A Permanent Research Platform for Ecological Studies in Intact Temperate Mountainous Forests from Slătioara UNESCO Site and Its Surroundings, Romania
Abstract
1. Introduction
2. Material and Methods
2.1. Rationale and Research Questions
- Slătioara Nature Reserve is well preserved and these intact forest stands are known as resistant to natural disturbances;
- long term studies have never been conducted till now in Romanian intact forests, based on successive inventories and intensive monitoring;
- the mixed temperate intact forests need extensive studies in terms of ecosystem functioning, to identify management patterns worth being applied to similar second-growth and cultivated forests;
- a wide range of forest ecosystem services provided by this forest need to be better understood through long term assessments; and
- real time identification of causes potentially affecting intact forest ecosystems in a rapidly changing climate.
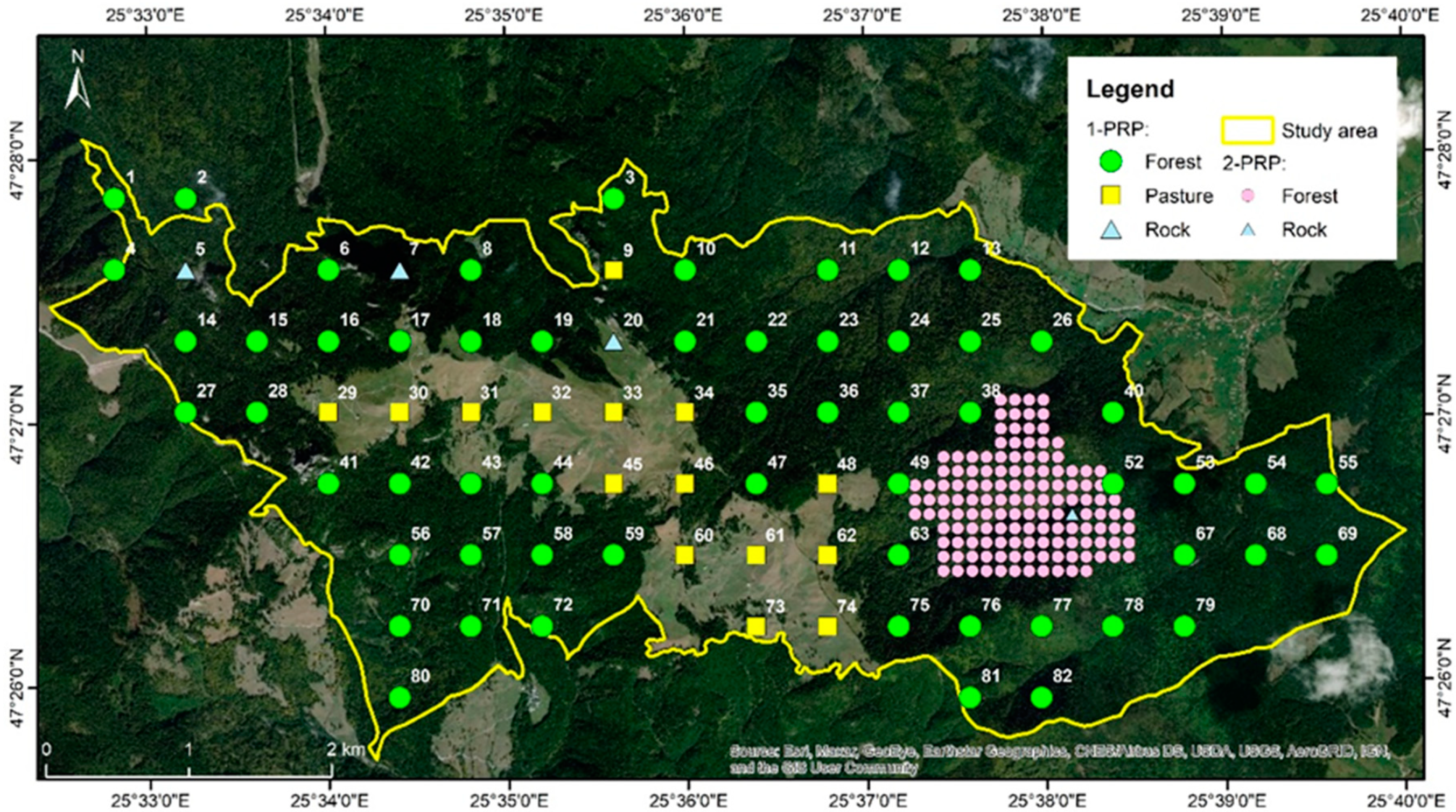
2.2. Design of Research Platform
- -
- Ensuring the accurate representation of the forest ecosystems state;
- -
- guaranteeing spatial positioning of the inventory plots within all habitat types within the studied area (randomly stratified schematic sampling);
- -
- the number of inventory plots should allow re-measurements of all studied parameters within an easy-to-establish period of time.
- -
- The 1st level of PRP (1-PRP) corresponds to a square grid which overlaps the entire study area. This resulted from the intersection of the network with the boundaries of the Rarău-Slătioara part of ROSCI0212 Rarău-Giumalău (2205.85 ha total area, of which 78% is covered by forests).
- -
- The 2nd level of PRP (2-PRP) resulted through increasing the density of the 1-PRP in the area previously described in literature as having higher ecosystem complexity and being relatively accessible for more detailed field research [38]. This component of the PRP covers approximately 136 ha, considering a 50 m width buffer area.
2.3. Establishment of Research Plots
- -
- Steep slopes, where the receiver required more time to receive signal from satellites;
- -
- forest stands traits, especially high density uneven-aged stands, where the positioning errors could not be reduced to less than ± 7 m; and
- -
- rarely, special geomorphology features (very steep slopes on valley bottom, abrupt rock formations) have urged the reconsideration of SP position.
2.4. Data Collection
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loreau, M.; Kinne, O. The Challenges of Biodiversity Science; International Ecology Institute: Oldendurf/Luhe, Germany, 2010; Volume 17, p. 120. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Arnell, A.P.; Contu, S.; De Palma, A.; Ferrier, S.; Hill, S.L.L.; Hoskins, A.J.; Lysenko, I.; Phillips, H.R.P.; et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 2016, 353, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F. Habitat destruction: Death by a thousand cuts. In Conservation Biology for All; Sodhi, N.S., Ehrlich, P.R., Eds.; Oxford University Press: Oxford, UK, 2010; Volume 1, pp. 73–88. [Google Scholar]
- Williams, B.A.; Grantham, H.S.; Watson, J.E.M.; Alvarez, S.J.; Simmonds, J.S.; Rogéliz, C.A.; Da Silva, M.; Forero-Medina, G.; Etter, A.; Nogales, J.; et al. Minimising the loss of biodiversity and ecosystem services in an intact landscape under risk of rapid agricultural development. Environ. Res. Lett. 2020, 15, 014001. [Google Scholar] [CrossRef]
- Pimm, S.L.; Raven, P. Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Martin, T.G.; Watson, J.E.M. Intact ecosystems provide best defence against climate change. Nat. Clim. Chang. 2016, 6, 122–124. [Google Scholar] [CrossRef]
- Palmer, M.A.; Filoso, S. Restoration of Ecosystem Services for Environmental Markets. Science 2009, 325, 575–576. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Li, B.V. How to protect half of Earth to ensure it protects sufficient biodiversity. Sci. Adv. 2018, 4, eaat2616. [Google Scholar] [CrossRef]
- Hassan, R.; Scholes, R.; Ash, N. Ecosystems and Human Well-Being: Current State and Trends: Findings of the Condition and Trends Working Group; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; p. 44. [Google Scholar]
- Myers, N. The world’s forests and their ecosystem services. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997; pp. 215–235. [Google Scholar]
- Summers, J.K.; Smith, L.M.; Case, J.L.; Linthurst, R.A. A Review of the Elements of Human Well-Being with an Emphasis on the Contribution of Ecosystem Services. AMBIO 2012, 41, 327–340. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Jucker, T.; Avăcăriței, D.; Bărnoaiea, I.; Duduman, G.; Bouriaud, O.; Coomes, D.A. Climate modulates the effects of tree diversity on forest productivity. J. Ecol. 2016, 104, 388–398. [Google Scholar] [CrossRef]
- Jucker, T.; Bouriaud, O.; Avacaritei, D.; Dănilă, I.; Duduman, G.; Valladares, F.; Coomes, D.A. Competition for light and water play contrasting roles in driving diversity–productivity relationships in Iberian forests. J. Ecol. 2014, 102, 1202–1213. [Google Scholar] [CrossRef]
- Potapov, P.; Yaroshenko, A.; Turubanova, S.; Dubinin, M.; Laestadius, L.; Thies, C.; Aksenov, D.; Egorov, A.; Yesipova, Y.; Glushkov, I.; et al. Mapping the World’s Intact Forest Landscapes by Remote Sensing. Ecol. Soc. 2008, 13. Available online: http://www.ecologyandsociety.org/vol13/iss2/art51/ (accessed on 10 July 2020). [CrossRef]
- Heino, M.; Kummu, M.; Makkonen, M.; Mulligan, M.; Verburg, P.H.; Jalava, M.; Räsänen, T.A. Forest Loss in Protected Areas and Intact Forest Landscapes: A Global Analysis. PLoS ONE 2015, 10, e0138918. [Google Scholar] [CrossRef]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef]
- Parviainen, J. Virgin and natural forests in the temperate zone of Europe. For. Snow Landsc. Res. 2005, 79, 9–18. [Google Scholar]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Ibisch, P.L. Romania’s key role in the conservation of European primary forests. In Potential Primary Forests Map of Romania; Ibisch, P.L., Ursu, A., Eds.; Centre for Econics and Ecosystem Management: Bucharest, Romania, 2017; pp. 11–12. [Google Scholar]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O.; et al. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef]
- Sălăgeanu, V. Why a Primary Forests Potential Map? In Potential Primary Forests Map of Romania; Ibisch, P.L., Ursu, A., Eds.; Centre for Econics and Ecosystem Management: Bucharest, Romania, 2017; pp. 13–23. [Google Scholar]
- Baeten, L.; Verheyen, K.; Wirth, C.; Bruelheide, H.; Bussotti, F.; Finér, L.; Jaroszewicz, B.; Selvi, F.; Valladares, F.; Allan, E.; et al. A novel comparative research platform designed to determine the functional significance of tree species diversity in European forests. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 281–291. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Fromin, N.; Milcu, A.; Buatois, B.; Pontoizeau, C.; Hättenschwiler, S. Higher tree diversity increases soil microbial resistance to drought. Commun. Biol. 2020, 3, 377. [Google Scholar] [CrossRef]
- ICP_Forests. Level II. Available online: http://icp-forests.net/page/level-ii (accessed on 12 July 2020).
- Ştefureac, T.I. “Slătioara old-growth forest” Reserve. In Geobotanical Guide for Northern Moldavia. Ghid Geobotanic Pentru Moldova de Nord; Societatea de Ştiinţe Naturale şi Geografie Din R.P.R.: Bucharest, Romania, 1965; pp. 51–65. [Google Scholar]
- Costea, C. Single Tree Selection System; Editura Agro-Silvică: Bucharest, Romania, 1962; p. 146. [Google Scholar]
- Anonymous. Emergency Ordinance no. 57/2007 on the regime of protected natural areas, conservation of natural habitats, wild flora and fauna. Off. J. Rom. 2007, I, 45. [Google Scholar]
- Marcean, M. The forests of Suceava county and the calamities between 1945 and 2002 [Pădurile Sucevei şi calamităţile din perioada 1945–2002]. Bucov. For. 2002, 10, 59–73. (In Romanian) [Google Scholar]
- Duduman, G.; Tomescu, C.; Dragoi, M.; Palaghianu, C. Tree size variability and plant diversity in mixed coniferous-beech forests in Slătioara Forest Reserve. Bucov. For. 2014, 14, 135–147. [Google Scholar]
- UNESCO. Ancient and Primeval Beech Forests of the Carpathians and Other Regions of Europe. Available online: http://whc.unesco.org/en/list/1133/multiple=1&unique_number=2152 (accessed on 12 July 2020).
- Duduman, G. An Ecological Approach for Establishing the Allowable Cut in Forests Where Single Tree Selection System Is Applied; Editura Universităţii Suceava: Suceava, Romania, 2009; p. 300. [Google Scholar]
- Staudhammer, C.L.; LeMay, V.M. Introduction and evaluation of possible indices of stand structural diversity. Can. J. For. Res. 2001, 31, 1105–1115. [Google Scholar] [CrossRef]
- Helms, J. The Dictionary of Forestry; Society of American Foresters: Bethesda, MD, USA, 1998; p. 210. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie; Springer: Berlin, Germany, 1964. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2020-2. Available online: https://www.iucnredlist.org (accessed on 9 July 2020).
- Duduman, G.; Drăgoi, M. Forest Management Planning—Spatial-Temporal Organization; Editura Universității “Ștefan cel Mare” Suceava: Suceava, Romania, 2019; X+209p. [Google Scholar]
- Diaci, J.; Rozenbergar, D.; Anic, I.; Mikac, S.; Saniga, M.; Kucbel, S.; Visnjic, C.; Ballian, D. Structural dynamics and synchronous silver fir decline in mixed old-growth mountain forests in Eastern and Southeastern Europe. For. Int. J. For. Res. 2011, 84, 479–491. [Google Scholar] [CrossRef]
- Klopcic, M.; Boncina, A. Stand dynamics of silver fir (Abies alba Mill.)-European beech (Fagus sylvatica L.) forests during the past century: A decline of silver fir? For. Int. J. For. Res. 2011, 84, 259–271. [Google Scholar] [CrossRef]
- Noss, R.F. Beyond Kyoto: Forest Management in a Time of Rapid Climate Change. Conserv. Biol. 2001, 15, 578–590. [Google Scholar] [CrossRef]
- White, J.C.; Coops, N.C.; Wulder, M.A.; Vastaranta, M.; Hilker, T.; Tompalski, P. Remote Sensing Technologies for Enhancing Forest Inventories: A Review. Can. J. Remote Sens. 2016, 42, 619–641. [Google Scholar] [CrossRef]
- Spracklen, B.D.; Spracklen, D.V. Identifying European Old-Growth Forests using Remote Sensing: A Study in the Ukrainian Carpathians. Forests 2019, 10, 127. [Google Scholar] [CrossRef]
- Pielke, R.A., Sr.; Pitman, A.; Niyogi, D.; Mahmood, R.; McAlpine, C.; Hossain, F.; Goldewijk, K.K.; Nair, U.; Betts, R.; Fall, S.; et al. Land use/land cover changes and climate: Modeling analysis and observational evidence. Wires Clim. Chang. 2011, 2, 828–850. [Google Scholar] [CrossRef]
- Angelstam, P.K. Maintaining and restoring biodiversity in European boreal forests by developing natural disturbance regimes. J. Veg. Sci. 1998, 9, 593–602. [Google Scholar] [CrossRef]
- Cenuşă, R. Problems of Forest Ecology—The Theory of Development Phases. Applications in Natural Stands from Bucovina; “Ștefan cel Mare” University: Suceava, Romania, 1996; p. 165. [Google Scholar]
- Cenuşă, R.; Popa, C.; Teodosiu, M. Researches regarding the relation structure-function and the evolution of natural forest ecosystems in Northern part of Romania. Ann. Icas 2002, 45, 9–19. [Google Scholar]
- Duduman, G.; Roibu, C.; Duduman, M.L.; Miron-Onciul, M. The influence of competition and dimensional-spatial characteristics of trees on their radial growth in Old-Growth Slătioara forest, Romania. Aes Bioflux 2010, 2, 215–230. [Google Scholar]
- Raianu, O.; Jeanrenaud, E.; Murariu, A.; Antohi, A.; Pisică-Donose, A.; Truşcă, M.; Vidraşcu, P. Dynamics of decomposing processes—Coniferous decomposing stocks in “Old-growth Slătioara Forest”. Annu. Suceava Cty. Mus. Nat. Sci. 1980, VI, 189–195. [Google Scholar]
- Cenuşă, R. Aspects regarding the dynamics and importance of dead wood in spruce natural stands. Bucov. For. 1995, 4, 62–63. [Google Scholar]
- Măciucă, A.; Roibu, C. Dead Wood—An important issue for forest biodiversity conservation. Present Environ. Sustain. Dev. 2012, 6, 299–308. [Google Scholar]
- Lassauce, A.; Paillet, Y.; Jactel, H.; Bouget, C. Deadwood as a surrogate for forest biodiversity: Meta-analysis of correlations between deadwood volume and species richness of saproxylic organisms. Ecol. Indic. 2011, 11, 1027–1039. [Google Scholar] [CrossRef]
- Cordonnier, T.; Berger, F.; Elkin, C.; Lamas, T.; Martinez, M. Models and linker functions (indicators) for ecosystem services. Deliv. D2 2013, 2, 94. [Google Scholar]
- Jeltsch, F.; Wissel, C. Modelling dieback phenomena in natural forests. Ecol. Model. 1994, 75–76, 111–121. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.Y.H. Observations from old forests underestimate climate change effects on tree mortality. Nat. Commun. 2013, 4, 1655. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Nagel, T.A.; Diaci, J.; Rozenbergar, D.; Rugani, T.; Firm, D. Old-growth forest reserves in Slovenia: The past, present, and future. Schweiz. Z. Fur Forstwes. 2012, 163, 240–246. [Google Scholar] [CrossRef]
- Steinkamp, J.; Hickler, T. Is drought-induced forest dieback globally increasing? J. Ecol. 2015, 103, 31–43. [Google Scholar] [CrossRef]
- Hawkes, C. Woody plant mortality algorithms: Description, problems and progress. Ecol. Model. 2000, 126, 225–248. [Google Scholar] [CrossRef]
- Hülsmann, L.; Bugmann, H.K.M.; Commarmot, B.; Meyer, P.; Zimmermann, S.; Brang, P. Does one model fit all? Patterns of beech mortality in natural forests of three European regions. Ecol. Appl. 2016, 26, 2465–2479. [Google Scholar] [CrossRef]
- Kucbel, S.; Jaloviar, P.; Saniga, M.; Vencurik, J.; Klimaš, V. Canopy gaps in an old-growth fir-beech forest remnant of Western Carpathians. Eur. J. For. Res. 2010, 129, 249–259. [Google Scholar] [CrossRef]
- Nagel, T.A.; Svoboda, M.; Rugani, T.; Diaci, J. Gap regeneration and replacement patterns in an old-growth Fagus–Abies forest of Bosnia–Herzegovina. Plant Ecol. 2010, 208, 307–318. [Google Scholar] [CrossRef]
- Garbarino, M.; Borgogno Mondino, E.; Lingua, E.; Nagel, T.A.; Dukić, V.; Govedar, Z.; Motta, R. Gap disturbances and regeneration patterns in a Bosnian old-growth forest: A multispectral remote sensing and ground-based approach. Ann. For. Sci. 2012, 69, 617–625. [Google Scholar] [CrossRef]
- Rugani, T.; Diaci, J.; Hladnik, D. Gap Dynamics and Structure of Two Old-Growth Beech Forest Remnants in Slovenia. PLoS ONE 2013, 8, e52641. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Bagnato, S.; Sidari, M.; Mercurio, R. A review of the roles of forest canopy gaps. J. For. Res. 2014, 25, 725–736. [Google Scholar] [CrossRef]
- Szewczyk, J.; Szwagrzyk, J. Spatial and temporal variability of natural regeneration in a temperate old-growth forest. Ann. For. Sci. 2010, 67, 202. [Google Scholar] [CrossRef]
- Rozenbergar, D.; Stjepan, M.; Igor, A.; Jurij, D. Gap regeneration patterns in relationship to light heterogeneity in two old-growth beech–fir forest reserves in South East Europe. For. Int. J. For. Res. 2007, 80, 431–443. [Google Scholar] [CrossRef]
- Liira, J.; Sepp, T.; Kohv, K. The ecology of tree regeneration in mature and old forests: Combined knowledge for sustainable forest management. J. Res. 2011, 16, 184–193. [Google Scholar] [CrossRef]
- Kuijper, D.P.J.; Cromsigt, J.P.G.M.; Jędrzejewska, B.; Miścicki, S.; Churski, M.; Jędrzejewski, W.; Kweczlich, I. Bottom-up versus top-down control of tree regeneration in the Białowieża Primeval Forest, Poland. J. Ecol. 2010, 98, 888–899. [Google Scholar] [CrossRef]
- Vandenberghe, C.; Freléchoux, F.; Gadallah, F.; Buttler, A. Competitive effects of herbaceous vegetation on tree seedling emergence, growth and survival: Does gap size matter? J. Veg. Sci. 2006, 17, 481–488. [Google Scholar] [CrossRef]
- Diaci, J.; Adamic, T.; Rozman, A. Gap recruitment and partitioning in an old-growth beech forest of the Dinaric Mountains: Influences of light regime, herb competition and browsing. For. Ecol. Manag. 2012, 285, 20–28. [Google Scholar] [CrossRef]
- Szewczyk, J.; Szwagrzyk, J. Tree regeneration on rotten wood and on soil in old-growth stand. Vegetatio 1996, 122, 37–46. [Google Scholar] [CrossRef]
- Takahashi, M.; Sakai, Y.; Ootomo, R.; Shiozaki, M. Establishment of tree seedlings and water-soluble nutrients in coarse woody debris in an old-growth Picea-Abies forest in Hokkaido, northern Japan. Can. J. For. Res. 2000, 30, 1148–1155. [Google Scholar] [CrossRef]
- Zielonka, T.; Niklasson, M. Dynamics of Dead Wood and Regeneration Pattern in Natural Spruce Forest in the Tatra Mountains, Poland. Ecol. Bull. 2001, 159–163. [Google Scholar] [CrossRef]
- Motta, R.; Berretti, R.; Lingua, E.; Piussi, P. Coarse woody debris, forest structure and regeneration in the Valbona Forest Reserve, Paneveggio, Italian Alps. For. Ecol. Manag. 2006, 235, 155–163. [Google Scholar] [CrossRef]
- Simon, A.; Gratzer, G.; Sieghardt, M. The influence of windthrow microsites on tree regeneration and establishment in an old growth mountain forest. For. Ecol. Manag. 2011, 262, 1289–1297. [Google Scholar] [CrossRef]
- Kint, V.; Aertsen, W.; Campioli, M.; Vansteenkiste, D.; Delcloo, A.; Muys, B. Radial growth change of temperate tree species in response to altered regional climate and air quality in the period 1901–2008. Clim. Chang. 2012, 115, 343–363. [Google Scholar] [CrossRef]
- Peñuelas, J.; Ogaya, R.; Boada, M.; S. Jump, A. Migration, invasion and decline: Changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 2007, 30, 829–837. [Google Scholar] [CrossRef]
- De Groot, M.; Diaci, J.; Ogris, N. Forest management history is an important factor in bark beetle outbreaks: Lessons for the future. For. Ecol. Manag. 2019, 433, 467–474. [Google Scholar] [CrossRef]
- Zubizarreta-Gerendiain, A.; Pellikka, P.; Garcia-Gonzalo, J.; Ikonen, V.-P.; Peltola, H. Factors affecting wind and snow damage of individual trees in a small management unit in Finland: Assessment based on inventoried damage and mechanistic modelling. Silva Fenn. 2012, 46, 181–196. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Hengeveld, G.; Reyer, C.; Hanewinkel, M.; Zimmermann, N.E.; Cullmann, D. Alternative forest management strategies to account for climate change-induced productivity and species suitability changes in Europe. Reg. Environ. Chang. 2015, 15, 1581–1594. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.; Düll, R.; Wirth, V.; Werner, W.D. PAULISSEN: Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 7–97. [Google Scholar]
- Cristea, V.; Gafta, D.; Pedrotti, F. Fitosociologie; Edit Presa Universitară Clujeană: Cluj Napoca, Romania, 2004; p. 394. [Google Scholar]
- He, P.; Maldonado-Chaparro, A.A.; Farine, D.R. The role of habitat configuration in shaping social structure: A gap in studies of animal social complexity. Behav. Ecol. Sociobiol. 2019, 73, 9. [Google Scholar] [CrossRef]
- Ampoorter, E.; Barbaro, L.; Jactel, H.; Baeten, L.; Boberg, J.; Carnol, M.; Castagneyrol, B.; Charbonnier, Y.; Dawud, S.M.; Deconchat, M.; et al. Tree diversity is key for promoting the diversity and abundance of forest-associated taxa in Europe. Oikos 2020, 129, 133–146. [Google Scholar] [CrossRef]
- Perault, D.R.; Lomolino, M.V. Corridors and Mammal Community Structure Across a Fragmented, Old-Growth Forest Landscape. Ecol. Monogr. 2000, 70, 401–422. [Google Scholar] [CrossRef]
- Gill, D.; Magin, G.; Bertram, E. Trees andClimate Change. A Guide to the Factors that Influence Species Vulnerability and a Summary of Adaptation Options; Fauna & Flora International: Cambridge, UK, 2013; p. 14. [Google Scholar]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martínez-Vilalta, J.; Lloret, F. Drought-Induced Multifactor Decline of Scots Pine in the Pyrenees and Potential Vegetation Change by the Expansion of Co-occurring Oak Species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Rigling, A.; Bigler, C.; Eilmann, B.; Feldmeyer-Christe, E.; Gimmi, U.; Ginzler, C.; Graf, U.; Mayer, P.; Vacchiano, G.; Weber, P.; et al. Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Chang. Biol. 2013, 19, 229–240. [Google Scholar] [CrossRef]
- Goebes, P.; Schmidt, K.; Seitz, S.; Both, S.; Bruelheide, H.; Erfmeier, A.; Scholten, T.; Kühn, P. The strength of soil-plant interactions under forest is related to a Critical Soil Depth. Sci. Rep. 2019, 9, 8635. [Google Scholar] [CrossRef]
- Uusitalo, J.; Ala-Ilomäki, J.; Lindeman, H.; Toivio, J.; Siren, M. Modelling Soil Moisture–Soil Strength Relationship of Fine-Grained Upland Forest Soils. Silva Fenn. 2019, 53. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Pisică-Donose, A.; Antohi, A.; Murariu, A.; Jeanrenaud, E.; Raianu, O.; Vidraşcu, P.; Truşcă, M. Researches regarding litter decomposing in “Old-growth Slătioara Forest”. Annu. Suceava Cty. Mus. Nat. Sci. 1980, VI, 173–187. [Google Scholar]
- Boulanger, V.; Bruchiamacchie, M.; Chauchard, S.; Dragicevic, A.; Dupouey, J.-L.; Stenger, A. Spatial dynamics of green corridors. In Proceedings of the 25th Annual Conference of the European Association of Evolutionary Political Economy (EAEPE), Paris, France, 7–9 November 2013. [Google Scholar]
- Karjalainen, E.; Sarjala, T.; Raitio, H. Promoting human health through forests: Overview and major challenges. Environ. Health Prev. Med. 2010, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Abadi, A.M. The application of Dempster–Shafer theory of evidence for assessing groundwater vulnerability at Galal Badra basin, Wasit governorate, east of Iraq. Appl. Water Sci. 2017, 7, 1725–1740. [Google Scholar] [CrossRef]
- Nelson, E. The Economics of Ecosystems and Biodiversity: Ecological and Economic Foundations, edited by Pushpam Kumar. J. Nat. Resour. Policy Res. 2013, 5. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]





| Land Use Type | Management Type | Area (ha) | Protected Area Type |
|---|---|---|---|
| Forests | Strictly protected | 1580.73 | Forest reserves: Codrul Secular Slătioara, Rarău-Pietrele Doamnei, Peştera Liliecilor |
| Highly protected (for erosion control) | 13.67 | ROSCI 0212 Rarău Giumalău | |
| Regular management | 130.17 | ROSCI 0212 Rarău Giumalău | |
| Pasture land and forested pasture | Regular management, for hay production and grazing | 435.3 | Scientific reserves Rarău-Pietrele Doamnei and Fâneţele Todirescu |
| Constructions | Regular management (touristic facilities, weather station, antennas) | 1.2 | Scientific reserve Rarău-Pietrele Doamnei |
| Powerlines | Limited height of trees | 3.75 | Scientific reserve Rarău-Pietrele Doamnei |
| Rock formations, partially covered with forest vegetation | Strictly protected | 41.03 | Forest reserves: Codrul Secular Slătioara, Rarău-Pietrele Doamnei, Peştera Liliecilor |
| Type of Research Network | Number of Plots Designed | Plot Numbering | Number of Plots Per Group of Habitat Type | No. of Plots Installed in the Field | ||
|---|---|---|---|---|---|---|
| Forest | Rock | Pasture | ||||
| 1-PRP | 82 * | Sl5L001 to Sl5L082 | 64 * | 3 | 15 | 58 ** |
| 2-PRP | 136 | S001 to S136 | 135 | 1 | - | 135 |
| Total | 218 | - | 199 * | 4 | 15 | 193 ** |
| Measured Elements | Sample Size (m2) | No. of Plots | No. of Stems/Trunks | No. of Species Per Sample | No. of Trees/Saplings Measured Per Plot | |
|---|---|---|---|---|---|---|
| Total | With Data | |||||
| Living trees | 500 | 193 | 193 | 8296 | 1 to 6 | 13 to 112 |
| Standing dead wood | 500 | 193 | 190 * | 1743 | 1 to 4 | 1 to 44 |
| Fallen dead wood | 500 | 193 | 188 ** | 1900 | 1 to 4 | 1 to 32 |
| Natural regeneration | 3.14 | 772 | 518 | 3214 | 1 to 5 | 1 to 67 |
| Herbaceous layer | 500 | 193 | 193 | - | 7 to 69 | - |
| Tree Species | No. of Plots | No. of Trees Per SP | Max. dbh (cm) | Max. Height (m) | Basal Area (m2) Per SP | Volume (m3) Per SP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Mean | Max. | Min. | Mean | Max. | Min. | Mean | Max. | ||||
| Acer pseudoplatanus L. | 54 | 1 | 3 | 10 | 102.0 | 36.5 | 0.003 | 0.111 | 0.817 | 0.02 | 1.20 | 12.36 |
| Abies alba Mill. | 162 | 1 | 19 | 59 | 118.7 | 51.4 | 0.007 | 1.016 | 3.462 | 0.02 | 13.20 | 56.90 |
| Betula pendula Roth | 7 | 1 | 3 | 13 | 23.2 | 21.3 | 0.006 | 0.048 | 0.187 | 0.02 | 0.30 | 0.96 |
| Fagus sylvatica L. | 156 | 1 | 14 | 46 | 102.0 | 43.3 | 0.002 | 0.629 | 2.088 | 0.01 | 8.88 | 37.90 |
| Larix decidua Mill. | 1 | 10 | 10 | 10 | 51.2 | 30.8 | 1.164 | 1.164 | 1.164 | 13.15 | 13.15 | 13.15 |
| Populus tremula L. | 3 | 1 | 1 | 1 | 44.2 | 26.1 | 0.024 | 0.072 | 0.153 | 0.20 | 0.81 | 1.97 |
| Picea abies (L.) H. Karst. | 187 | 1 | 15 | 98 | 101.1 | 56.1 | 0.006 | 1.105 | 3.187 | 0.01 | 14.64 | 48.86 |
| Pinus sylvestris L. | 1 | 9 | 9 | 9 | 42.1 | 29.3 | 0.864 | 0.864 | 0.864 | 8.85 | 8.85 | 8.85 |
| Sorbus aucuparia L. | 6 | 1 | 2 | 5 | 19.2 | 16.2 | 0.018 | 0.025 | 0.037 | 0.09 | 0.16 | 0.24 |
| Taxus baccata L. | 4 | 1 | 1 | 1 | 18.6 | 10.7 | 0.004 | 0.012 | 0.027 | 0.01 | 0.05 | 0.15 |
| Ulmus glabra Huds. | 8 | 1 | 1 | 2 | 47.0 | 29.2 | 0.007 | 0.047 | 0.173 | 0.05 | 0.53 | 2.31 |
| All species | 193 | 13 | 43 | 112 | 118.7 | 56.1 | 0.161 | 2.479 | 4.846 | 0.99 | 32.95 | 72.04 |
| Species | No. of Plots | Dead Wood Volume (m3) Per SP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standing | Lying | Total | Standing | Lying | Total | |||||||
| Min. | Mean | Max. | Min. | Mean | Max. | Min. | Mean | Max. | ||||
| Acer pseudoplatanus L. | 12 | 3 | 14 | 0.007 | 0.128 | 0.568 | 0.102 | 0.212 | 0.352 | 0.007 | 0.155 | 0.920 |
| Abies alba Mill. | 134 | 100 | 147 | 0.008 | 1.498 | 11.134 | 0.014 | 3.117 | 16.871 | 0.008 | 3.486 | 24.642 |
| Fagus sylvatica L. | 65 | 58 | 89 | 0.007 | 0.193 | 2.384 | 0.009 | 0.737 | 5.119 | 0.008 | 0.621 | 5.415 |
| Larix decidua Mill. | 1 | 1 | 1 | 0.098 | 0.098 | 0.098 | 0.022 | 0.022 | 0.022 | 0.120 | 0.120 | 0.120 |
| Populus tremula L. | 1 | 1 | 1 | 0.088 | 0.088 | 0.088 | 0.113 | 0.113 | 0.113 | 0.201 | 0.201 | 0.201 |
| Picea abies (L.) H. Karst. | 172 | 168 | 188 | 0.007 | 1.434 | 12.175 | 0.010 | 3.334 | 25.619 | 0.016 | 4.291 | 26.448 |
| Pinus sylvestris L. | 1 | 2 | 2 | 0.939 | 0.939 | 0.939 | 0.089 | 0.300 | 0.511 | 0.089 | 0.770 | 1.450 |
| Salix caprea L. | 1 | 1 | 1 | 0.012 | 0.012 | 0.012 | 0.105 | 0.105 | 0.105 | 0.117 | 0.117 | 0.117 |
| Coniferous species * | 9 | 22 | 23 | 0.127 | 1.325 | 7.261 | 0.125 | 5.123 | 14.157 | 0.533 | 5.419 | 17.829 |
| Deciduous species * | - | 3 | 3 | - | - | - | 0.052 | 0.235 | 0.350 | 0.052 | 0.235 | 0.350 |
| Unidentified species ** | - | 7 | 7 | - | - | - | 0.135 | 2.617 | 6.431 | 0.135 | 2.617 | 6.431 |
| All species | 190 | 188 | 193 | 0.013 | 2.498 | 17.545 | 0.038 | 5.603 | 30.549 | 0.138 | 7.887 | 31.445 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duduman, G.; Duduman, M.-L.; Avăcăriței, D.; Barnoaiea, I.; Barbu, C.-O.; Ciornei, I.; Clinovschi, F.; Coșofreț, V.C.; Cotos, M.-G.; Dănilă, G.; et al. A Permanent Research Platform for Ecological Studies in Intact Temperate Mountainous Forests from Slătioara UNESCO Site and Its Surroundings, Romania. Forests 2020, 11, 1004. https://doi.org/10.3390/f11091004
Duduman G, Duduman M-L, Avăcăriței D, Barnoaiea I, Barbu C-O, Ciornei I, Clinovschi F, Coșofreț VC, Cotos M-G, Dănilă G, et al. A Permanent Research Platform for Ecological Studies in Intact Temperate Mountainous Forests from Slătioara UNESCO Site and Its Surroundings, Romania. Forests. 2020; 11(9):1004. https://doi.org/10.3390/f11091004
Chicago/Turabian StyleDuduman, Gabriel, Mihai-Leonard Duduman, Daniel Avăcăriței, Ionuț Barnoaiea, Cătălina-Oana Barbu, Ioan Ciornei, Florin Clinovschi, Vasile Cosmin Coșofreț, Mihai-Gabriel Cotos, Gabriel Dănilă, and et al. 2020. "A Permanent Research Platform for Ecological Studies in Intact Temperate Mountainous Forests from Slătioara UNESCO Site and Its Surroundings, Romania" Forests 11, no. 9: 1004. https://doi.org/10.3390/f11091004
APA StyleDuduman, G., Duduman, M.-L., Avăcăriței, D., Barnoaiea, I., Barbu, C.-O., Ciornei, I., Clinovschi, F., Coșofreț, V. C., Cotos, M.-G., Dănilă, G., Dănilă, I.-C., Drăgoi, M., Flocea, M.-N., Horodnic, S.-A., Iacobescu, O., Mazăre, G. C., Măciucă, A., Mursa, A., Palaghianu, C., ... Scriban, R.-E. (2020). A Permanent Research Platform for Ecological Studies in Intact Temperate Mountainous Forests from Slătioara UNESCO Site and Its Surroundings, Romania. Forests, 11(9), 1004. https://doi.org/10.3390/f11091004










