Allometric Equations for Volume, Biomass, and Carbon in Commercial Stems Harvested in a Managed Forest in the Southwestern Amazon: A Case Study
Abstract
1. Introduction
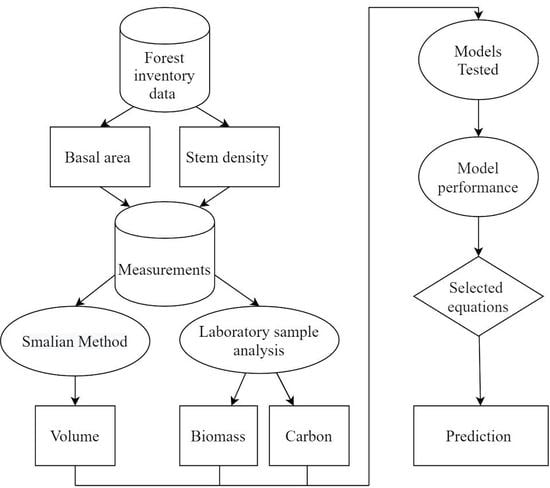
2. Materials and Methods
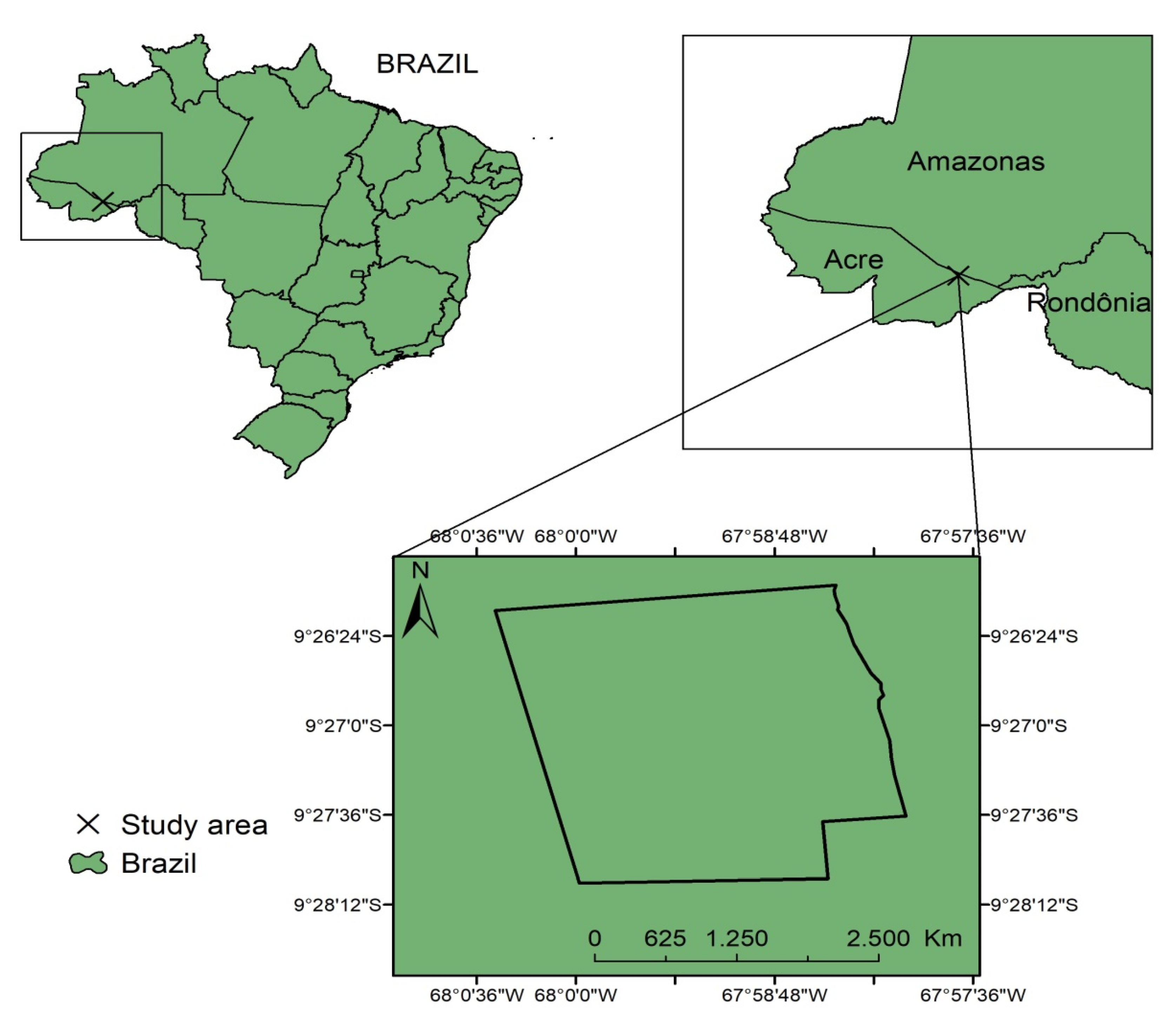
2.1. Study Area
2.2. Selection Criteria for Commercial Tree Species
2.3. Determination of Volume and Collection of Stem Wood Disks
2.4. Determination of Wood Density and Carbon Content of the Stem
2.5. Models Tested
3. Results
Models for Volume, Biomass, and Carbon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brazil, CONAMA (Conselho Nacional do Meio Ambiente). Resolução no 406, de 02 de fevereiro de 2009. Diário Oficial da União no 26, 2 February 2009. Brasília, DF, Brazil. Available online: http://www.tjpa.jus.br/CMSPortal/VisualizarArquivo?idArquivo=8372 (accessed on 3 August 2020).
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, Z.; Cienciala, E.; Mäkipää, R.; Muukkonen, P.; Lehtonen, A.; Weiss, P. Indirect methods of large-scale forest biomass estimation. Eur. J. For. Res. 2007, 126, 197–207. [Google Scholar] [CrossRef]
- Vidal, E.; West, T.A.P.; Putz, F.E. Recovery of biomass and merchantable timber volumes twenty years after conventional and reduced-impact logging in Amazonian Brazil. For. Ecol. Manag. 2016, 376, 1–8. [Google Scholar] [CrossRef]
- Alvarez, E.; Duque, A.; Saldarriaga, J.; Cabrera, K.; de, G.; Lema, A.; Moreno, F.; Orrego, S.; Rodríguez, L. Tree above-ground biomass allometries for carbon stocks estimation in the natural forests of Colombia. For. Ecol. Manag. 2012, 267, 297–308. [Google Scholar] [CrossRef]
- Basuki, T.M.; van Laake, P.E.; Skidmore, A.K.; Hussin, Y.A. Allometric equations for estimating the aboveground biomass in tropical lowland Dipterocarp forests. For. Ecol. Manag. 2009, 257, 1684–1694. [Google Scholar] [CrossRef]
- Brown, I.F.; Martinelli, L.A.; Thomas, W.W.; Moreira, M.Z.; Cid Ferreira, C.A.; Victoria, R.A. Uncertainty in the biomass of Amazonian forests: An example from Rondônia, Brazil. For. Ecol. Manag. 1995, 75, 175–189. [Google Scholar] [CrossRef]
- Chambers, J.Q.; dos Santos, J.; Ribeiro, R.J.; Higuchi, N. Tree damage, allometric relationships, and aboveground net primary production in central Amazon forest. For. Ecol. Manag. 2001, 152, 73–84. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Aguilar, S.; Hernandez, A.; Lao, S.; Perez, R. Error propagation and scaling for tropical forest biomass estimates. Philos Trans R Soc Lond B Biol Sci. 2004, 359, 409–420. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.; Chambers, J.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Goodman, R.C.; Phillips, O.L.; Baker, T.R. The importance of crown dimensions to improve tropical tree biomass estimates. Ecol. Appl. 2014, 24, 680–698. [Google Scholar] [CrossRef]
- Higuchi, N.; Ramm, W. Developing stem wood volume equations for a group of tree species of Central Amazon (Brazil). Commonw. For. Rev. 1985, 64, 33–41. Available online: https://www.jstor.org/stable/42608005 (accessed on 3 August 2020).
- Nelson, B.W.; Mesquita, R.; Pereira, J.L.G.; de Souza, S.G.A.; Batista, G.T.; Couto, L.B. Allometric regressions for improved estimates of secondary forest biomass in the central Amazon. For. Ecol. Manag. 1999, 117, 149–167. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Fearnside, P.M.; Nelson, B.W.; Barbosa, R.I.; Keizer, E.W.H. Estimates of forest biomass in the Brazilian Amazon: New allometric equations and adjustments to biomass from wood-volume inventories. For. Ecol. Manag. 2008, 256, 1853–1867. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Brancalion, P.H.S.; Laestadius, L.; Bennett-Curry, A.; Buckingham, K.; Kumar, C.; Moll-Rocek, J.; Vieira, I.C.G.; Wilson, S.J. When is a forest a forest? Forest concepts and definitions in the era of forest and landscape restoration. Ambio 2016, 45, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, E.O.; d’Oliveira, M.V.N.; Braz, E.M.; de Almeida Papa, D.; Fearnside, P.M. LIDAR-based estimation of boles biomass for precision management of an Amazonian forest: Comparisons of ground-based and remotely sensed estimates. Remote Sens. Environ. 2016, 187, 281–293. [Google Scholar] [CrossRef]
- Salimon, C.I.; Putz, F.E.; Menezes-Filho, L.; Anderson, A.; Silveira, M.; Brown, I.F.; Oliveira, L.C. Estimating state-wide biomass carbon stocks for a REDD plan in Acre, Brazil. For. Ecol. Manag. 2011, 262, 555–560. [Google Scholar] [CrossRef]
- Rockwell, C.A.; Kainer, K.A.; d’Oliveira, M.V.N.; Staudhammer, C.L.; Baraloto, C. Logging in bamboo-dominated forests in southwestern Amazonia: Caveats and opportunities for smallholder forest management. For. Ecol. Manag. 2014, 315, 202–210. [Google Scholar] [CrossRef]
- Lewis, L.S.; Brando, P.M.; Phillips, O.L.; van der Heijden, G.M.F.; Nepstad, D. The 2010 Amazon drought. Science 2011, 331, 554. [Google Scholar] [CrossRef]
- da Silva, S.S.; Fearnside, P.M.; Graça, P.M.L.A.; Brown, I.F.; Alencar, A.; de Melo, A.W.F. Dynamics of forest fires in the southwestern Amazon. For. Ecol. Manag. 2018, 424, 312–322. [Google Scholar] [CrossRef]
- Corlett, R.T. The impacts of droughts in tropical forests. Trends Plant Sci. 2016, 21, 584–593. [Google Scholar] [CrossRef]
- Phillips, O.L.; Aragão, L.E.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef]
- Ziccardi, L.G.; Graça, P.M.L.A.; Figueiredo, E.O.; Fearnside, P.M. Decline of large-diameter trees in a bamboo-dominated forest following anthropogenic disturbances in southwestern Amazonia. Ann. For. Sci. 2019, 76, 110. [Google Scholar] [CrossRef]
- de Avila, A.L.; Schwartz, G.; Ruschel, A.R.; Lopes, J.C.; Silva, J.N.M.; Carvalho, J.O.P.; Dormann, C.F.; Mazzei, L.; Soares, M.H.M.; Bauhus, J. Recruitment, growth and recovery of commercial tree species over 30 years following logging and thinning in a tropical rain forest. For. Ecol. Manag. 2017, 385, 225–235. [Google Scholar] [CrossRef]
- Fearnside, P.M. Brazil’s Amazonian forest carbon: The key to Southern Amazonia’s significance for global climate. Reg. Environ. Chang. 2018, 18, 47–61. [Google Scholar] [CrossRef]
- Brazil, SFB (Serviço Florestal Brasileiro). Estoque dasFlorestas. 2014. Available online: http://snif.florestal.gov.br/pt-br/estoques-das-florestas (accessed on 21 August 2019).
- d’Oliveira, M.V.N.; Reutebuch, S.E.; McGaughey, R.J.; Andersen, H.E. Estimating forest biomass and identifying low-intensity logging areas using airborne scanning lidar in Antimary State Forest, Acre State, Western Brazilian Amazon. Remote Sens. Environ. 2012, 124, 479–491. [Google Scholar] [CrossRef]
- de Melo, A.W.F. Alometria de Árvores e Biomassa Florestal na Amazônia Sul-Ocidental. Ph.D. Thesis, Tropical Forest Science, Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Amazonas, Brazil, 2017. Available online: https://bdtd.inpa.gov.br/bitstream/tede/2389/5/20170911_tese_willian_v05_Final.pdf (accessed on 3 August 2020).
- Romero, F.M. Contribuição do manejo sustentável em floresta do bioma amazônico para minimização de gases de efeito estufa. Ph.D. Thesis, Forest Science, Universidade Federal de Viçosa (UFV), Viçosa, Minas Gerais, Brazil, 2018. Available online: https://www.locus.ufv.br/bitstream/handle/123456789/23560/texto%20completo.pdf (accessed on 3 August 2020).
- Selivon, C.A. Plano de Operação Anual-POA, UPA-002; Fazenda Antimari I e II. Fox Laminados Ltd.a: Rio Branco, Acre, Brazil, 2014. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef]
- d’Oliveira, M.V.N.; Braz, E.M. Estudo da dinâmica da floresta manejada no projeto de manejo florestal comunitário do PC Pedro Peixoto na Amazônia Ocidental. Acta Amaz. 2006, 36, 177–182. [Google Scholar] [CrossRef]
- Acre, SEMA (Secretaria do Meio Ambiente). Guia para o uso da terra acreana com sabedoria: Resumo educativo do Zoneamento Ecológico-Econômico do Acre: Fase II (escala 1: 250.000). Doc. Síntese do ZEE, Secretaria do Meio Ambiente (SEMA) Rio Branco, Acre, Brazil. 2010, p. 152. Available online: https://gcftaskforce-database.org/public/assets/downloads/GCFF/database/Acre%20-%20ZEE%20Resumen.pdf (accessed on 3 August 2020).
- Radambrasil, P. Levantamento dos Recursos Naturais; Departamento Nacional de Produção Mineral: Rio de Janeiro, Brazil, 1976; p. 454. [Google Scholar]
- d’Souza, A.L.; Soares, C.P.B. Florestas Nativas—Estruturas, dinâmica e manejo, 1st ed.; Editora da Universidade Federal de Viçosa (UFV): Viçosa, Minas Gerais, Brazil, 2013. [Google Scholar]
- REFLORA. Flora do Brasil 2020—Algas, fungos e plantas. 2019. Available online: http://floradobrasil.jbrj.gov.br/reflora/listaBrasil/PrincipalUC/PrincipalUC.do;jsessionid=21250D75FD7F1FEE1A402F5E30D08648#CondicaoTaxonCP (accessed on 16 July 2019).
- Soares, C.P.B.; Neto, P.F.; Souza, L.A. Dendrometria e Inventário Florestal, 2nd ed.; Editora da Universidade Federal de Viçosa (UFV): Viçosa, Minas Gerais, Brazil, 2011; p. 272. [Google Scholar]
- Ellis, P.W.; Gopalakrishna, T.; Putz, F.E.; Roopsind, A.; Umunay, P.M.; Zalman, J.; Ellis, E.A.; Mo, K.; Gregoire, T.G.; Griscom, B.W. Reduced-impact logging for climate change mitigation (RIL-C) can halve selective logging emissions from tropical forests. For. Ecol. Manag. 2019, 438, 255–266. [Google Scholar] [CrossRef]
- Campos, J.C.C.; Leite, H.G. Mensuração Florestal, 5th ed.; Editora da Universidade Federal de Viçosa (UFV): Viçosa, Minas Gerais, Brazil, 2017. [Google Scholar]
- Husch, B.; Beers, T.W.; Kershaw, J.A. Forest Mensuration; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Silva, H.F.; Ribeiro, S.C.; Botelho, S.A.; Faria, R.A.V.B.; Teixeira, M.B.R.; Mello, J.M. Estimativa do estoque de carbono por métodos indiretos em área de restauração florestal em Minas Gerais. Sci. For. 2015, 43, 943–953. [Google Scholar] [CrossRef]
- Husch, B. Forest Mensuration and Statistics; Ronald Press: New York, NY, USA, 1963. [Google Scholar]
- Spurr, S.H. Forest Inventory; Ronald: New York, NY, USA, 1952. [Google Scholar]
- Schumacher, F.X.; Hall, F.S. Logarithmic expression of timber-tree volume. J. Agric. Res. 1933, 47, 719–734. Available online: https://naldc.nal.usda.gov/download/IND43968352/PDF (accessed on 3 August 2020).
- Loetsch, F.; Zöhrer, F.; Haller, K.E. Forest Inventory; BLV Verlagsgesellschaft: Munich, Germany, 1973. [Google Scholar]
- Draper, N.R.; Smith, H. Applied Regression Analysis; Wiley: New York, NY, USA, 1966; p. 407. [Google Scholar]
- Gunst, R.F. Applied regression analysis. Technometrics 2012, 41, 265–266. [Google Scholar] [CrossRef]
- Shewhart, W.; Wilks, S.S. Applied Regression Analysis; Wiley-Interscience: New York, NY, USA, 1967. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Gujarati, D.N.; Porter, D.C. Econometria Básica, 5th ed.; AMGH Editora Ltda.: Porto Alegre, Brazil, 2011. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2019. Available online: https://www.R-project.org/ (accessed on 3 August 2020).
- Feldpausch, T.R.; Banin, L.; Phillips, O.L.; Baker, T.R.; Lewis, S.L.; Quesada, C.A.; Affum-Baffoe, K.; Arets, E.J.M.M.; Berry, N.J.; Bird, M.; et al. Height-diameter allometry of tropical forest trees. Biogeosciences 2011, 8, 1081–1106. [Google Scholar] [CrossRef]
- Feldpausch, T.R.; Lloyd, J.; Lewis, S.L.; Brienen, R.J.; Gloor, M.; Monteagudo Mendoza, A.; Lopez-Gonzalez, G.; Banin, L.; Salim, K.A.; Affum-Baffoe, K.; et al. Tree height integrated into pantropical forest biomass estimates. Biogeosciences 2012, 9, 3381–3403. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Nelson, B.W.; Fearnside, P.M.; França, M.B.; de Oliveira, Á.C.A. Tree height in Brazil’s “arc of deforestation”: Shorter trees in south and southwest Amazonia imply lower biomass. For. Ecol. Manag. 2008, 255, 2963–2972. [Google Scholar] [CrossRef]
- Baker, T.R.; Phillips, O.L.; Malhi, Y.; Almeida, S.; Arroyo, L.; Di Fiore, A.; Erwin, T.; Killeen, J.T.; Laurance, G.S. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob. Chang. Biol. 2004, 10, 1–18. [Google Scholar] [CrossRef]
- Chave, J.; Muller-Landau, H.C.; Baker, T.R.; Easdale, T.A.; ter Steege, H.; Webb, C.O. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol. Appl. 2006, 16, 2356–2367. [Google Scholar] [CrossRef]
- Silprandi, N.C.; Nogueira, E.M.; Toledo, J.J.; Fearnside, P.M.; Nascimento, H.E.M. Inter-site variation in allometry and wood density of Goupia glabra Aubl. in Amazonia. Braz. J. Biol. 2016, 76, 268–276. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Nelson, B.W.; Fearnside, P.M. Wood density in dense forest in central Amazonia, Brazil. For. Ecol. Manag. 2005, 208, 261–286. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Fearnside, P.M.; Nelson, B.W.; França, M.B. Wood density in forests of Brazil’s “arc of deforestation”: Implications for biomass and flux of carbon from land-use change in Amazonia. For. Ecol. Manag. 2007, 248, 119–135. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Fearnside, P.M.; Nelson, B.W. Normalization of wood density in biomass estimates of Amazon forests. For. Ecol. Manag. 2008, 256, 990–996. [Google Scholar] [CrossRef]
- GlobAllomeTree, N.D. GlobAllomeTree is an international web platform to share data for assessing volume, biomass and carbon stock of trees and forests. Available online: http://globallometree.org/ (accessed on 16 July 2019).
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Global Wood Density Database. 2009. Available online: https://dryad.figshare.com/articles/Global_Wood_Density_Database/4172847 (accessed on 3 August 2020).
- Fearnside, P.M. Wood density for estimating forest biomass in Brazilian Amazonia. For. Ecol. Manag. 1997, 90, 59–89. [Google Scholar] [CrossRef]
- Higuchi, N.; Santos, J.; Ribeiro, R.J.; Minette, L.; Biot, Y. Biomassa da parte aérea da vegetação da floresta tropical úmida de terra-firme da Amazônia Brasileira. Acta Amaz. 1998, 28, 153–166. [Google Scholar] [CrossRef]
- Brown, S.; Gillespie, A.J.R.; Lugo, A.E. Biomass estimation methods for tropical forests with applications to forest inventory data. For. Sci. 1989, 35, 881–902. [Google Scholar] [CrossRef]
- Brown, I.F.; Nepstad, D.C.; Pires, I.O.; Luz, M.L.; Alechandre, A.S. Carbon storage and land-use in extractive reserves, Acre, Brazil. For. Sci. 2009, 19, 307–315. [Google Scholar] [CrossRef]
- Brown, S. Estimating Biomass and Biomass Change of Tropical Forests: A Primer. In FAO Forestry Paper 134; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1997; Available online: http://www.fao.org/3/w4095e/w4095e00.htm (accessed on 3 August 2020).
- da Silva, R.P. Alometria, estoque e dinâmica da biomassa de florestas primárias e secundárias na região de Manaus (AM). Ph.D. Thesis, Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Amazonas, Brazil, 2007. Available online: https://www.inpa.gov.br/arquivos/Tese_Biomassa_Roseana_Silva.pdf (accessed on 3 August 2020).
- MCT (Ministério da Ciência e Tecnologia). Second National Communication of Brazil to the United Nations Framework Convention on Climate Change. MCT: Brasília, DF, Brazil, 2010. Available online: https://unfccc.int/resource/docs/natc/branc3v1.pdf and https://unfccc.int/resource/docs/natc/branc3v2.pdf (accessed on 3 August 2020).
- França, M.B. Modelagem de Biomassa Através do Padrão Espectral no Sudoeste da Amazônia. Master’s Thesis, Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Amazonas, Brazil, 2002. Available online: https://bdtd.inpa.gov.br/handle/tede/2953 (accessed on 3 August 2020).
- IPCC (Intergovernmental Panel on Climate Change). Forest Lands. In Intergovernmental Panel on Climate Change Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006; p. 83. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/ (accessed on 3 August 2020).
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Niklas, K.J. Size-dependent allometry of tree height, diameter and trunk-taper. Ann. Bot. 1995, 75, 217–227. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the structure and allometry of plant vascular systems. Nature 1999, 400, 664–667. [Google Scholar] [CrossRef]
- Sileshi, G.W. A critical review of forest biomass estimation models, common mistakes and corrective measures. For. Ecol. Manag. 2014, 329, 237–254. [Google Scholar] [CrossRef]
- Cox, P.M.; Harris, P.P.; Huntingford, C.; Betts, R.A.; Collins, M.; Jones, C.D.; Jupp, T.E.; Marengo, J.A.; Nobre, C.A. Increasing risk of Amazonian drought due to decreasing aerosol pollution. Nature 2008, 453, 212–215. [Google Scholar] [CrossRef]
- Evan, A.T.; Vimont, D.J.; Heidinger, A.K.; Kossin, J.P.; Bennartz, R. The role of aerosols in the evolution of tropical North Atlantic Ocean temperature anomalies. Science 2009, 324, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Wood, D.; Baker, T.R.; Wright, J.; Phillips, O.L.; Cochrane, T.; Meir, P.; Chave, J.; Almeida, S.; Arroyo, L.; et al. The regional variation of aboveground live biomass in old-growth Amazonian forests. Glob. Chang. Biol. 2006, 12, 1107–1138. [Google Scholar] [CrossRef]
- Goodman, R.C.; Aramburu, M.H.; Gopalakrishna, T.; Putz, F.E.; Gutiérrez, N.; Mena Alvarez, J.L.; Aguilar-Amuchastegui, N.; Ellis, P.W. Carbon emissions and potential emissions reductions from low-intensity selective logging in southwestern Amazonia. For. Ecol. Manag. 2019, 439, 18–27. [Google Scholar] [CrossRef]
- Fearnside, P.M.; Nogueira, E.M.; Yanai, A.M. Maintaining carbon stocks in extractive reserves in Brazilian Amazonia. Desenvolv. e Meio Ambiente 2018, 48, 446–476. [Google Scholar] [CrossRef]
| No | Model | Type | Author |
|---|---|---|---|
| Volume | |||
| MV1 | ln(V) = β0 + β1 ln(d) + ε | Linear | Husch (1963; logarithmic) [42] |
| MV2 | ln(V) = β0 + β1 ln(d2l) + ε | Linear | Spurr (1952; logarithmic) [43] |
| MV3 | ln(V) = β0 + β1 ln(d) + β2ln(l) + ε | Linear | Schumacher and Hall (1933; logarithmic) [44] |
| MV4 | V = β0 + ε | Linear | Husch (1963) [42] |
| MV5 | V = β0 (d2l) β1 + ε | Nonlinear | Spurr (1952) [43] |
| MV6 | V = β0 + ε | Nonlinear | Schumacher and Hall (1933) [44] |
| Biomass | |||
| MB1 | ln(B) = β0 + β1 ln(d) + β2 ln(p) + ε | Linear | Chave et al. (2005) [10] |
| MB2 | ln(B) = β0 + β1 ln(d2l) + β2 ln(p) + ε | Linear | Loetsch et al. (1973) [45] |
| MB3 | ln(B) = β0 + β1 ln(d) + β2ln(l) + β3ln(p) + ε | Linear | Schumacher and Hall (1933; logarithmic modified) [44] |
| MB4 | ln(B) = β0 + β1 ln(d) + β2ln(l) + ε | Linear | Schumacher and Hall (1933; logarithmic) [44] |
| MB5 | B = β0 + ε | Nonlinear | Schumacher and Hall (1933) [44] |
| MB6 | B = β0 + ε | Nonlinear | Schumacher and Hall (1933; modified) [44] |
| Carbon | |||
| MC1 | ln(C) = β0 + β1ln(d) + β2ln(l) + β3ln(p) + β4ln(t) + ε | Linear | Schumacher and Hall (1933; logarithmic) [44] |
| MC2 | ln(C) = β0 + β1ln(d2l) + β2ln(p) + β3ln(t) + ε | Linear | Loetsch et al. (1973; modified) [45] |
| MC3 | ln(C) = β0 + β1ln(d2l) + β2ln(pt) + ε | Linear | Loetsch et al. (1973; modified) [45] |
| MC4 | ln(C) = β0 + β1ln(d) + β2ln(l) + β3ln(p) + ε | Linear | Schumacher and Hall (1933; logarithmic modified) [44] |
| MC5 | ln(C) = β0 + β1ln(d) + β2ln(l) +ε | Linear | Schumacher and Hall (1933; logarithmic modified) [44] |
| Family | Scientific Name | N | DBH (cm) | p (g cm−3) | ||
|---|---|---|---|---|---|---|
| Range | Mean (±SD) | Range | Mean (±SD) | |||
| Bignoniaceae Juss. | Handroanthus serratifolius (Vahl) S.Grose | 8 | 50.9–78 | 61.8 ± 9.5 | 0.76–0.87 | 0.82 ± 0.04 |
| Combretaceae R.Br. | Buchenavia tetraphylla (Aubl.) R.A.Howard | 9 | 50.4–89.1 | 70.7 ± 12.9 | 0.64–0.76 | 0.69 ± 0.04 |
| Euphorbiaceae Juss. | Hura crepitans L. | 6 | 74.9–121 | 96.5 ± 17.1 | 0.27–0.43 | 0.36 ± 0.05 |
| Fabaceae Lindl. | Albizia niopoides (Spruce ex Benth.) Burkart | 7 | 54.7–79.3 | 65.8 ± 8.1 | 0.61–0.68 | 0.64 ± 0.03 |
| Apuleia leiocarpa (Vogel) J.F.Macbr. | 13 | 64.3–130.5 | 957 ± 17.6 | 0.71–0.83 | 0.77 ± 0.03 | |
| Barnebydendron riedelii (Tul.) J.H.Kirkbr. | 5 | 66.8–85.9 | 77 ± 7.6 | 0.54–0.62 | 0.57 ± 0.03 | |
| Copaifera multijuga Hayne | 6 | 78.9–136.9 | 97.8 ± 21.8 | 0.47–0.60 | 0.52 ± 0.05 | |
| Dipteryx odorata (Aubl.) Willd. | 11 | 70–123.5 | 90.4 ± 16.2 | 0.75–0.89 | 0.80 ± 0.04 | |
| Hymenaea courbaril L. | 8 | 66.2–121 | 93.5 ± 17.6 | 0.71–0.84 | 0.76 ± 0.04 | |
| Parkia paraensis Ducke | 20 | 51.2–149.6 | 86.9 ± 27 | 0.38–0.56 | 0.46 ± 0.06 | |
| Schizolobium parahyba var. amazonicum (Huber ex Ducke) Barneby | 16 | 50.9–89.1 | 62.8 ± 10.8 | 0.31–0.65 | 0.48 ± 0.08 | |
| Lecythidaceae A.Rich. | Eschweilera grandiflora (Aubl.) Sandwith | 13 | 55.4–111.4 | 76.4 ± 16.4 | 0.69–0.79 | 0.73 ± 0.03 |
| Malvaceae Juss. | Ceiba pentandra (L.) Gaertn. | 4 | 99.9–149.9 | 130.2 ± 24.4 | 0.27–0.32 | 0.29 ± 0.03 |
| Ceiba samauma (Mart.) K.Schum. | 22 | 66.5–111.4 | 78.9 ± 10.3 | 0.42–0.65 | 0.51 ± 0.06 | |
| Sterculia apetala (Jacq.) H.Karst. | 5 | 70–82.8 | 75.9 ± 5.3 | 0.31–0.47 | 0.38 ± 0.06 | |
| Meliaceae A.Juss. | Cedrela odorata L. | 8 | 57.3–118.1 | 70.7 ± 20.2 | 0.34–0.47 | 0.43 ± 0.044 |
| Moraceae Gaudich. | Castilla ulei Warb. | 37 | 56.7–121 | 79.7 ± 15.1 | 0.34–0.48 | 0.41 ± 0.04 |
| Ficus insipida Willd. | 4 | 74.8–99.9 | 82.5 ± 11.8 | 0.34–0.39 | 0.35 ± 0.03 | |
| Anacardiaceae R.Br. | Astronium lecointei Ducke | 6 | 52.6–96.4 | 62.7 ± 16.7 | 0.73–0.85 | 0.82 ± 0.05 |
| Lecythidaceae A.Rich. | Eschweilera bracteosa (Poepp. ex O.Berg) Miers | 15 | 54.1–95.5 | 68.1 ± 10.2 | 0.54–0.72 | 0.65 ± 0.05 |
| Total | 223 | 50.4–149.9 | 79.6 ± 19.8 | 0.27–0.89 | 0.56 ± 0.16 | |
| Model | RMSE | R2 | AIC | MAD | CF | |||
|---|---|---|---|---|---|---|---|---|
| MV1 | −6.51250 * | 1.88558 * | 2.318 | 0.67 | 1011.70 | 1.621 | 1.0527 | |
| MV2 | −8.23500 * | 0.8734 * | 1.637 | 0.83 | 856.53 | 1.059 | 1.0242 | |
| MV3 | −8.23250 * | 1.74399 * | 0.87702 * | 1.641 | 0.83 | 858.71 | 1.059 | 1.0243 |
| MV4 | 0.0014095 * | 1.909563 * | 2.317 | - | 1011.52 | 1.615 | - | |
| MV5 | 0.000322 * | 0.859100 * | 1.635 | - | 856.16 | 1.068 | - | |
| MV6 | 0.000313 * | 1.7610 * | 0.80000 * | 1.634 | - | 856.76 | 1.066 | - |
| Model | RMSE | R2 | AIC | MAD | CF | ||||
|---|---|---|---|---|---|---|---|---|---|
| MB1 | −6.51456 * | 1.93113 * | 1.317114 * | 1.296 | 0.78 | 753.60 | 0.857 | 1.048296 | |
| MB2 | −8.26306 * | 0.87461 * | 0.97690 * | 1.047 | 0.86 | 658.12 | 0.609 | 1.024296 | |
| MB3 | −8.26077 * | 1.73728 * | 0.89154 * | 0.96957 * | 1.052 | 0.86 | 661.39 | 0.611 | 1.024398 |
| MB4 | −9.16151 * | 1.52337 * | 1.35403 * | 1.696 | 0.62 | 873.64 | 1.027 | 1.059213 | |
| MB5 | 0.0002996 * | 1.367517 * | 1.254061 * | 1.679 | - | 869.06 | 1.049 | - | |
| MB6 | 0.0003331 * | 1.821004 * | 0.712642 * | 1.15938 * | 1.025 | - | 649.79 | 0.615 | - |
| Model | RMSE | R2 | AIC | MAD | CF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MC1 | −6.59205 * | 1.73329 * | 0.90503 * | 0.91298 * | 3.45897 * | 0.503 | 0.87 | 333.95 | 0.294 | 1.023900 |
| MC2 | −6.62824 * | 0.87661 * | 0.92627 * | 3.411260 * | 0.500 | 0.87 | 330.55 | 0.293 | 1.023819 | |
| MC3 | −8.26837 * | 0.87431 * | 0.98260 * | 0.530 | 0.85 | 355.15 | 0.304 | 1.024306 | ||
| MC4 | −8.93939 * | 1.73890 * | 0.88605 * | 0.99259 * | 0.551 | 0.84 | 373.57 | 0.310 | 1.212228 | |
| MC5 | −9.86152 * | 1.51991 * | 1.35952 * | 0.883 | 0.59 | 582.37 | 0.526 | 1.061534 |
| Author | Equation (a) | CBB | DEV | PDO | SAE |
|---|---|---|---|---|---|
| This study | ln(CBB) = −8.26306 + 0.87461 ln(d2l) + 0.97690 ln(p) | 784.51 | −24.04 | −2.97 | |
| Higuchi et al., 1998 [65] | FAGB = 0.0009 × d1.585 × h2.651 | 229.0 | −579.5 | −71.7 | |
| Chave et al., 2014 [2] | AGB = 0.0673 × (pd2h)0.976 | 384.42 | −424.13 | −52.46 | |
| Nogueira et al., 2008 [14] | ln(TBB) = −1.929 + 2.335 × ln(d) | 956..89 | +148.34 | +18.35 | |
| Nogueira et al., 2008 [14] | ln(AGB) = −1.716 + 2.413 × ln(d) | 1045.10 | +236.55 | +29.26 | d’Oliveira et al., 2012 [27] |
| S. Brown et al.,1989 [66] | AGB = 34.4703 − 8.0671 × d + 0.6589 × d2 | 181.90 | −626.65 | −77.50 | I.F. Brown et al., 2009 [67] |
| S. Brown, 1997 [68] | AGB = 42.69 − 12.800 × d + 1.242 × d2 | 973.70 | +165.15 | +20.43 | Salimon et al., 2011 [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, F.M.B.; Jacovine, L.A.G.; Ribeiro, S.C.; Torres, C.M.M.E.; Silva, L.F.d.; Gaspar, R.d.O.; Rocha, S.J.S.S.d.; Staudhammer, C.L.; Fearnside, P.M. Allometric Equations for Volume, Biomass, and Carbon in Commercial Stems Harvested in a Managed Forest in the Southwestern Amazon: A Case Study. Forests 2020, 11, 874. https://doi.org/10.3390/f11080874
Romero FMB, Jacovine LAG, Ribeiro SC, Torres CMME, Silva LFd, Gaspar RdO, Rocha SJSSd, Staudhammer CL, Fearnside PM. Allometric Equations for Volume, Biomass, and Carbon in Commercial Stems Harvested in a Managed Forest in the Southwestern Amazon: A Case Study. Forests. 2020; 11(8):874. https://doi.org/10.3390/f11080874
Chicago/Turabian StyleRomero, Flora Magdaline Benitez, Laércio Antônio Gonçalves Jacovine, Sabina Cerruto Ribeiro, Carlos Moreira Miquelino Eleto Torres, Liniker Fernandes da Silva, Ricardo de Oliveira Gaspar, Samuel José Silva Soares da Rocha, Christina Lynn Staudhammer, and Philip Martin Fearnside. 2020. "Allometric Equations for Volume, Biomass, and Carbon in Commercial Stems Harvested in a Managed Forest in the Southwestern Amazon: A Case Study" Forests 11, no. 8: 874. https://doi.org/10.3390/f11080874
APA StyleRomero, F. M. B., Jacovine, L. A. G., Ribeiro, S. C., Torres, C. M. M. E., Silva, L. F. d., Gaspar, R. d. O., Rocha, S. J. S. S. d., Staudhammer, C. L., & Fearnside, P. M. (2020). Allometric Equations for Volume, Biomass, and Carbon in Commercial Stems Harvested in a Managed Forest in the Southwestern Amazon: A Case Study. Forests, 11(8), 874. https://doi.org/10.3390/f11080874