Appl. Ecol. 1996, 33, 1551–1560. [Google Scholar] [CrossRef]
- Maithani, K.; Tripathi, R.S.; Arunachalam, A.; Pandey, H.N. Seasonal dynamics of microbial biomass C, N and P during regrowth of a disturbed subtropical humid forest in north-east India. Appl. Soil Ecol. 1996, 4, 73–80. [Google Scholar] [CrossRef]
- Mylliemngap, W.; Nath, D.; Barik, S.K. Changes in vegetation and nitrogen mineralization during recovery of a montane subtropical broadleaved forest in North-eastern India following anthropogenic disturbance. Ecol. Res. 2016, 31, 21–38. [Google Scholar] [CrossRef]
- Maithani, K.; Arunachalam, A.; Tripathi, R.S.; Pandey, H.N. Influence of leaf litter quality on N mineralization in soils of subtropical humid forest regrowths. Biol. Fertil. Soils 1998, 27, 44–50. [Google Scholar] [CrossRef][Green Version]
- Arunachalam, A.; Arunachalam, K.; Pandey, H.N.; Tripathi, R.S. Fine litterfall and nutrient dynamics during forest regrowth in the humid subtropics of north-eastern India. For. Ecol. Manage. 1998, 110, 209–219. [Google Scholar] [CrossRef]
- Sarma, K.; Kushwaha, S.P.S.; Singh, K.J. Impact of coal mining on plant diversity and tree population structure in Jaintia Hills district of Meghalaya, North East India. New York Sci. J. 2010, 3, 79–85. [Google Scholar]
- Forest Survey of India. India State of Forest Report; Forest Survey of India: Dehradun, India, 2009. [Google Scholar]
- Forest Survey of India. India State of Forest Report; Forest Survey of India: Dehradun, India, 2017. [Google Scholar]
- Shankar, U.; Boral, L.; Pandey, H.N.; Tripathi, R.S. Degradation of land due to coal mining and its natural recovery pattern. Curr. Sci. 1993, 65, 680–687. [Google Scholar]
- Pao, N.T.; Upadhaya, K. Effect of fragmentation and anthropogenic disturbances on floristic composition and structure of subtropical broad leaved humid forest in Meghalaya, Northeast India. Appl. Ecol. Environ. Res. 2017, 15, 385–407. [Google Scholar] [CrossRef]
- Poffenberger, M. Land Tenure and Forest Carbon Management in India: A Khasi Approach to REDD+ Project Development. In Local responses to global issues; International Union of Forest Research Organizations (IUFRO): Vienna, Austria, 2014; pp. 229–240. [Google Scholar]
- Forest Survey of India. India State of Forest Report; Forest Survey of India: Dehradun, India, 2019. [Google Scholar]
- Sarma, K. Land ownership, administration and status of forests of Khasi Hills Autonomous District Council of Meghalaya. NeBIO 2010, 1, 8–13. [Google Scholar]
- Chandramouli, C.; General, R. Census of India; Government of India: New Delhi, India, 2011.
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2002, 403, 853–858. [Google Scholar] [CrossRef]
- Balakrishnan, N.P. Flora of Jowai and vicinity, Meghalaya; Botanical Survey of India: Howrah, India, 1981; Volume I.
- Balakrishnan, N.P. Flora of Jowai and vicinity, Meghalaya; Botanical Survey of India: Howrah, India, 1983; Volume II.
- Haridasan, K.; Rao, R.R. Forest Flora of Meghalaya; Bishen Singh and Mahendrapal Singh: Dehradun, India, 1985; Volume I. [Google Scholar]
- Haridasan, K.; Rao, R.R. Forest Flora of Meghalaya; Bishen Singh Mahendra Pal Singh: Dehra Dun, India, 1985; Volume II. [Google Scholar]
- Cordonnier, T.; Kunstler, G. The Gini index brings asymmetric competition to light. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 107–115. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1974; ISBN 0632003219. [Google Scholar]
- Misra, R.; Misra, R. Ecology Workbook; Oxford and IBH Publishing Co.: Calcutta, India, 1968. [Google Scholar]
- Muller-Dombois, D.; Ellenberg, H. Measuring Species Quantites. In Aims and Methods of Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1974; pp. 67–92. [Google Scholar]
- Lei, P.; Scherer-Lorenzen, M.; Bauhus, J. The effect of tree species diversity on fine-root production in a young temperate forest. Oecologia 2012, 169, 1105–1115. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Gini, C. Variabilità e mutabilità. Reprinted in E. Pizetti & T. Salvemini. Mem. Metodol. Stat. 1912. [Google Scholar] [CrossRef]
- Lexerød, N.L.; Eid, T. An evaluation of different diameter diversity indices based on criteria related to forest management planning. For. Ecol. Manage. 2006, 222, 17–28. [Google Scholar] [CrossRef]
- Shankar, U. A case of high tree diversity in a sal (Shorea robusta) -dominated lowland forest of Eastern Himalaya: Floristic composition, regeneration and conservation. Curr. Sci. 2001, 81, 776–786. [Google Scholar]
- Saha, S.; Kuehne, C.; Bauhus, J. Intra- and interspecific competition differently influence growth and stem quality of young oaks (Quercus robur L. and Quercus petraea (Mattuschka) Liebl.). Ann. For. Sci. 2014, 71, 381–393. [Google Scholar] [CrossRef]
- Chakraborty, T.; Saha, S.; Matzarakis, A.; Reif, A. Influence of multiple biotic and abiotic factors on the crown die-back of European beech trees at their drought limit. Flora 2017, 229, 58–70. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Echeverría, C.; Newton, A.C.; Lara, A.; Benayas, J.M.R.; Coomes, D.A. Impacts of forest fragmentation on species composition and forest structure in the temperate landscape of southern Chile. Glob. Ecol. Biogeogr. 2007, 16, 426–439. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Tabarelli, M.; Cardoso da Silva, J.M.; Gascon, C. Forest fragmentation, synergisms and the impoverishment of neotropical forests. Biodivers. Conserv. 2004, 13, 1419–1425. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; van Dijk, P.P.; Rhodin, A.G.J.; Nash, S.D. Turtle Hotspots: An Analysis of the Occurrence of Tortoises and Freshwater Turtles in Biodiversity Hotspots, High-Biodiversity Wilderness Areas, and Turtle Priority Areas. Chelonian Conserv. Biol. 2015, 14, 2–10. [Google Scholar] [CrossRef]
- Li, X.-S.; Liu, W.-Y.; Chen, J.-W.; Tang, C.Q.; Yuan, C.-M. Regeneration pattern of primary forest species across forest-field gradients in the subtropical Mountains of Southwestern China. J. Plant Res. 2010, 123, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, K.; Pandey, H.N.; Law, P.S.; Tripathi, R.S. Tree diversity in sacred groves of the Jaintia hills in Meghalaya, northeast India. Biodivers. Conserv. 2003, 12, 583–597. [Google Scholar] [CrossRef]
- Shaw, T.E. Species diversity in restoration plantings: Important factors for increasing the diversity of threatened tree species in the restoration of the Araucaria forest ecosystem. Plant Divers. 2019, 41, 84–93. [Google Scholar] [CrossRef]
- Lu, Y.; Ranjitkar, S.; Harrison, R.D.; Xu, J.; Ou, X.; Ma, X.; He, J. Selection of Native Tree Species for Subtropical Forest Restoration in Southwest China. PLoS ONE 2017, 12, e0170418. [Google Scholar] [CrossRef]
- Aldrich, P.R.; Parker, G.R.; Romero-severson, J.; Michler, C.H. Confirmation of Oak Recruitment Failure in Indiana Old-Growth Forest: 75 Years of Data. For. Sci. 2005, 51, 406–416. [Google Scholar]
- Watt, A.A.S. On the Causes of Failure of Natural Regeneration in British Oakwoods. J. Ecol. 1919, 7, 173–203. [Google Scholar] [CrossRef]
- Li, Y.; Lo, Y.; Lin, Y.; Guan, B.; Blanco, J.; You, C. Bringing the Natives Back: Identifying and Alleviating Establishment Limitations of Native Hardwood Species in a Conifer Plantation. Forests 2018, 9, 3. [Google Scholar] [CrossRef]
- Loewenstein, E.F.; Johnson, P.S.; Garrett, H.E. Age and diameter structure of a managed uneven-aged oak forest. Can. J. For. Res. 2011, 30, 1060–1070. [Google Scholar] [CrossRef]
- Manjaribe, C.; Frasier, C.L.; Rakouth, B.; Louis, E.E. Ecological Restoration and Reforestation of Fragmented Forests in Kianjavato, Madagascar. Int. J. Ecol. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Kang, H.; Zheng, Y.; Liu, S.; Chai, Z.; Chang, M.; Hu, Y.; Li, G.; Wang, D. Population structure and spatial pattern of predominant tree species in a pine–oak mosaic mixed forest in the Qinling Mountains, China. J. Plant Interact. 2017, 12, 78–86. [Google Scholar] [CrossRef]
- Fashing, P.J.; Forrestel, A.; Scully, C.; Cords, M. Long-term tree population dynamics and their implications for the conservation of the Kakamega Forest, Kenya. Biodivers. Conserv. 2004, 13, 753–771. [Google Scholar] [CrossRef]
- Rawat, Y.S.; Singh, J.S. Structure and Function of Oak Forests in Central Himalaya. I. Dry Matter Dynamics. Ann. Bot. 1988, 62, 397–411. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J. Structure and function of the Central Himalayan oak forests. Proc. Plant Sci. 1986, 96, 159–189. [Google Scholar]
- Bellingham, A.P.J.; Tanner, E.V.J.; Healey, J.R. Sprouting of Trees in Jamaican Montane Forests, after a Hurricane. J. Ecol. 2009, 82, 747–758. [Google Scholar] [CrossRef]
- Sagar, R.; Singh, J.S. Tree density, basal area and species diversity in a disturbed dry tropical forest of northern India: implications for conservation. Environ. Conserv. 2006, 33, 256–262. [Google Scholar] [CrossRef]
- Enquist, B.J.; West, G.B.; Charnov, E.L.; Brown, J.H. Allometric scaling of production and life-history variation in vascular plants. Nature 1999, 401, 907–911. [Google Scholar] [CrossRef]
- Keren, S.; Medarević, M.; Obradović, S.; Zlokapa, B. Five Decades of Structural and Compositional Changes in Managed and Unmanaged Montane Stands: A Case Study from South-East Europe. Forests 2018, 9, 479. [Google Scholar] [CrossRef]
- Benton, A.H.; Werner, W.E. Field Biology and Ecology; McGraw-Hill: New York, NY, USA, 1974. [Google Scholar]
- Vlam, M.; van der Sleen, P.; Groenendijk, P.; Zuidema, P.A. Tree Age Distributions Reveal Large-Scale Disturbance-Recovery Cycles in Three Tropical Forests. Front. Plant Sci. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Odum, E.P.; Barrett, G.W. Fundamentals of Ecology; Saunders Philadelphia: Philadelphia, PA, USA, 1971; Volume 3. [Google Scholar]
- Singh, S.P.; Tewari, J.C.; Yadav, S.; Ralhan, P.K. Population structure of tree species in forests as an indicator of regeneration and future stability. Proc. Plant Sci. 1986, 96, 443–455. [Google Scholar]
- Manral, V.; Padalia, K.; Karki, H. Plant diversity and regeneration potential of three different forests of Central Himalaya. Curr. World Environ. 2018, 13, 113–123. [Google Scholar] [CrossRef]
- Lundqvist, L.; Nilson, K. Regeneration dynamics in an uneven-aged virgin Norway spruce forest in northern Sweden. Scand. J. For. Res. 2007, 22, 304–309. [Google Scholar] [CrossRef]
- Singh, S.; Malik, Z.A.; Sharma, C.M. Tree species richness, diversity, and regeneration status in different oak (Quercus spp.) dominated forests of Garhwal Himalaya, India. J. Asia-Pacific Biodivers. 2016, 9, 293–300. [Google Scholar] [CrossRef]
- Murali, K.S.; Shankar, U.; Shaanker, R.U.; Ganeshaiah, K.N.; Bawa, K.S. Extraction of non-timber forest products in the forests of Biligiri Rangan Hills, India. 2. Impact of NTFP extraction on regeneration, population structure, and species composition. Econ. Bot. 1996, 50, 252–269. [Google Scholar] [CrossRef]
- Götmark, F.; Berglund, Å.; Wiklander, K. Browsing damage on broadleaved trees in semi-natural temperate forest in Sweden, with a focus on oak regeneration. Scand. J. For. Res. 2005, 20, 223–234. [Google Scholar] [CrossRef]
- Weigel, D.R.; Peng, C.-Y.J. Predicting stump sprouting and competitive success of five oak species in southern Indiana. Can. J. For. Res. 2002, 32, 703–712. [Google Scholar] [CrossRef]
- Retana, J.; Riba, M.; Castell, C.; Espelta, J.M. Regeneration by Sprouting of Holm-Oak ( Quercus ilex ) Stands Exploited by Selection Thinning. Vegetatio 1992, 99, 355–364. [Google Scholar] [CrossRef]
- Keyser, T.L.; Zarnoch, S.J. Stump sprout dynamics in response to reductions in stand density for nine upland hardwood species in the southern Appalachian Mountains. For. Ecol. Manag. 2014, 319, 29–35. [Google Scholar] [CrossRef]
- Negreros-Castillo, P.; Hall, R.B. Sprouting capability of 17 tropical tree species after overstory removal in Quintana Roo, Mexico. For. Ecol. Manag. 2000, 126, 399–403. [Google Scholar] [CrossRef]
- Oliver, C.D. Forest development in North America following majir disturbances. Forest Ecology and Management. For. Ecol. Manag. 1981, 3, 153–168. [Google Scholar] [CrossRef]
- White, P.S. Pattern, process, and natural disturbance in vegetation. Bot. Rev. 1979, 45, 229–299. [Google Scholar] [CrossRef]
- Tiwari, O.P.; Rana, Y.S.; Krishan, R.; Sharma, C.M.; Bhandari, B.S. Regeneration dynamics, population structure, and forest composition in some ridge forests of the Western Himalaya, India. Forest Sci. Technol. 2018, 14, 66–75. [Google Scholar] [CrossRef]

| Site | Laitkynsew | Jarain | Pynursla | Weiloi | Laitryngew | Upper Shillong |
|---|---|---|---|---|---|---|
| Elevation range (meters above sea level (m.a.s.l.) | 800–1200 | 1200–1600 | 1600–2000 | |||
| Elevation (m.a.s.l.) | 903 | 1132 | 1368 | 1595 | 1636 | 1873 |
| Stand Size (ha) | 7.8 | 2.4 | 15.7 | 17.6 | 11.7 | 60.4 |
| Latitude (N) | 25°13.16′ | 25°18.55′ | 25°18.26′ | 25°21.32′ | 25°19.94′ | 25°32.35′ |
| Longitude (E) | 91°40.27′ | 92°7.88′ | 91°54.1′ | 91°36.53′ | 91°43.89′ | 91°51.18′ |
| Distance from the nearest village (km) | 0.83 | 1.26 | 0.47 | 0.59 | 0.51 | 1.07 |
| Parameter | Laitkynsew | Jarain | Pynursla | Weiloi | Laitryngew | Upper Shillong |
|---|---|---|---|---|---|---|
| Elevation (m) | 903 | 1132 | 1368 | 1599 | 1636 | 1873 |
| Species richness | 37 | 51 | 39 | 10 | 45 | 27 |
| Number of families | 23 | 24 | 24 | 8 | 22 | 17 |
| Number of genera | 37 | 40 | 32 | 10 | 32 | 23 |
| Tree basal area (m2 ha−1) | 35.3 | 20.69 | 16.48 | 28.69 | 24.7 | 20.6 |
| Tree density ha−1 | 338 | 524 | 608 | 276 | 868 | 478 |
| Shannon diversity index | 2.49 | 2.75 | 2.72 | 0.48 | 2.46 | 1.76 |
| Simpson dominance index | 0.12 | 0.10 | 0.11 | 0.81 | 0.16 | 0.26 |
| Pielou’s Evenness index | 0.46 | 0.46 | 0.54 | 0.24 | 0.36 | 0.31 |
| Bulk density (g/cm3) | 0.94 | 1.06 | 1.39 | 1.47 | 0.99 | 0.65 |
| Sites | Trees ha−1 | Stems ha−1 | Basal Area ha−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pole | Middle-Sized | Mature | Pole | Middle-Sized | Mature | Pole | Middle-Sized | Mature | ||
| Laitkynsew | Fag | 16 | 18 | 8 | 16 | 18 | 12 | 0.5 | 2.5 | 4 |
| Non-Fag | 202 | 72 | 22 | 214 | 76 | 26 | 6.7 | 9.9 | 11.7 | |
| Total | 218 (64.5) | 90 (26.6) | 30 (8.9) | 230 (63.5) | 94 (26.0) | 38 (10.5) | 7.1 (20.2) | 12.5 (35.4) | 15.7 (44.4) | |
| Jarain | Fag | 170 | 40 | 0 | 214 | 82 | 0 | 5.4 | 4 | 0 |
| Non-Fag | 290 | 22 | 2 | 320 | 26 | 2 | 7.6 | 2.8 | 0.8 | |
| Total | 460 (87.8) | 62 (11.8) | 2 (0.4) | 534 (82.9) | 108 (16.8) | 2 (0.3) | 13 (63.0) | 6.8 (32.9) | 0.8 (4.1) | |
| Pynursla | Fag | 170 | 18 | 0 | 236 | 56 | 0 | 3.5 | 2.9 | 0 |
| Non-Fag | 404 | 16 | 0 | 482 | 18 | 0 | 8.3 | 1.8 | 0 | |
| Total | 574 (94.4) | 34 (5.6) | 0 | 718 (90.7) | 74 (9.3) | 0 | 11.8 (72.6) | 4.7 (28.4) | 0 | |
| Weiloi | Fag | 98 | 116 | 18 | 148 | 396 | 84 | 3.9 | 15.8 | 6.5 |
| Non-Fag | 30 | 14 | 0 | 36 | 50 | 0 | 0.5 | 1.5 | 0.6 | |
| Total | 128 (46.4) | 130 (47.1) | 18 (6.5) | 184 (25.8) | 446 (62.5) | 84 (11.8) | 4.3 (15.1) | 17.3 (60.4) | 7 (24.4) | |
| Laitryngew | Fag | 226 | 40 | 0 | 428 | 190 | 0 | 6.9 | 4.2 | 0 |
| Non-Fag | 580 | 22 | 0 | 708 | 46 | 0 | 11.9 | 1.8 | 0 | |
| Total | 806 (92.9) | 62 (7.1) | 0 | 1136 (82.8) | 236 (17.2) | 0 | 18.8 (75.9) | 6 (24.1) | 0 | |
| Upper Shillong | Fag | 194 | 30 | 2 | 224 | 34 | 2 | 5 | 4.2 | 0.6 |
| Non-Fag | 204 | 48 | 0 | 236 | 58 | 0 | 5.3 | 5.6 | 0 | |
| Total | 398 (83.3) | 78 (16.3) | 2 (0.4) | 460 (83.0) | 92 (16.6) | 2 (0.4) | 10.2 (49.7) | 9.8 (47.5) | 0.6 (2.8) | |
| Total Fagaceae | 874 (28.3) | 262 (8.5) | 28 (0.9) | 1266 (28.5) | 776 (17.5) | 98 (2.2) | 25.2 (17.2) | 33.6 (22.9) | 11.1 (7.6) | |
| Total Non-Fagaceae | 1710 (55.3) | 194 (6.3) | 24 (0.8) | 1996 (45.0) | 274 (6.2) | 28 (0.6) | 40.3 (27.5) | 23.4 (16.0) | 13.1 (8.9) | |
| Total (Fag+ Non-Fag) | 2584 (83.6) | 456 (14.7) | 52 (1.7) | 3262 (73.5) | 1050 (23.7) | 126 (2.8) | 65.3 (44.6) | 57.0 (38.9) | 24.1 (16.5) | |
| Study Sites | Species Name | Basal Area (%) | Trees ha−1 | Regeneration Potential | ||||
|---|---|---|---|---|---|---|---|---|
| Seed | Sm. Sap. | L. Sap. | Pole | L. Tr. | ||||
| Laitkynsew | Castanopsis indica | 14.7 | 22 | 154 | 58 | 4 | 18 | Good |
| Castanopsis lanceifolia | - | 2 | 48 | 18 | New | |||
| Lithocarpus dealbatus | 3.3 | 2 | 44 | 6 | 12 | 6 | Good | |
| Lithocarpus elegans | - | 4 | 46 | 34 | New | |||
| Quercus semiserrata | 1.7 | 6 | 78 | 48 | 2 | Good | ||
| Relative Proportion (%) | 19.7 | 5.9 | 60.5 | 26.8 | 2.6 | 4.2 | ||
| Jarain | Castanopsis purpurella | 1.2 | 2 | 26 | 6 | Good | ||
| Castanopsis tribuloides | 13.3 | 46 | 120 | 62 | 44 | 14 | Good | |
| Lithocarpus dealbatus | 21.2 | 10 | 98 | 286 | 80 | 20 | Good | |
| Lithocarpus elegans | 1.4 | 14 | 84 | 4 | 2 | Poor | ||
| Quercus glauca | 8.4 | 2 | 18 | 26 | 36 | 4 | Good | |
| Relative Proportion (%) | 45.6 | 6 | 27.5 | 45.6 | 16.9 | 4 | ||
| Pynursla | Castanopsis purpurella | 2.3 | 8 | 22 | 16 | 4 | 2 | Good |
| Castanopsis tribuloides | 3.9 | 4 | 2 | None | ||||
| Lithocarpus dealbatus | 26.6 | 72 | 122 | 220 | 118 | 14 | Good | |
| Lithocarpus elegans | 5.6 | 34 | 38 | 14 | 42 | Good | ||
| Quercus glauca | 0.3 | 2 | None | |||||
| Relative Proportion (%) | 38.9 | 15.5 | 24.8 | 34.1 | 23.2 | 2.5 | ||
| Weiloi | Castanopsis tribuloides | - | 2 | 2 | New | |||
| Lithocarpus dealbatus | 1.4 | 26 | 62 | 24 | 6 | 2 | Good | |
| Quercus lineata | 89.8 | 64 | 34 | 22 | 92 | 132 | Fair | |
| Relative Proportion (%) | 91.2 | 19.2 | 20.9 | 10.3 | 20.9 | 28.6 | ||
| Laitryngew | Castanopsis tribuloides | 3.5 | 18 | 2 | None | |||
| Lithocarpus dealbatus | 35.6 | 42 | 218 | 206 | 178 | 32 | Good | |
| Quercus glauca | 0.2 | 4 | 4 | Poor | ||||
| Quercus lineata | 5.7 | 4 | 18 | 44 | 26 | 6 | Good | |
| Relative Proportion (%) | 44.9 | 5.7 | 29.4 | 31.7 | 28.2 | 5 | ||
| Upper Shillong | Castanopsis tribuloides | - | 2 | New | ||||
| Lithocarpus dealbatus | 44.1 | 58 | 290 | 240 | 190 | 30 | Good | |
| Quercus griffithii | 3.3 | 2 | 12 | 12 | 4 | 2 | Good | |
| Relative Proportion (%) | 47.4 | 7.1 | 35.9 | 30.2 | 23 | 3.8 | ||
| Stands | Disturbance Score Class * | Distance from the Nearest Village (Km) | Proportion of Stumps (%) | Proportion of Cut Stems (%) | Gini Index |
|---|---|---|---|---|---|
| Laitkynsew | 1 | 0.83 | 7.08 | 1.00 | 0.59 |
| Jarain | 3 | 1.26 | 17.84 | 18.42 | 0.44 |
| Pynursla | 3 | 0.47 | 24.06 | 10.51 | 0.46 |
| Weiloi | 3 | 0.59 | 35.20 | 71.28 | 0.47 |
| Laitryngew | 2 | 0.51 | 18.59 | 32.80 | 0.40 |
| Upper Shillong | 2 | 1.07 | 32.28 | 23.87 | 0.47 |
| Components | Mean tree ha−1 (T) | Mean Stems ha−1 (S) | Mean Healthy Stems ha−1 | Mean Cut Stems ha−1 | Mean Natural Damage Stem ha−1 | Sprouts (S-T) | Sprouting Percentage (S-T)/Total Sprouts X 100 | Sprouting Stem per Tree Ratio (S/T) |
|---|---|---|---|---|---|---|---|---|
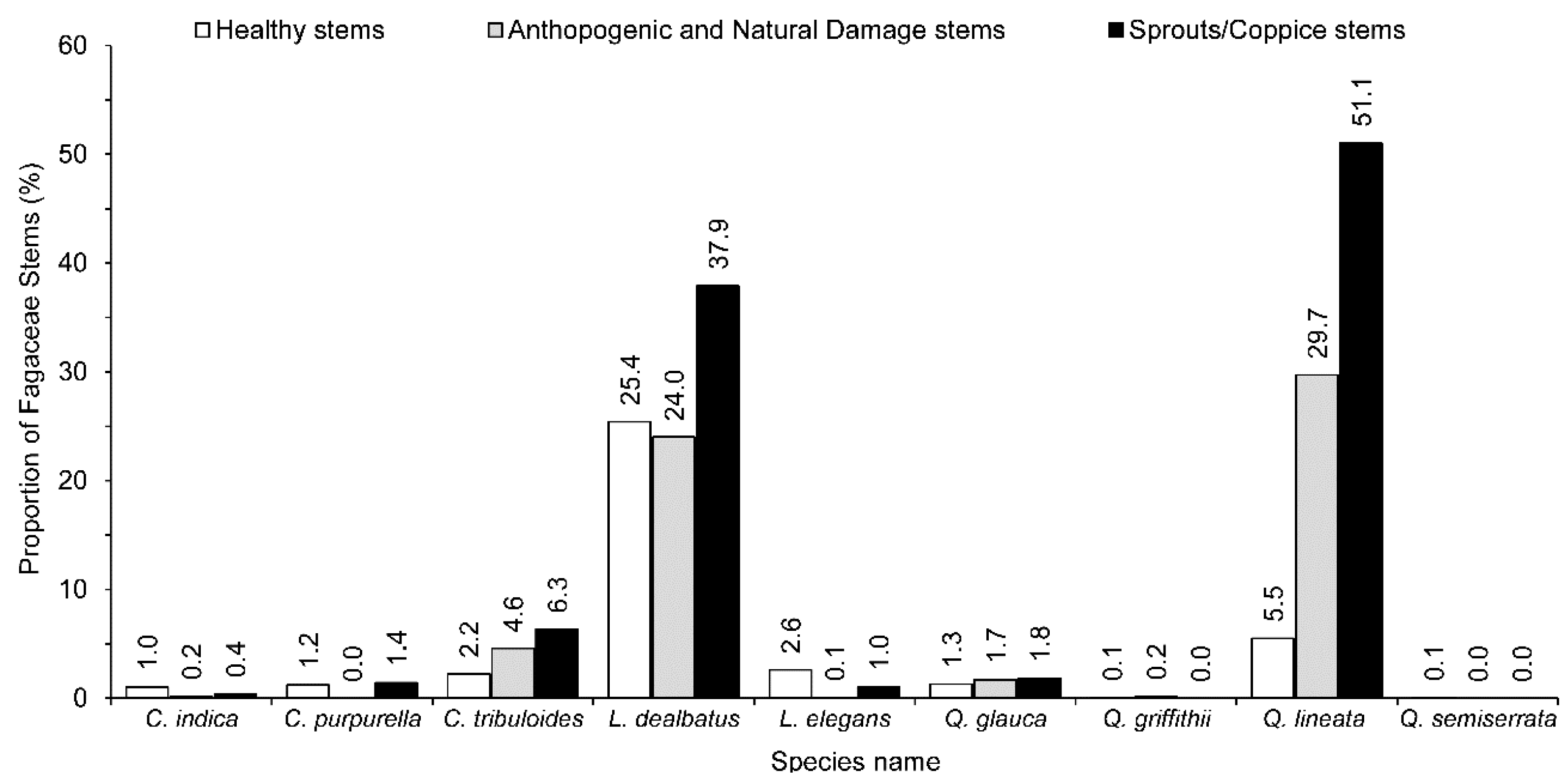
| Lithocarpus dealbatus | 114.7 | 176.3 | 90.7 (51.4) | 76.0 (43.1) | 9.7 (5.5) | 61.6 | 37.9 | 1.54 |
| Quercus lineata | 42.7 | 125.7 | 19.7 (15.7) | 102.3 (81.4) | 3.7 (2.9) | 83.0 | 51.1 | 2.94 |
| Castanopsis tribuloides | 14.0 | 24.3 | 8.0 (32.9) | 16.3 (67.1) | 0 (0.0) | 10.3 | 6.3 | 1.74 |
| Quercus glauca | 7.7 | 10.7 | 4.7 (43.9) | 4.3 (40.2) | 1.7 (15.9) | 3.0 | 1.8 | 1.39 |
| Lithocarpus elegans | 8.0 | 9.7 | 9.3 (95.9) | 0.3 (3.1) | 0 (0) | 1.7 | 1.0 | 1.21 |
| Castanopsis indica | 3.7 | 4.3 | 3.7 (86.0) | 0 (0) | 0.7 (16.3) | 0.6 | 0.4 | 1.16 |
| Castanopsis purpurella | 2.0 | 4.3 | 4.3 (100) | 0 (0) | 0 (0) | 2.3 | 1.4 | 2.15 |
| Quercus griffithii | 1.0 | 1.0 | 0.3 (33) | 0.7 (70.0) | 0 (0) | 0.0 | 0.0 | 1.00 |
| Quercus semiserrata | 0.3 | 0.3 | 0.3 (100) | 0 (0) | 0 (0) | 0.0 | 0.0 | 1.00 |
| Fagaceae Total | 194.1 (37.7) | 356.7 (48.2) | 141 (19.1) | 200 (27.0) | 15.8 (2.1) | 162.6 (45.6) | 45.6 | 1.84 |
| Non Fagaceae Total | 321.3 (62.4) | 383 (51.8) | 310 (41.9) | 64.7 (8.7) | 8.3 (1.1) | 61.7 (16.1) | 16.1 | 1.20 |
| Grand Total | 515.3 | 739.7 | 451 (61.0) | 264.7 (35.8) | 24.0 (3.2) | 224.4 | 30.3 | 1.44 |
| Target Variable | Independent Variables | Parameter Estimates | SE of Parameter Estimates | 95% Wald Confidence Interval of SE | Hypothesis Test | |||
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Wald Chi-Square | df | Sig. | ||||
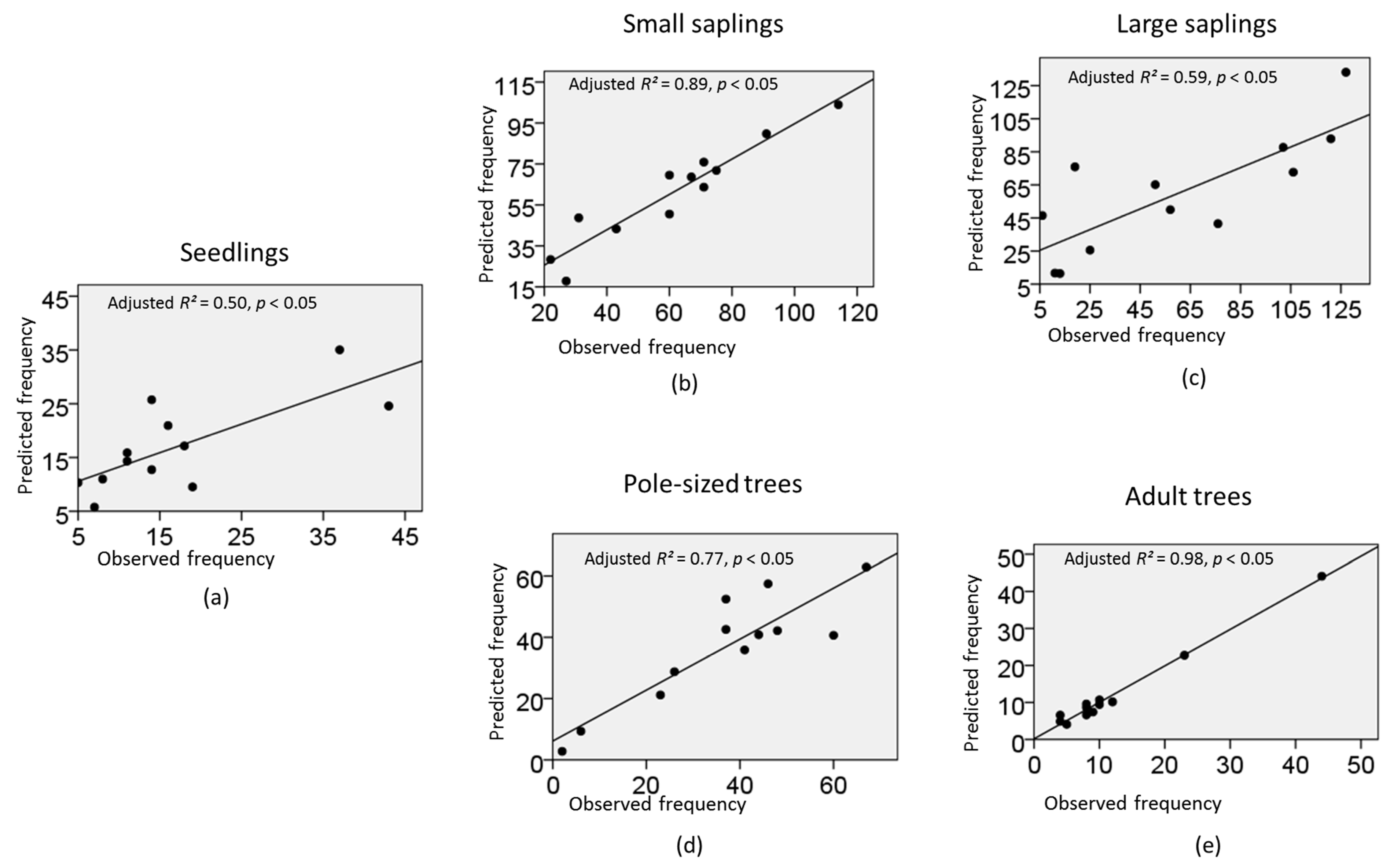
| Frequency of Fagaceae seedlings per plot | Intercept | −0.2924 | 1.3844 | −3.0058 | 2.4210 | 0.0446 | 1 | 0.8327 |
| Disturbance intensity | 0.5444 | 0.1342 | 0.2814 | 0.8075 | 16.4556 | 1 | 0.0000 | |
| Elevation * % of stumps * % of cut stem | −0.000001 | 0.0000002 | −0.0000014 | −0.0000004 | 13.0753 | 1 | 0.0003 | |
| Distance to nearest village | 0.1500 | 0.4537 | −0.7392 | 1.0392 | 0.1094 | 1 | 0.7409 | |
| Bulk density | 0.0565 | 0.4864 | −0.8969 | 1.0098 | 0.0135 | 1 | 0.9075 | |
| Gini index * Stem density | 0.0593 | 0.0176 | 0.0248 | 0.0937 | 11.3527 | 1 | 0.0008 | |
| Tree species diversity * Tree density | −0.0057 | 0.0018 | −0.0092 | −0.0022 | 10.2920 | 1 | 0.0013 | |
| % of basal area of Fagaceae trees × % of cut stem | 0.0005 | 0.0001 | 0.0003 | 0.0008 | 16.8173 | 1 | 0.0000 | |
| Omnibus test: Likelihood Ratio Chi Square = 43.38, df = 7, p < 0.05, Akaike information criterion (AIC) value = 103.47 | ||||||||
| Frequency of Fagaceae small saplings per plot | Intercept | 4.0018 | 0.5353 | 2.9526 | 5.0510 | 55.8836 | 1 | 0.0000 |
| Disturbance intensity | −0.0899 | 0.0651 | −0.2175 | 0.0376 | 1.9109 | 1 | 0.1669 | |
| Elevation * % of stumps * % of cut stem | −0.0000002 | 0.0000001 | −0.0000004 | 0.0000001 | 1.6553 | 1 | 0.1982 | |
| Distance to nearest village | 0.3997 | 0.1929 | 0.0215 | 0.7778 | 4.2909 | 1 | 0.0383 | |
| Bulk density | −0.2580 | 0.2303 | −0.7095 | 0.1934 | 1.2549 | 1 | 0.2626 | |
| Gini index * Stem density | 0.0188 | 0.0077 | 0.0036 | 0.0340 | 5.8792 | 1 | 0.0153 | |
| Tree species diversity * Tree density | −0.0022 | 0.0009 | −0.0039 | −0.0005 | 6.1223 | 1 | 0.0133 | |
| % of basal area of Fagaceae trees * % of cut stem | −0.0001 | 0.0001 | −0.0002 | 0.0001 | 0.4266 | 1 | 0.5137 | |
| Omnibus test: Likelihood Ratio Chi Square = 118.79, df = 7, p < 0.05, AIC value = 104.52 | ||||||||
| Frequency of Fagaceae large saplings per plot | Intercept | −0.9063 | 0.6198 | −2.1211 | 0.3084 | 2.1385 | 1 | 0.1436 |
| Disturbance intensity | 0.2723 | 0.0652 | 0.1446 | 0.4000 | 17.4605 | 1 | 0.0000 | |
| Elevation * % of stumps * % of cut stem | 0.0000001 | 0.0000002 | −0.0000002 | 0.0000004 | 0.1817 | 1 | 0.6699 | |
| Distance to nearest village | 1.7619 | 0.1753 | 1.4182 | 2.1055 | 100.9570 | 1 | 0.0000 | |
| Bulk density | 0.8462 | 0.1967 | 0.4606 | 1.2317 | 18.5036 | 1 | 0.0000 | |
| Gini index * Stem density | 0.0482 | 0.0094 | 0.0297 | 0.0667 | 26.0291 | 1 | 0.0000 | |
| Tree species diversity * Tree density | −0.0023 | 0.0010 | −0.0042 | −0.0003 | 4.9953 | 1 | 0.0254 | |
| % of basal area of Fagaceae trees * % of cut stem | −0.00021 | 0.00010 | −0.00040 | −0.00002 | 4.4837 | 1 | 0.0342 | |
| Omnibus test: Likelihood Ratio Chi Square = 253.58, df = 7, p < 0.05, AIC value = 250.88 | ||||||||
| Frequency of Fagaceae pole−sized trees per plot | Intercept | −3.6595 | 1.2668 | −6.1424 | −1.1766 | 8.3450 | 1 | 0.0039 |
| Disturbance intensity | 0.4936 | 0.1191 | 0.2602 | 0.7269 | 17.1798 | 1 | 0.0000 | |
| Elevation * % of stumps * % of cut stem | 0.000001 | 0.0000002 | 0.0000006 | 0.0000013 | 27.6601 | 1 | 0.0000 | |
| Distance to nearest village | 1.7098 | 0.3327 | 1.0577 | 2.3618 | 26.4131 | 1 | 0.0000 | |
| Bulk density | 0.4754 | 0.2948 | −0.1023 | 1.0532 | 2.6014 | 1 | 0.1068 | |
| Gini index * Stem density | 0.0558 | 0.0136 | 0.0291 | 0.0825 | 16.7853 | 1 | 0.0000 | |
| Tree species diversity * Tree density | 0.0005 | 0.0011 | −0.0017 | 0.0027 | 0.2204 | 1 | 0.6387 | |
| % of basal area of Fagaceae trees * % of cut stem | −0.0003 | 0.0001 | −0.0005 | −0.0001 | 7.2466 | 1 | 0.0071 | |
| Omnibus test: Likelihood Ratio Chi Square = 126.08, df = 7, p < 0.05, AIC value = 98.14 | ||||||||
| Frequency of Fagaceae large trees per plot | Intercept | 1.8435 | 1.5448 | −1.1842 | 4.8712 | 1.4241 | 1 | 0.2327 |
| Disturbance intensity | −0.2652 | 0.1896 | −0.6367 | 0.1064 | 1.9567 | 1 | 0.1619 | |
| Elevation * % of stumps * % of cut stem | −0.0000001 | 0.0000003 | −0.0000007 | 0.0000004 | 0.3044 | 1 | 0.5811 | |
| Distance to nearest village | 0.5935 | 0.5339 | −0.4529 | 1.6400 | 1.2358 | 1 | 0.2663 | |
| Bulk density | 0.0171 | 0.5853 | −1.1300 | 1.1642 | 0.0009 | 1 | 0.9767 | |
| Gini index * Stem density | −0.0108 | 0.0216 | −0.0532 | 0.0316 | 0.2487 | 1 | 0.6180 | |
| Tree species diversity * Tree density | 0.0019 | 0.0024 | −0.0028 | 0.0066 | 0.6174 | 1 | 0.4320 | |
| % of basal area of Fagaceae trees * % of cut stem | 0.0004 | 0.0001 | 0.0001 | 0.0007 | 8.5302 | 1 | 0.0035 | |
| Omnibus test: Likelihood Ratio Chi Square = 81.16, df = 7, p < 0.05, AIC value = 67.96 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.P.; Chakraborty, T.; Dermann, A.; Dermann, F.; Adhikari, D.; Gurung, P.B.; Barik, S.K.; Bauhus, J.; Fassnacht, F.E.; Dey, D.C.; et al. Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India. Forests 2020, 11, 1008. https://doi.org/10.3390/f11091008
Singh PP, Chakraborty T, Dermann A, Dermann F, Adhikari D, Gurung PB, Barik SK, Bauhus J, Fassnacht FE, Dey DC, et al. Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India. Forests. 2020; 11(9):1008. https://doi.org/10.3390/f11091008
Chicago/Turabian StyleSingh, Prem Prakash, Tamalika Chakraborty, Anna Dermann, Florian Dermann, Dibyendu Adhikari, Purna B. Gurung, Saroj Kanta Barik, Jürgen Bauhus, Fabian Ewald Fassnacht, Daniel C. Dey, and et al. 2020. "Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India" Forests 11, no. 9: 1008. https://doi.org/10.3390/f11091008
APA StyleSingh, P. P., Chakraborty, T., Dermann, A., Dermann, F., Adhikari, D., Gurung, P. B., Barik, S. K., Bauhus, J., Fassnacht, F. E., Dey, D. C., Rösch, C., & Saha, S. (2020). Assessing Restoration Potential of Fragmented and Degraded Fagaceae Forests in Meghalaya, North-East India. Forests, 11(9), 1008. https://doi.org/10.3390/f11091008







