Survival of Quercus alba (White Oak) Advance Reproduction in Small Group and Single Tree Openings
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Study Establishment, Measurements and Treatments
- U1, no control of competing reproduction;
- U2, mechanical control of all competing woody stems greater than 30.5 cm tall and less than or equal to 14 cm d.b.h. (cut stems);
- U3, chemical control of all competing woody stems greater than 30.5 cm tall and less than or equal to 14 cm d.b.h. (cut stems sprayed with an herbicide).
2.3. Data Analysis and Modeling
3. Results
3.1. Survival Model
3.2. Competition Control Treatments
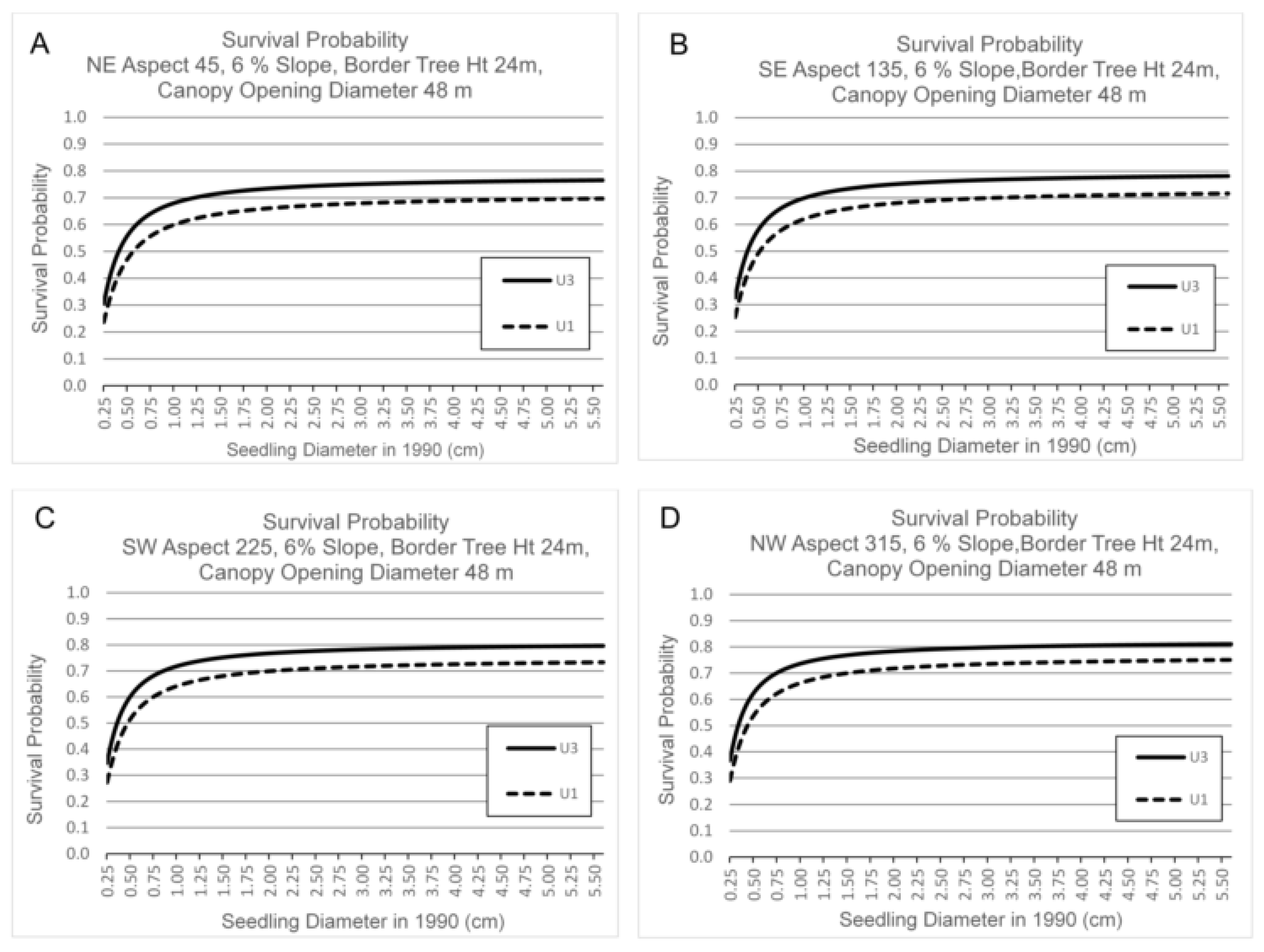
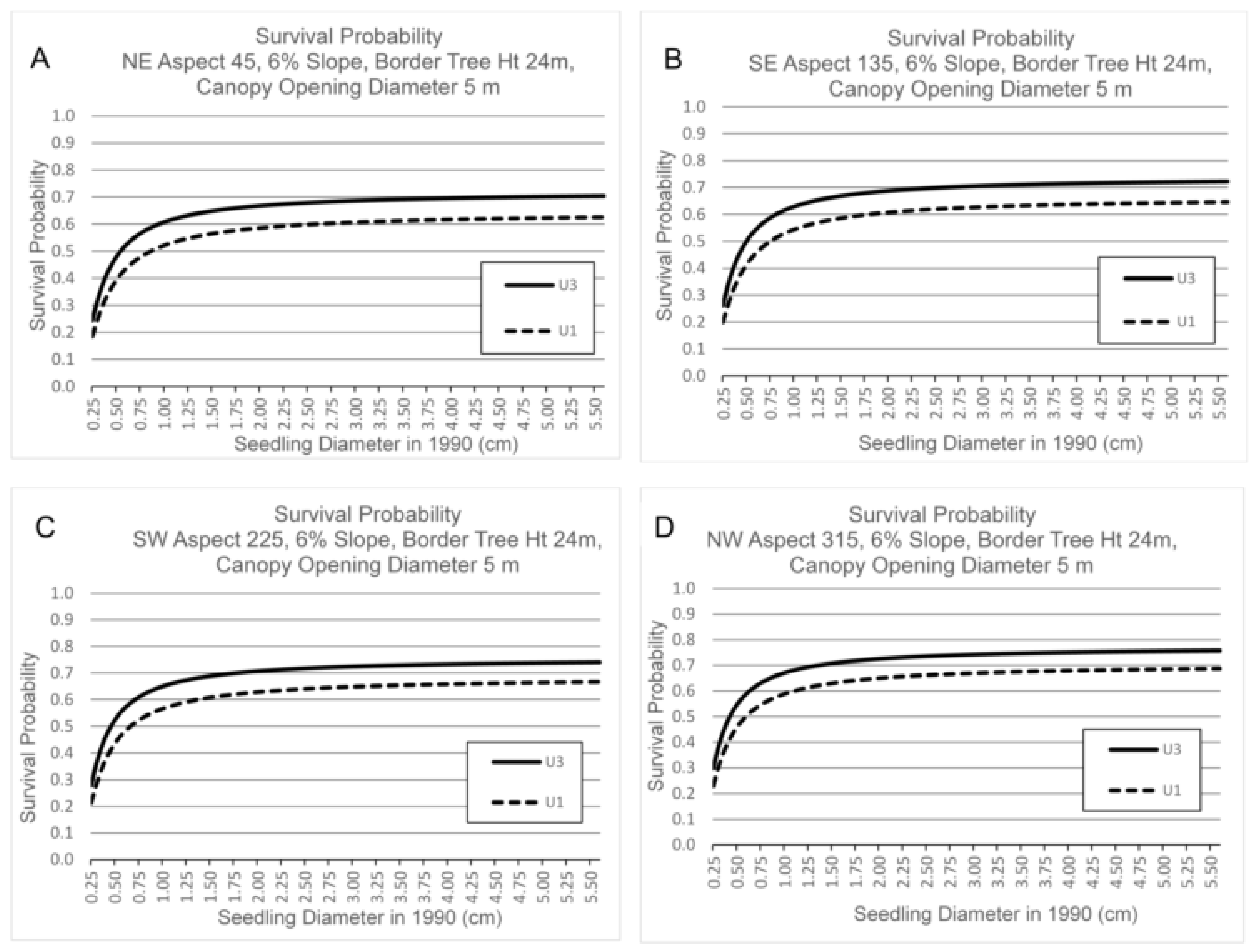
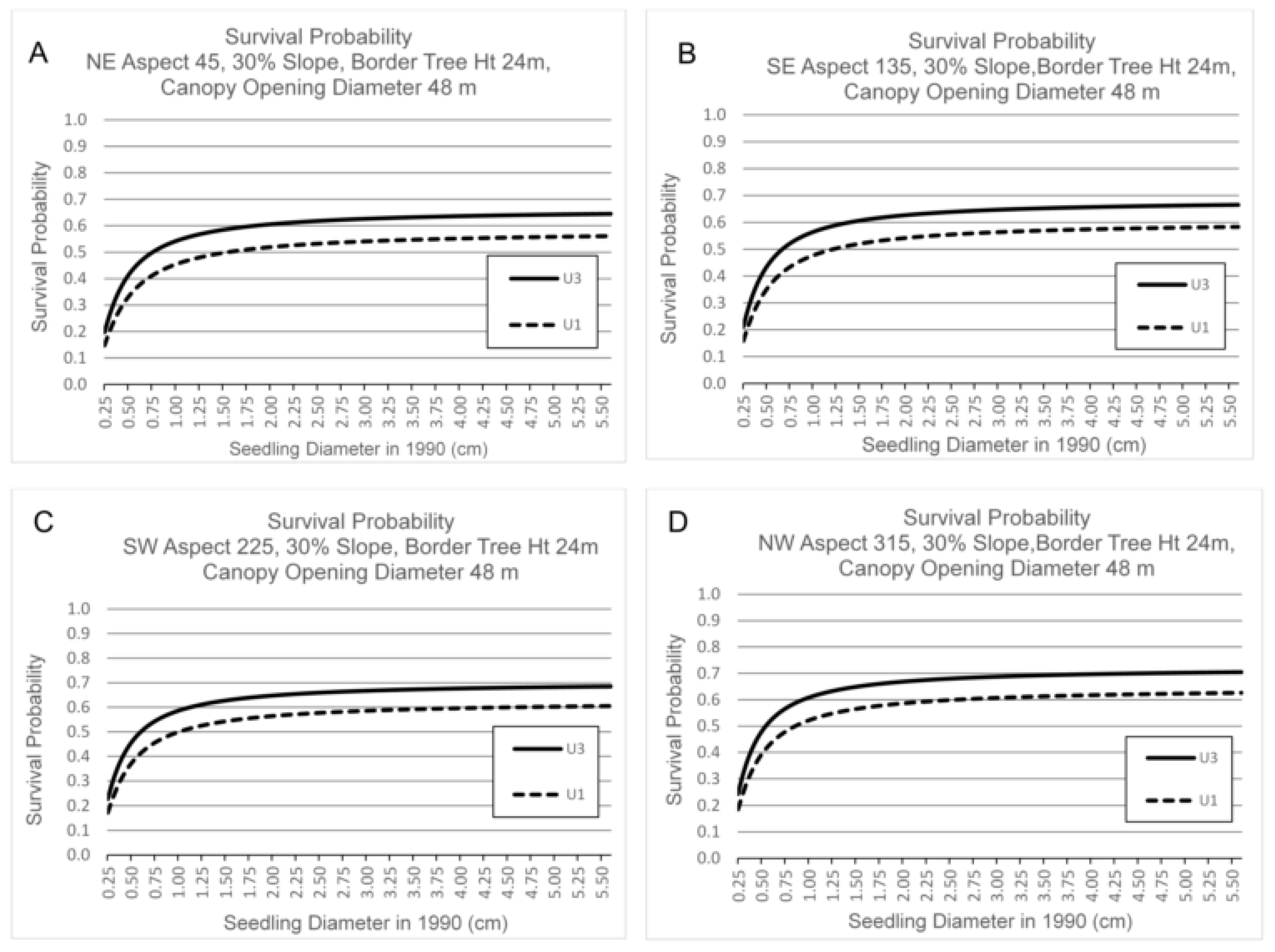
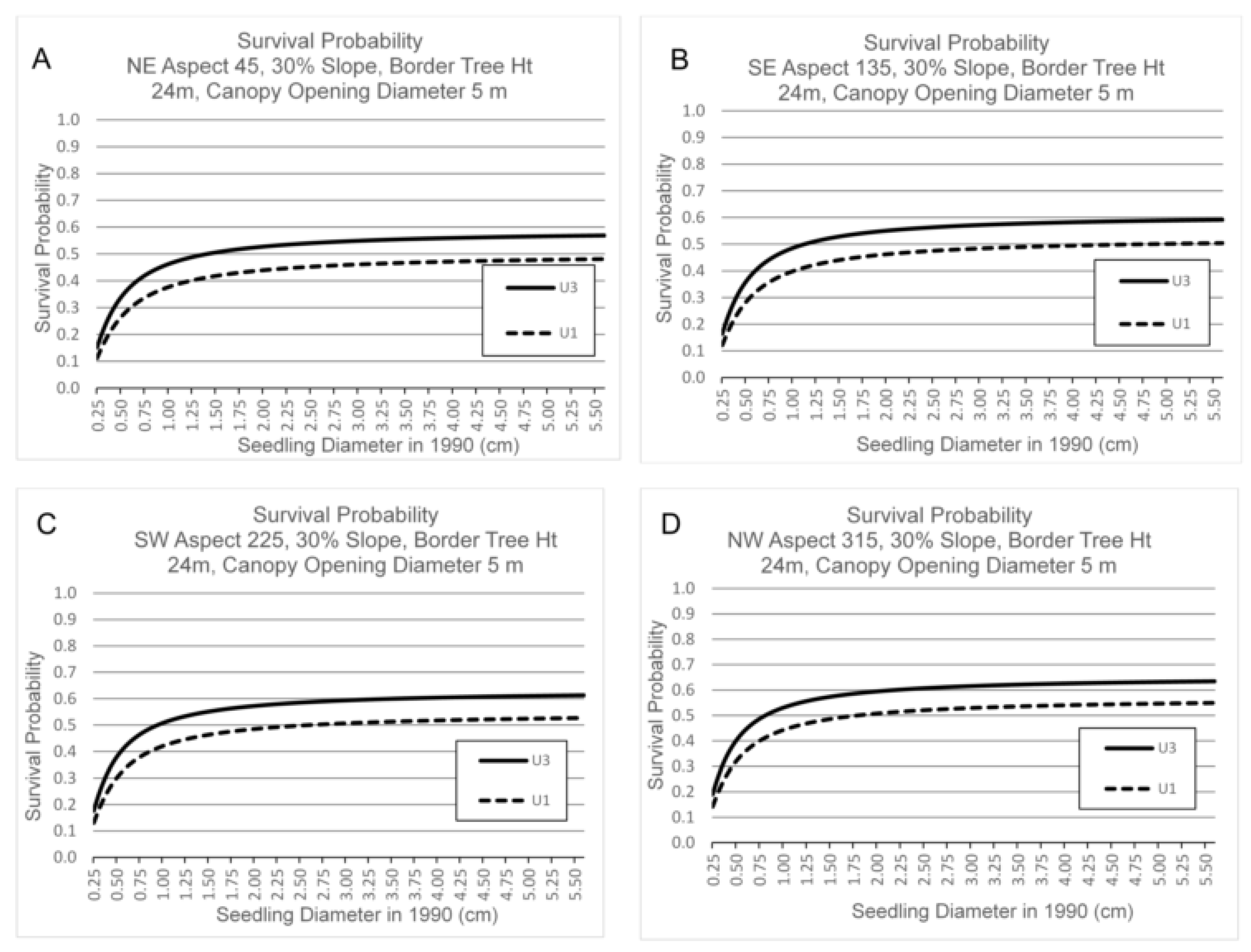
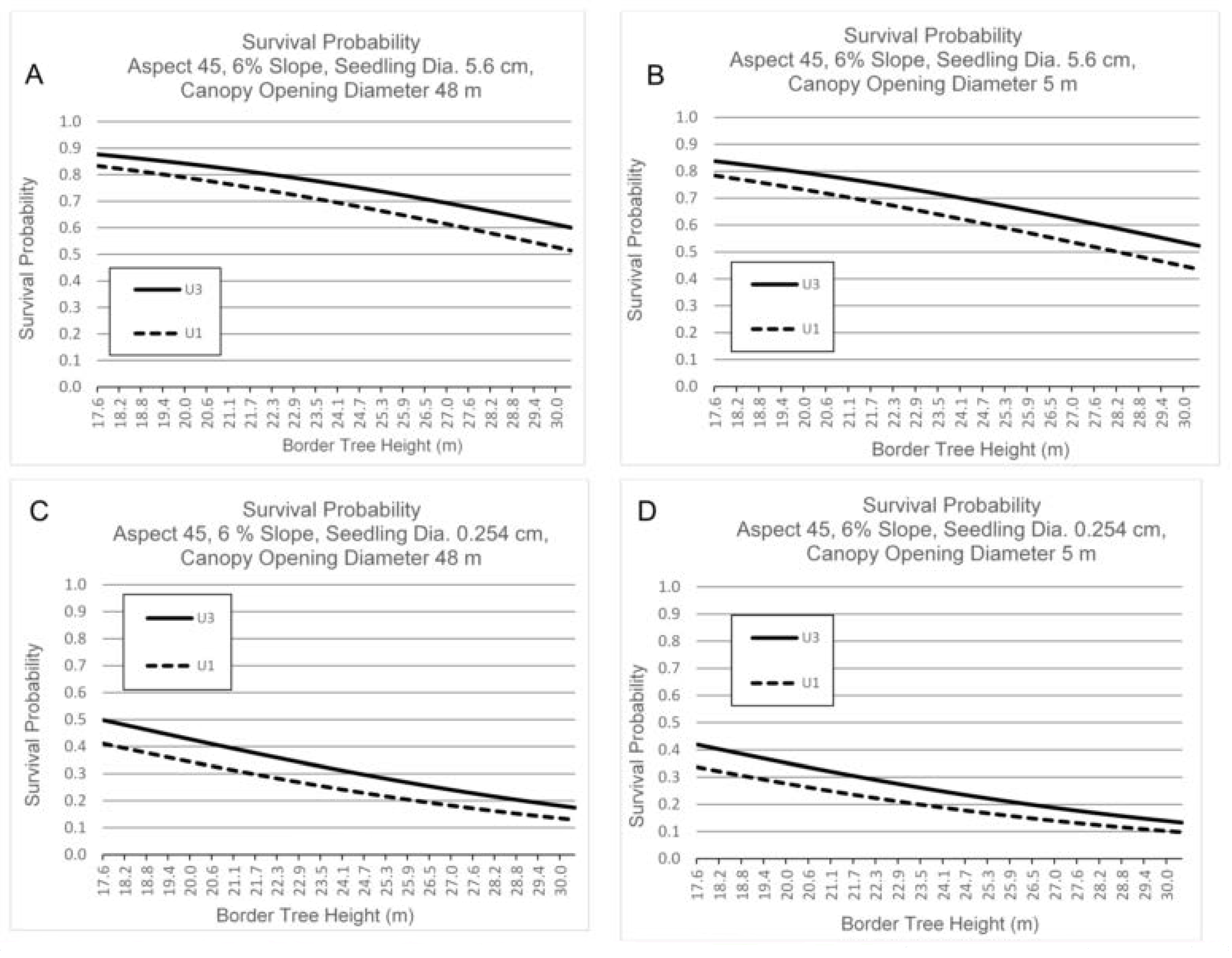
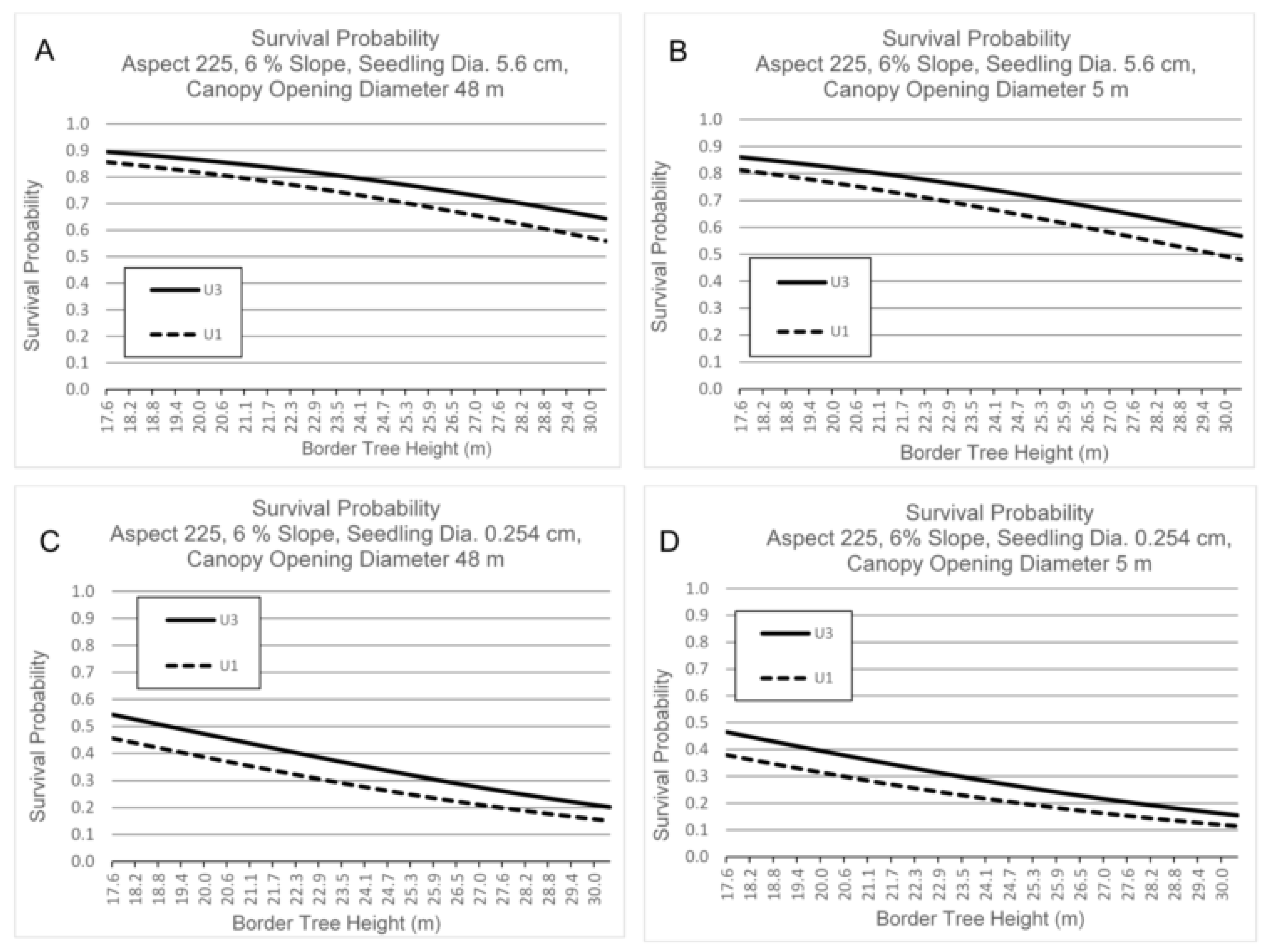
3.3. Survival by Canopy Opening Diameter, Border Tree Height, Seedling Basal Diameter, Aspect and Slope
4. Discussion
4.1. Competition Control
4.2. Reproduction Size and Survival
4.3. Canopy Opening Diameter, Border Tree Height, Aspect and Slope
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Johnson, P.S.; Shifley, S.R.; Rogers, R.; Dey, D.C.; Kabrick, J.M. The Ecology and Silviculture of Oaks, 3rd ed.; CABI Publishing: Wallingford Oxon, UK, 2019; p. 628. [Google Scholar]
- Dey, D.C.; Royo, A.A.; Brose, P.H.; Hutchinson, T.F.; Spetich, M.A.; Stoleson, S.H. An ecologically based approach to oak silviculture: A synthesis of 50 years of oak ecosystem research in North America. Rev. Columbia For. 2010, 13, 201–222. [Google Scholar]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture, Applied Forest Ecology, 9th ed.; John Wiley and Sons: New York, NY, USA, 1997; p. 560. ISBN 0-471-10941-X. [Google Scholar]
- Spetich, M.A.; Dey, D.C.; Johnson, P.S.; Graney, D.L. Competitive capacity of Quercus rubra L. planted in Arkansas’ Boston Mountains. For. Sci. 2002, 48, 504–517. [Google Scholar]
- Lorimer, C.G. The Oak Regeneration Problem: New Evidence on Causes and Possible Solutions (Forest Resource Analyses No. 8, Publ. R3484); Dept. of Forestry, University of Wisconsin-Madison: Madison, WI, USA, 1989; p. 31. [Google Scholar]
- Oak, S.W.; Spetich, M.A.; Morin, R.S. Chapter 3: Oak decline in central hardwood forests: Frequency, spatial extent, and scale. In Natural Disturbances and Historic Range of Variation: Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C.H., Collins, B.S., Eds.; Managing Forest Ecosystems; Springer International Publishing: Cham, Switzerland, 2015; Volume 32, p. 400. [Google Scholar]
- Spetich, M.A.; Fan, Z.; Sui, Z.; Crosby, M.K.; He, H.S.; Shifley, S.R.; Leininger, T.D.; Moser, W.K. Tree mortality estimates and species distribution probabilities in southeastern United States forests. In Forest Health Monitoring: National Status, Trends, and Analysis 2016; General Technical Report SRS-222; Potter, K.M., Conkling, B.L., Eds.; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2017; pp. 175–181. Available online: https://www.srs.fs.fed.us/pubs/54586 (accessed on 25 December 2017).
- Fan, Z.; Fan, X.; Crosby, M.K.; Moser, W.K.; He, H.S.; Spetich, M.A.; Shifley, S.R. Spatio-temporal trends of oak decline and mortality under periodic regional drought in the Ozark Highlands of Arkansas and Missouri. Forests 2012, 3, 614–631. [Google Scholar] [CrossRef]
- Norby, R.J.; Wullschleger, S.D.; Gunderson, C.A.; Nietch, C.T. Increased growth efficiency of Quercus alba trees in a CO2-enriched atmosphere. New Phytol. 1995, 131, 91–97. [Google Scholar] [CrossRef]
- Murphy, P.A.; Shelton, M.G.; Graney, D.L. Group selection—problems and possibilities and for the more shade-intolerant species. 9th Cent. Hardwood For. Conf. Gen. Tech. Rep. NC-161 St. Paul MN 1993, 229–247. [Google Scholar]
- Fan, Z.; Yao, Q.; Dey, D.; Spetich, M.A.; Ezell, A.; Shifley, S.S.; Kabrick, J.; Jensen, R. Efficacy and associated factors of even- and uneven-aged management to promote oak regeneration in the Missouri Ozarks. For. Sci. 2015, 61, 397–408. [Google Scholar] [CrossRef]
- Jenkins, M.A.; Parker, G.R. Composition and diversity of woody vegetation in silvicultural openings of southern Indiana forests. For. Ecol. Manag. 1998, 109, 57–74. [Google Scholar] [CrossRef]
- Wang, W.J.; He, H.S.; Spetich, M.A.; Shifley, S.R.; Thompson, F.R., III; Fraser, J.S. Modeling the effects of harvest alternatives on mitigating oak decline in a Central Hardwood Forest landscape. PLoS ONE 2013, 8, e66713. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Merritt, C. The central region. In Regional Silviculture of the United States, 2nd ed.; Barrett, J.W., Ed.; Wiley: New York, NY, USA, 1980; pp. 107–143. [Google Scholar]
- Bailey, R.G.; Avers, P.E.; King, T.; McNab, W.H. Ecoregions and subregions of the United States (map). U.S. Geological Survey, Scale 1:7,5000,000; colored. In Accompanied by a Supplementary Table of Map Unit Descriptions Compiled; McNab, W.H., Bailey, R.G., Eds.; USDA Forest Service: Washington, DC, USA, 1994. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; John Wiley and Sons Inc.: New York, NY, USA, 1989; p. 307. [Google Scholar]
- Lowell, K.E.; Mitchell, R.J.; Johnson, P.S.; Garrett, H.E.; Cox, G.S. Predicting growth and “Success” of coppice-regenerated oak stems. For. Sci. 1987, 33, 740–749. [Google Scholar]
- Johnson, P.S.; Rogers, R. A method for estimating the contribution of planted hardwoods to future stocking. For. Sci. 1985, 3, 883–891. [Google Scholar]
- Dey, D.C.; Johnson, P.S.; Garrett, H.E. Modeling the regeneration of oak stands in the Missouri Ozark Highlands. Can. J. For. Res. 1996, 6, 573–583. [Google Scholar] [CrossRef]
- Spetich, M.A.; Dey, D.C.; Johnson, P.S. Shelterwood-planted northern red oaks: Integrated costs and options. South J. Appl. For. 2009, 33, 182–186. [Google Scholar] [CrossRef][Green Version]
- Thomas-Van Gundy, M.A.; Wood, K.U.; Rentch, J.S. Impacts of wildfire recency and frequency on an Appalachian oak forest. J. For. 2015, 113, 393–403. [Google Scholar] [CrossRef]
- Brose, P.H. Long-term effects of single prescribed fires on hardwood regeneration in oak shelterwood stands. For. Ecol. Manag. 2010, 260, 1516–1524. [Google Scholar] [CrossRef]
- Craig, J.M.; Lhotka, J.M.; Stringer, J.W. Response of naturally regenerated and underplanted white oak (Quercus alba L.) seedlings 6-years following midstory removal. In Proceedings of the 18th Central Hardwood Forest Conference, Morgantown, WV, USA, 26–28 March 2012; Miller, G.W., Schuler, T.M., Gottschalk, K.W., Brooks, J.R., Grushecky, S.T., Spong, B.D., Rentch, J.S., Eds.; General Technical Report NRS-P-117; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2013; pp. 365–372. [Google Scholar]
- Brockway, D.G.; Outcalt, K.W. Gap-phase regeneration in longleaf pine wiregrass ecosystems. For. Ecol. Manag. 1998, 106, 125–139. [Google Scholar] [CrossRef]
- McGuire, J.P.; Mitchell, R.J.; Moser, E.B.; Picot, S.D.; Gjerstad, D.H.; Hedman, C.W. Gaps in a gappy forest: Plant resources, longleaf pine regeneration, and understory response to tree removal in longleaf pine savannas. Can. J. For. Res. 2001, 31, 765–778. [Google Scholar] [CrossRef]
- Wang, W.J.; Thompson, F.R.; He, H.S.; Fraser, J.S.; Dijak, W.D.; Spetich, M.A. Population dynamics has greater effects than climate change on tree species distribution in a temperate forest region. J. Biogeogr. 2018, 45, 2766–2778. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Silvics of North America: 1. Conifers; 2. hardwoods. In Agriculture Handbook 654; USDA Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Spetich, M.A.; Fan, Z.; He, H.S.; Wang, W.J.; Crosby, M.K.; Shifley, S.R. Oak decline across the Ozark Highlands- from stand to landscape and regional scale processes. In Proceedings of the 18th Biennial Southern Silvicultural Research Conference, Knoxville, TN, USA, 2–5 March 2015; General Technical Report SRS-222. U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2016; p. 641. [Google Scholar]
- Fischer, B.C. Designing forest openings for the group selection method. In Proceedings of the First Biennial Southern Silviculture Research Conference, Atlanta, GA, USA, 6–7 November 1980; Barnett, J., Ed.; USDA Forest Service General Technical Report SO-34; USDA Forest Service: Washington, DC, USA, 1981; pp. 274–277. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spetich, M.A. Survival of Quercus alba (White Oak) Advance Reproduction in Small Group and Single Tree Openings. Forests 2020, 11, 889. https://doi.org/10.3390/f11080889
Spetich MA. Survival of Quercus alba (White Oak) Advance Reproduction in Small Group and Single Tree Openings. Forests. 2020; 11(8):889. https://doi.org/10.3390/f11080889
Chicago/Turabian StyleSpetich, Martin A. 2020. "Survival of Quercus alba (White Oak) Advance Reproduction in Small Group and Single Tree Openings" Forests 11, no. 8: 889. https://doi.org/10.3390/f11080889
APA StyleSpetich, M. A. (2020). Survival of Quercus alba (White Oak) Advance Reproduction in Small Group and Single Tree Openings. Forests, 11(8), 889. https://doi.org/10.3390/f11080889



