Soil Resistance to Burn Severity in Different Forest Ecosystems in the Framework of a Wildfire
Abstract
1. Introduction
2. Materials and Methods
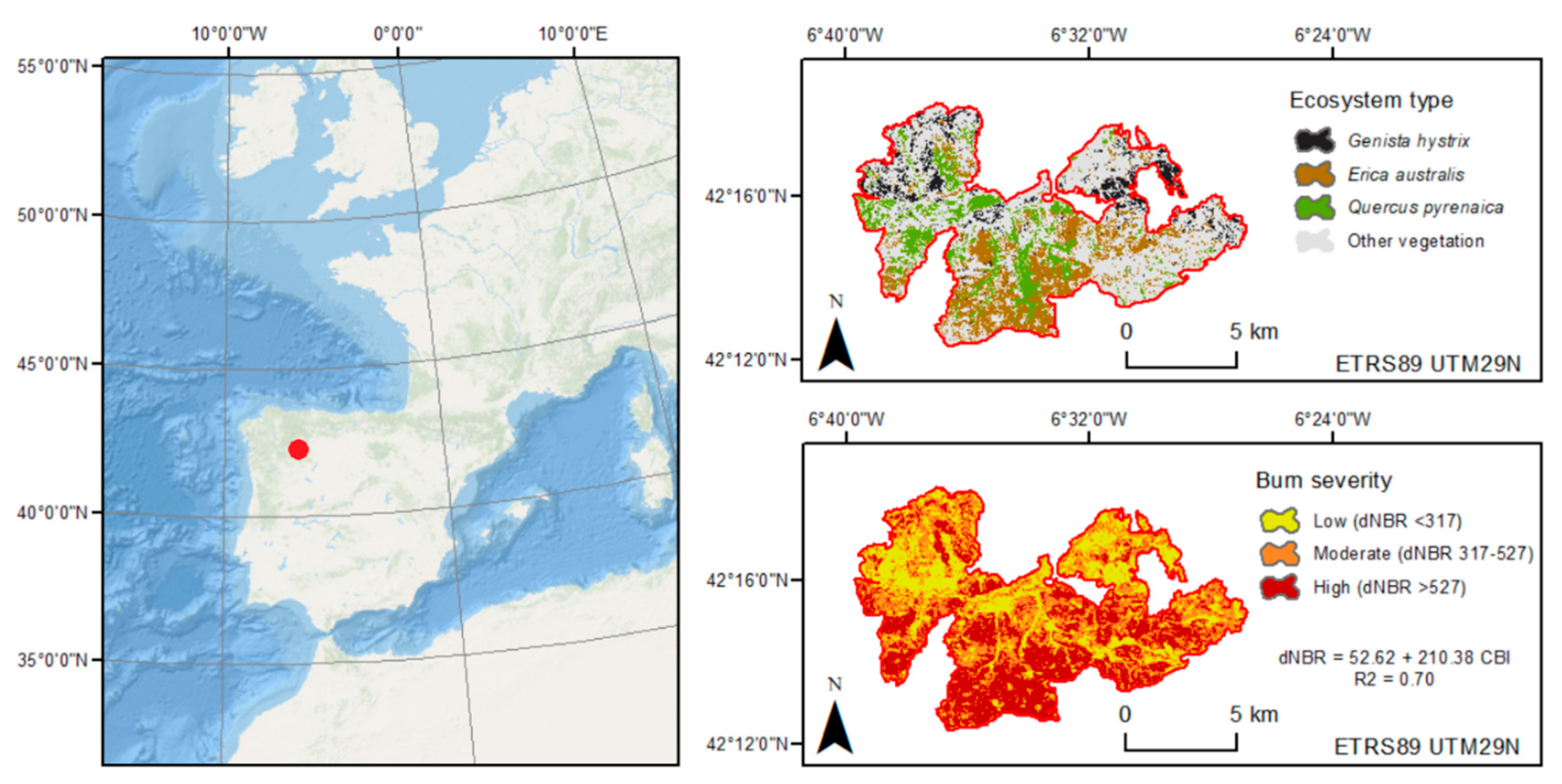
2.1. Study Area
2.2. Field Sampling
2.3. Soil Analysis
2.4. Resistance Calculations
2.5. Data Analysis
3. Results
3.1. General Values
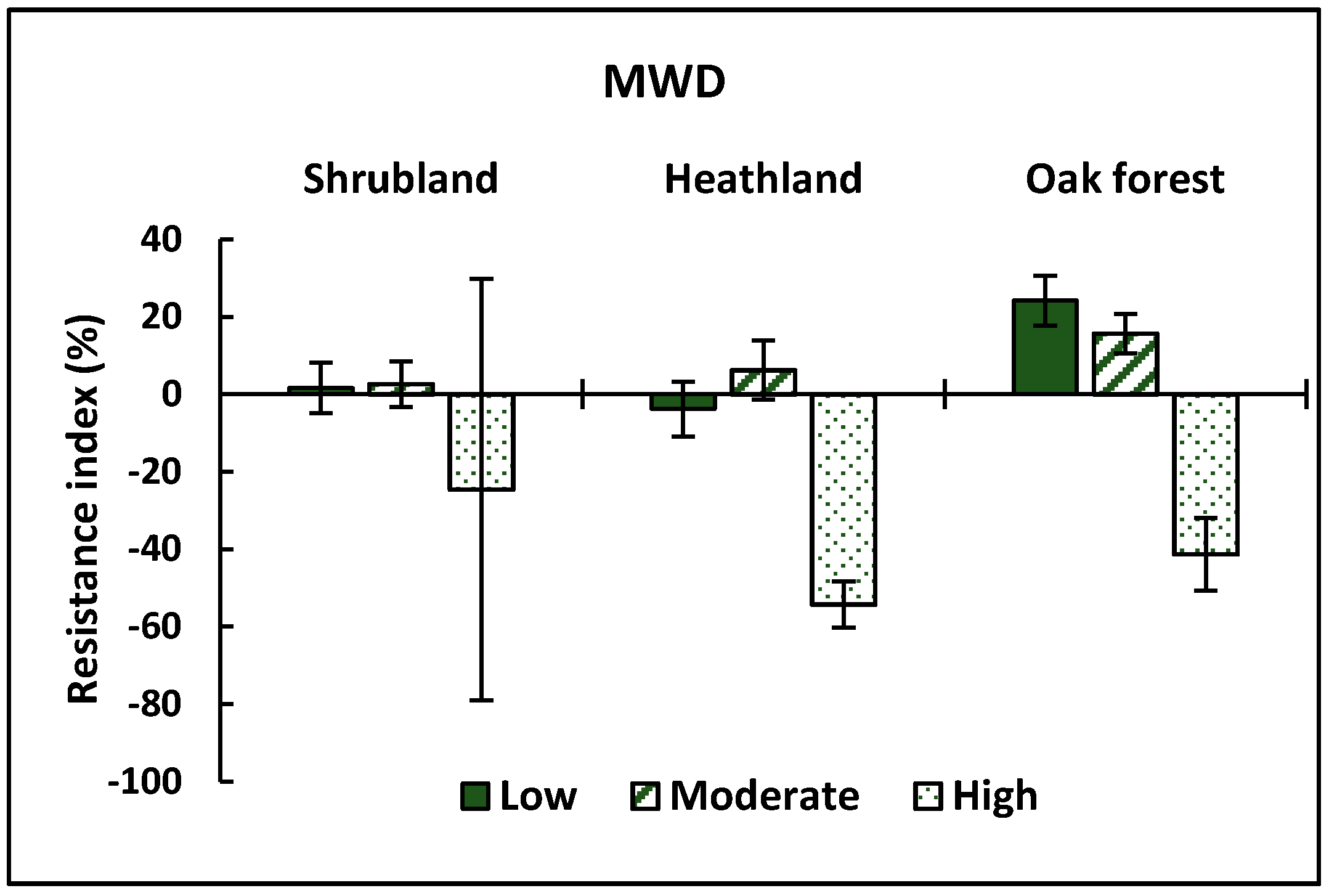
3.2. Physical Properties
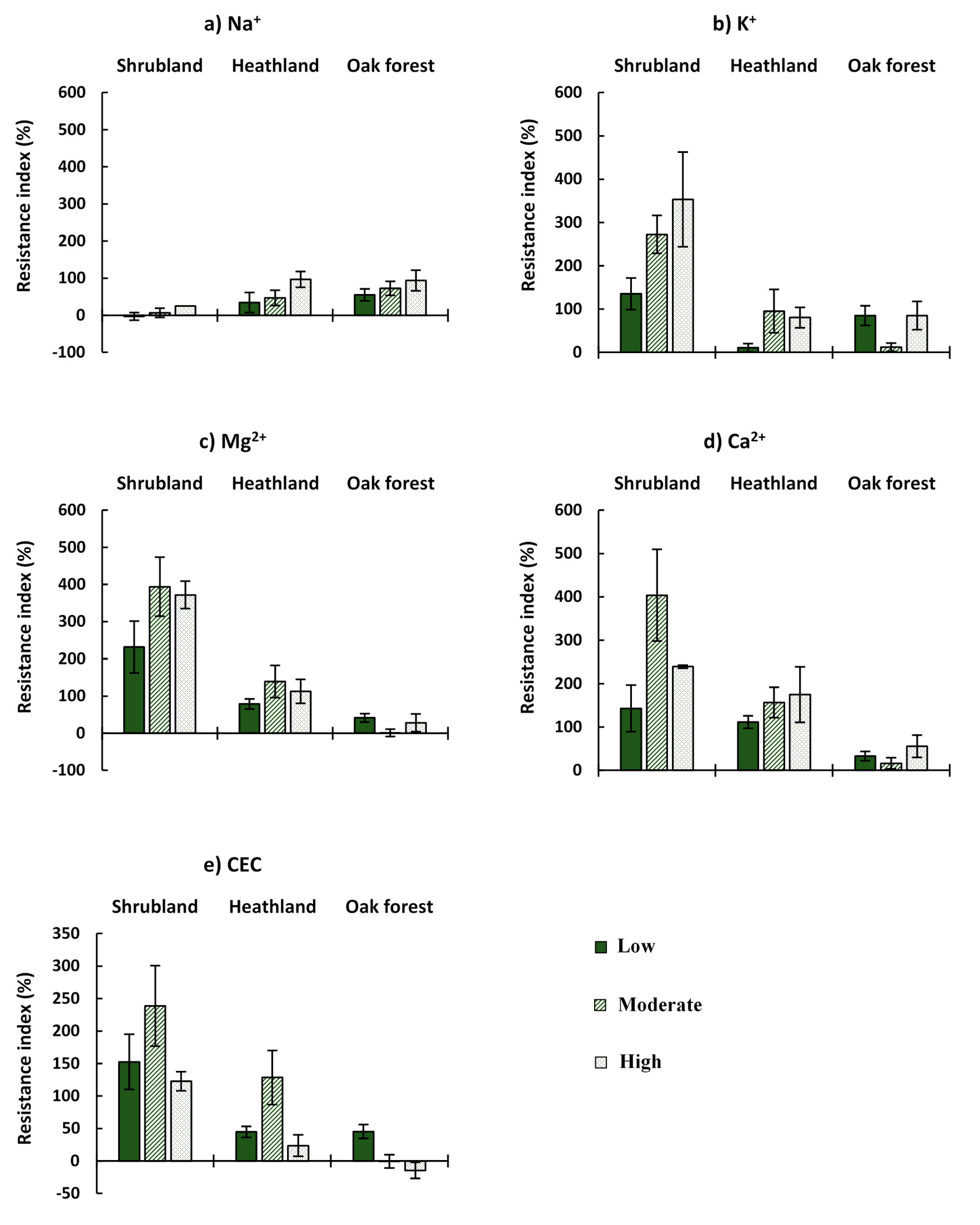
3.3. Chemical Properties
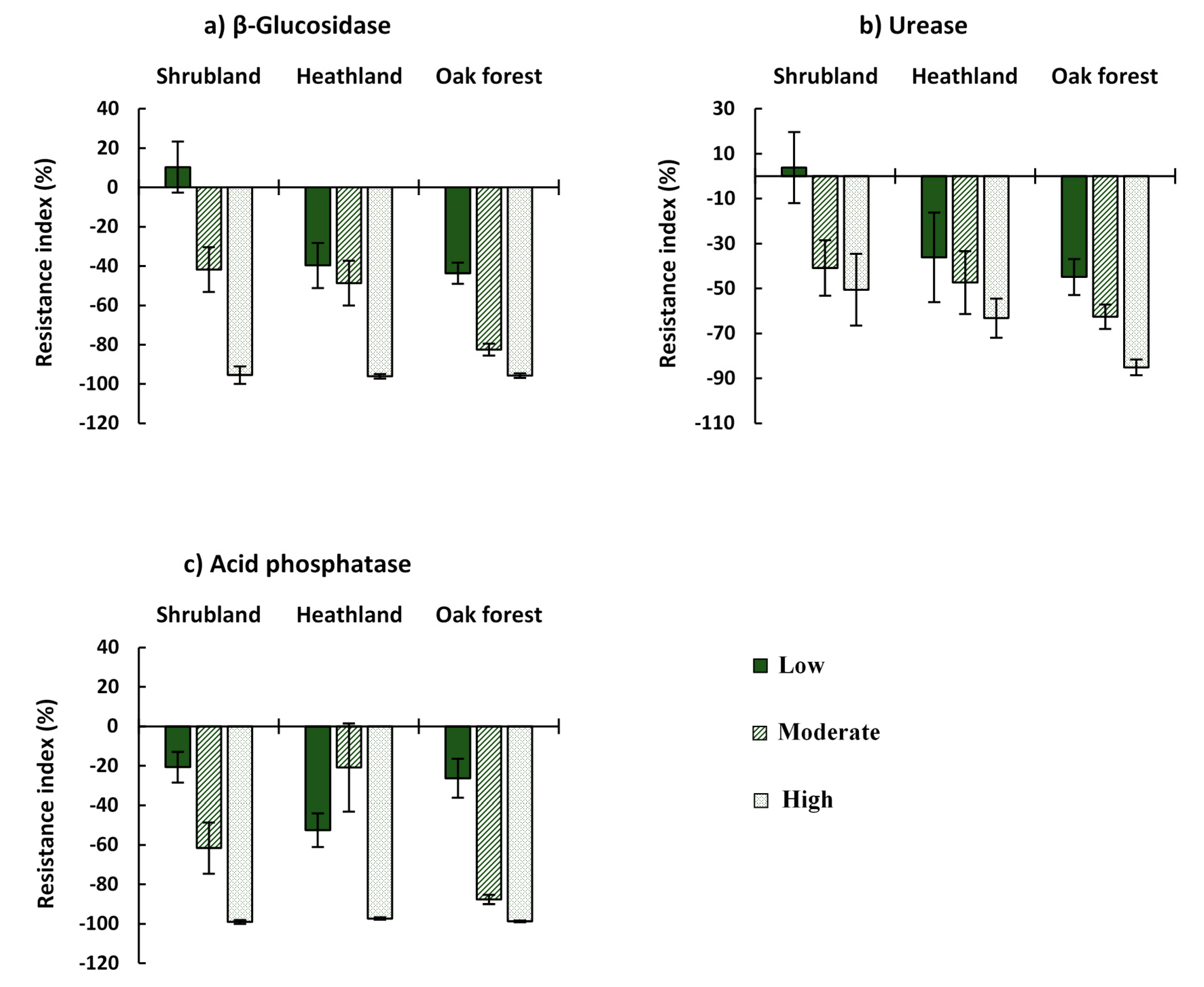
3.4. Biochemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire effects on soil aggregation: A review. Earth Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Hernández, T.; García, C.; Reinhardt, I. Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biol. Fertil. Soils 1997, 25, 109–116. [Google Scholar] [CrossRef]
- Pausas, J.G. Changes in fire and climate in the eastern Iberian peninsula (Mediterranean Basin). Clim. Chang. 2004, 63, 337–350. [Google Scholar] [CrossRef]
- Pausas, J.G.; Vallejo, V.R. The role of fire in European Mediterranean ecosystems. In Remote Sensing of Large Wildfires in the European Mediterranean Basin; Chuvieco, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 3–16. ISBN 978-3-642-60164-4. [Google Scholar]
- Pausas, J.G.; Fernández-Muñoz, S. Fire regime changes in the Western Mediterranean Basin: From fuel-limited to drought-driven fire regime. Clim. Chang. 2012, 110, 215–226. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Abrupt climate-independent fire regime changes. Ecosystems 2014, 17, 1109–1120. [Google Scholar] [CrossRef]
- Hedo, J.; Lucas-Borja, M.E.; Wic, C.; Andrés-Abellán, M.; de Las Heras, J. Soil microbiological properties and enzymatic activities of long-term post-fire recovery in dry and semiarid Aleppo pine (Pinus halepensis M.) forest stands. Solid Earth 2015, 6, 243–252. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Parra, A.; Laudicina, V.A.; Moreno, J.M. Post-fire soil functionality and microbial community structure in a Mediterranean shrubland subjected to experimental drought. Sci. Total Environ. 2016, 573, 1178–1189. [Google Scholar] [CrossRef]
- Moreno, M.V.; Conedera, M.; Chuvieco, E.; Pezzatti, G.B. Fire regime changes and major driving forces in Spain from 1968 to 2010. Environ. Sci. Policy 2014, 37, 11–22. [Google Scholar] [CrossRef]
- Amatulli, G.; Camia, A.; San-Miguel-Ayanz, J. Estimating future burned areas under changing climate in the EU-Mediterranean countries. Sci. Total Environ. 2013, 450, 209–222. [Google Scholar] [CrossRef]
- Pereira, P.; Rein, G.; Martin, D. Past and present post-fire environments. Sci. Total Environ. 2016, 573, 1275–1277. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Balch, J.; Artaxo, P.; Bond, W.J.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.; Johnston, F.H.; Keeley, J.E.; Krawchuk, M.A.; et al. The human dimension of fire regimes on Earth. J. Biogeogr. 2011, 38, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Román, M.V.; Azqueta, D.; Rodrígues, M. Methodological approach to assess the socio-economic vulnerability to wildfires in Spain. For. Ecol. Manag. 2013, 294, 158–165. [Google Scholar] [CrossRef]
- Brotons, L.; Aquilué, N.; de Cáceres, M.; Fortin, M.J.; Fall, A. How fire history, fire suppression practices and climate change affect wildfire regimes in mediterranean landscapes. PLoS ONE 2013, 8, e62392. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ruano, A.; Mimbrero, M.R.; de la Riva Fernández, J. Understanding wildfires in mainland Spain. A comprehensive analysis of fire regime features in a climate-human context. Appl. Geogr. 2017, 89, 100–111. [Google Scholar] [CrossRef]
- Moreira, F.; Viedma, O.; Arianoutsou, M.; Curt, T.; Koutsias, N.; Rigolot, E.; Barbati, A.; Corona, P.; Vaz, P.; Xanthopoulos, G.; et al. Landscape—wildfire interactions in Southern Europe: Implications for landscape management. J. Environ. Manag. 2011, 92, 2389–2402. [Google Scholar] [CrossRef]
- Fidelis, A.; Alvarado, S.T.; Barradas, A.C.S.; Pivello, V.R. The year 2017: Megafires and management in the Cerrado. Fire 2018, 1, 49. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- DeBano, L.F.; Neary, D.G.; Ffolliott, P.F. Fire’s Effects on Ecosystems; Wiley: New York, NY, USA, 1998; ISBN 0-471-16356-2. [Google Scholar]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Neary, D.G.; Debano, L.F.; Ffolliott, P.F. Fire impacts on forest soils: A comparison to mechanical and chemical site preparation. In Fire and Forest Ecology: Innovative Silviculture and Vegetation Management; Moser, W.K., Moser, C.F., Eds.; Tall Timbers Ecology Conference Proceedings. Tall Timbers Research Station: Tallahassee, FL, USA, 2000; Volume 21, pp. 85–94. [Google Scholar]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Farguell, J.; Úbeda, X. Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí Massif, Catalonia, Spain). Sci. Total Environ. 2016, 572, 1329–1335. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America Special Publication: Madison, WI, USA, 1994; pp. 3–31. ISBN 9780891189305. [Google Scholar]
- Doran, J.W.; Jones, A.J. Methods for Assessing Soil Quality; Soil Science Society of America Special Publication: Madison, WI, USA, 1996; Volume 49, p. 410. [Google Scholar]
- Schoenholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Heydari, M.; Rostamy, A.; Najafi, F.; Dey, D.C. Effect of fire severity on physical and biochemical soil properties in Zagros oak (Quercus brantii Lindl.) forests in Iran. J. For. Res. 2017, 28, 95–104. [Google Scholar] [CrossRef]
- Francos, M.; Úbeda, X.; Pereira, P.; Alcañiz, M. Long-term impact of wildfire on soils exposed to different fire severities. A case study in Cadiretes Massif (NE Iberian Peninsula). Sci. Total Environ. 2018, 615, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, V.; Santamarta, M.; Fernández-Mando, A.; Quintaro, C.; Marcos, E.; Calvo, L. Burn severity metrics in fire-prone pine ecosystems along a climatic gradient using Landsat imagery. Remote Sens. Environ. 2018, 206, 205–217. [Google Scholar] [CrossRef]
- Moya, D.; González-De Vega, S.; García-Orenes, F.; Morugán-Coronado, A.; Arcenegui, V.; Mataix-Solera, J.; Lucas-Borja, M.E.; De las Heras, J. Temporal characterisation of soil-plant natural recovery related to fire severity in burned Pinus halepensis Mill. forests. Sci. Total Environ. 2018, 640, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, V.; Marcos, E.; Fernández-Guisuraga, J.M.; Taboada, A.; Suárez-Seoane, S.; Calvo, L. Impact of burn severity on soil properties in a Pinus pinaster ecosystem immediately after fire. Int. J. Wildland Fire 2019, 28, 354–364. [Google Scholar] [CrossRef]
- Fernández-García, V.; Miesel, J.; Baeza, M.J.; Marcos, E.; Calvo, L. Wildfire effects on soil properties in fire-prone pine ecosystems: Indicators of burn severity legacy over the medium term after fire. Appl. Soil Ecol. 2019, 135, 147–156. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A. Incendios forestales en España. Ecosistemas terrestres y suelos. In Efectos de Los Incendios Forestales Sobre Los Suelos en España. El Estado de La Cuestión Visto Por Los Científicos Españoles; Cerdà, A., Mataix-Solera, J., Eds.; Càtedra de Divulgació de la Ciència. Universitat de Valencia: Valencia, Spain, 2009; pp. 25–53. [Google Scholar]
- Jordán, A.; Gordillo-Rivero, Á.J.; García-Moreno, J.; Zavala, L.M.; Granged, A.J.P.; Gil, J.; Neto-Paixão, H.M. Post-fire evolution of water repellency and aggregate stability in Mediterranean calcareous soils: A 6-year study. Catena 2014, 118, 115–123. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Gómez, I.; Navarro-Pedreño, J.; Guerrero, C.; Moral, R. Soil organic matter and aggregates affected by wildfire in Pinus halepensis forest in a Mediterranean environment. Int. J. Wildland Fire 2002, 11, 107–114. [Google Scholar] [CrossRef]
- Redin, M.; dos Santos, G.D.; Miguel, P.; Denega, G.L.; Lupatini, M.; Doneda, A.; de Souza, E.L. Impacts of burning on chemical, physical and biological attributes of soil. Cienc. Florest. 2011, 21, 381–392. [Google Scholar] [CrossRef]
- Cerdà, A. Changes in overland flow and infiltration after a rangeland fire in a Mediterranean scrubland. Hydrol. Process. 1998, 12, 1031–1042. [Google Scholar] [CrossRef]
- García-Corona, R.; Benito, E.; de Blas, E.; Varela, M.E. Effects of heating on some soil physical properties related to its hydrological behaviour in two north-western Spanish soils. Int. J. Wildland Fire 2004, 13, 195–199. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; Debano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soil and Water; Gen. Tech. Rep. RMRS-GTR-42-Vol.4; USDA Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005.
- Smith, D.W. Concentrations of soil nutrients before and after fire. Can. J. Soil Sci. 1970, 50, 17–29. [Google Scholar] [CrossRef]
- Viro, P.J. Effects of forest fire on soil. In Fire and Ecosystems; Kozlowski, T.T., Ahlgren, C.E., Eds.; Academic Press, Inc.: New York, NY, USA, 1974; pp. 7–45. ISBN 0-12-424255-3. [Google Scholar]
- Pietikäinen, J.; Fritze, H. Clear-cutting and prescribed burning in coniferous forest: Comparision of effects on soil fungal and total microbial biomass, respiration activity and nitrification. Soil Biol. Biochem. 1995, 27, 101–109. [Google Scholar] [CrossRef]
- Khanna, P.K.; Raison, R.J. Effect of fire intensity on solution chemistry of surface soil under a Eucalyptus pauciflora forest. Aust. J. Soil Res. 1986, 24, 423–434. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aguirre, A.J.; Aznar, J.M.; González-Pérez, J.A.; De la Rosa, J.M.; León, J.; Ibarra, P.; Echeverría, T. Wildfire effects on nutrients and organic carbon of a Rendzic Phaeozem in NE Spain: Changes at cm-scale topsoil. Catena 2014, 113, 267–275. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Guerrero, C.; García-Orenes, F.; Bárcenas, G.M.; Torres, M.P. Forest fire effects on soil microbiology. In Fire Effects on Soils and Restoration Strategies; Cerdà, A., Robichaud, P., Eds.; Science Publishers, Inc.: Enfield, NH, USA, 2009; pp. 133–175. [Google Scholar]
- Mayor, Á.G.; Goirán, S.B.; Vallejo, V.R.; Bautista, S. Variation in soil enzyme activity as a function of vegetation amount, type, and spatial structure in fire-prone Mediterranean shrublands. Sci. Total Environ. 2016, 573, 1209–1216. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Reyes, O.; Calvo, L. Do fire regime attributes affect soil biochemical properties in the same way under different environmental conditions? Forests 2020, 11, 274. [Google Scholar] [CrossRef]
- Ladd, J.N. Soil Enzymes. In Soil Organic Matter and Biological Activity. Developments in Plant and Soil Sciences; Vaughan, D., Malcolm, R.E., Eds.; Springer: Dordrecht, The Netherlands, 1985; Volume 16, pp. 175–221. ISBN 978-94-009-5105-1. [Google Scholar]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America Special Publication: Madison, WI, USA, 1996; Volume 49, pp. 247–272. ISBN 9780891189442. [Google Scholar]
- Dick, R.P. Soil enzyme activities as indicators of soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America Special Publication: Madison, WI, USA, 1994; Volume 35, pp. 107–124. ISBN 9780891189305. [Google Scholar]
- Trasar-Cepeda, C.; Leirós, C.; Gil-Sotres, F.; Seoane, S. Towards a biochemical quality index for soils: An expression relating several biological and biochemical properties. Biol. Fertil. Soils 1998, 26, 100–106. [Google Scholar] [CrossRef]
- Zornoza, R.; Mataix-Solera, J.; Guerrero, C.; Arcenegui, V.; García-Orenes, F.; Mataix-Beneyto, J.; Morugán, A. Evaluation of soil quality using multiple lineal regression based on physical, chemical and biochemical properties. Sci. Total Environ. 2007, 378, 233–237. [Google Scholar] [CrossRef]
- Saha, J.K.; Selladurai, R.; Coumar, M.V.; Dotaniya, M.L.; Kundu, S.; Patra, A.K. Soil Pollution—An Emerging Threat to Agriculture; Springer: Singapore, 2017; pp. 59–60. ISBN 978-981-10-4274-4. [Google Scholar]
- Herrick, J.E. Soil quality: An indicator of sustainable land management? Appl. Soil Ecol. 2000, 15, 75–83. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Bonkowski, M.; Roy, J.; Ritz, K. Functional stability, substrate utilisation and biological indicators of soils following environmental impacts. Appl. Soil Ecol. 2001, 16, 49–61. [Google Scholar] [CrossRef]
- Fialho, J.S.; Aguiar, M.I.; Maia, L.S.; Magalhães, R.B.; Araújo, F.C.S.; Campanha, M.M.; Oliveira, T.S. Soil quality, resistance and resilience in traditional agricultural and agroforestry ecosystems in Brazil’s semiarid region. Afr. J. Agric. Res. 2013, 8, 5020–5031. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- McNaughton, S.J. Biodiversity and function of grazing ecosystems. In Biodiversity and Ecosystem Function; Schulze, E.D., Mooney, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 361–383. ISBN 978-3-642-58001-7. [Google Scholar]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Bürgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.H.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbio. 2012, 3, 417. [Google Scholar] [CrossRef] [PubMed]
- Orwin, K.H.; Wardle, D.A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Sørensen, S.J.; Bååth, E.; Bloem, J.; De Ruiter, P.C.; et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity-ecosystem function relationship. Oikos 2000, 90, 279–294. [Google Scholar] [CrossRef]
- Banning, N.C.; Murphy, D.V. Effect of heat-induced disturbance on microbial biomass and activity in forest soil and the relationship between disturbance effects and microbial community structure. Appl. Soil Ecol. 2008, 40, 109–119. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Vega, J.A.; Fontúrbel, T.; Merino, A.; Fernández, C.; Ferreiro, A.; Jiménez, E. Testing the ability of visual indicators of soil burn severity to reflect changes in soil chemical and microbial properties in pine forests and shrubland. Plant Soil 2013, 369, 73–91. [Google Scholar] [CrossRef]
- Gimeno-García, E.; Andreu, V.; Rubio, J. Changes in organic matter, nitrogen, phosphorus and cations in soil as the result of fire and water erosion in a Mediterranean landscape. Eur. J. Soil Sci. 2000, 51, 201–210. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest wildfire and grassland prescribed fire effects on soil biogeochemical processes and microbial communities: Two case studies in the semi-arid Southwest. Appl. Soil Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Air Temperature and Precipitation (1971–2000). Iberian Climate Atlas; Agencia Estatal de Meteorología, Ministerio de Medio Ambiente y Medio Rural y Marino; Instituto de Meteorologia de Portugal: Lisbon, Portugal, 2011; pp. 1–80. ISBN 978-84-7837-079-5. [Google Scholar]
- Ninyerola, M.; Pons, X.; Roure, J.M. Atlas Climático Digital de la Península Ibérica. Metodología y Aplicaciones en Bioclimatología y Geobotánica; Universidad Autónoma de Barcelona: Bellaterra, Spain, 2005; pp. 1–44. ISBN 932860-8-7. [Google Scholar]
- Fernández-García, V.; Beltrán-Marcos, D.; Pinto-Prieto, R.; Fernández-Guisuraga, J.M.; Calvo, L. Uso de técnicas de teledetección para determinar la relación entre la historia de incendios y la severidad del fuego. In Teledetección. Hacia Una Visión Global del Cambio Climático; Fernández, L.Á.R., Cremades, J.E., Montes, A.C., Sánchez, J.C.A., Eds.; Ediciones Universidad de Valladolid: Madrid, Spain, 2019; pp. 135–138. ISBN 978-84-1320-038-5. [Google Scholar]
- Instituto Geológico y Minero de España (IGME). GEODE. Mapa Geológico Digital Continuo de España. Available online: http://mapas.igme.es/gis/rest/services/Cartografia_Geologica/IGME_Geode_50/MapServer (accessed on 19 February 2019).
- Instituto Tecnológico y Agrario de Castilla y León (ITACyL). Portal de Suelos. Visor de Datos de Suelos. Available online: http://suelos.itacyl.es/visor_datos (accessed on 7 June 2019).
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods; Kluter, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Dumas, J.B.A. Procedes de l’analyse Organic. Ann. Chem. Phys. 1831, 247, 198–213. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Frank, S.W.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; The United States Department of Agriculture (USDA) Circular. US Government Printing Office: Washington, DC, USA, 1954; Volume 939.
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2: Microbial and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.J., Bezdicek, D.F., Smith, S., Tabatabai, M.A., Wollum, A.G., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. ISBN 9780891188650. [Google Scholar]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Varela, M.E.; Benito, E.; Keizer, J.J. Influence of wildfire severity on soil physical degradation in two pine forest stands of NW Spain. Catena 2015, 133, 342–348. [Google Scholar] [CrossRef]
- Varela, M.E.; Benito, E.; Keizer, J.J. Wildfire effects on soil erodibility of woodlands in NW Spain. Land Degrad. Dev. 2010, 21, 75–82. [Google Scholar] [CrossRef]
- Soto, B.; Benito, E.; Diaz-Fierros, F. Heat-induced degradation processes in forest soils. Int. J. Wildland Fire 1991, 1, 147–152. [Google Scholar] [CrossRef]
- Oades, J.M. The role of biology in the formation, stabilization and degradation of soil structure. Geoderma 1993, 56, 377–400. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Nix, B.; Jacobs, K.A.; Bowles, M.L. Two decades of low-severity prescribed fire increases soil nutrient availability in a Midwestern, USA oak (Quercus) forest. Geoderma 2012, 183, 80–91. [Google Scholar] [CrossRef]
- Chandler, C.; Cheney, P.; Thomas, P.; Trabaud, L.; Williams, D. Fire in Forestry. Volume I. Forest Fire Behavior and Effects; Wiley: New York, NY, USA, 1983; ISBN 0471874426. [Google Scholar]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter —A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Almendros, G.; González-Vila, F.J.; Martin, F. Fire-induced transformation of soil organic matter from an oak forest. An experimental approach to the effects of fire on humic substances. Soil Sci. 1990, 149, 158–168. [Google Scholar] [CrossRef]
- Neill, C.; Patterson, W.A.; Crary, D.W. Responses of soil carbon, nitrogen and cations to the frequency and seasonality of prescribed burning in a Cape Cod oak-pine forest. For. Ecol. Manag. 2007, 250, 234–243. [Google Scholar] [CrossRef]
- Lombao, A.; Barreiro, A.; Carballas, T.; Fontúrbel, M.T.; Martín, A.; Vega, J.A.; Fernández, C.; Díaz-Raviña, M. Changes in soil properties after a wildfire in Fragas do Eume Natural Park (Galicia, Spain). Catena 2015, 135, 409–418. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S.; Gawroński, S. Impact of fire severity on soil properties and the development of tree and shrub species in a Scots pine moist forest site in southern Poland. For. Ecol. Manag. 2015, 342, 56–63. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.; Chen, H. Effects of a wildfire on selected physical, chemical and biochemical soil Properties in a Pinus massoniana forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X.; Martin, D.A. Fire severity effects on ash chemical composition and water-extractable elements. Geoderma 2012, 191, 105–114. [Google Scholar] [CrossRef]
- Cade-Menun, B.J.; Berch, S.M.; Preston, C.M.; Lavkulich, L.M. Phosphorus forms and related soil chemistry of Podzolic soils on northern Vancouver Island. II. The effects of clear-cutting and burning. Can. J. For. Res. 2000, 30, 1726–1741. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Plant ash and heat intensity effects on chemical and physical properties of two contrasting soils. Arid Land Res. Manag. 2003, 17, 23–41. [Google Scholar] [CrossRef]
- Kutiel, P.; Shaviv, A. Effects of soil type, plant composition and leaching on soil nutrients following a simulated forest fire. For. Ecol. Manag. 1992, 53, 329–343. [Google Scholar] [CrossRef]
- Franklin, S.B.; Robertson, P.A.; Fralish, J.S. Prescribed burning effects on upland Quercus forest structure and function. For. Ecol. Manag. 2003, 184, 315–335. [Google Scholar] [CrossRef]
- Marcos, E.; Luis-Calabuig, E.; Tárrega, R. Chemical soil changes in shrubland after experimental fire. In Fire Management and Landscape Ecology; Trabaud, L., Ed.; International Association of Wildland Fire: Fairfield, WA, USA, 1998; pp. 3–11. [Google Scholar]
- Marcos, E.; Villalón, C.; Calvo, L.; Luis-Calabuig, E. Short-term effects of experimental burning on soil nutrients in the Cantabrian heathlands. Ecol. Eng. 2009, 35, 820–828. [Google Scholar] [CrossRef]
- Pereira, P.; Cerda, A.; Martin, D.; Úbeda, X.; Depellegrin, D.; Novara, A.; Martínez-Murillo, J.F.; Brevik, E.C.; Menshov, O.; Rodrigo Comino, J.; et al. Short-term low-severity spring grassland fire impacts on soil extractable elements and soil ratios in Lithuania. Sci. Total Environ. 2017, 578, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Härdtle, W.; Jirjahn, B.; Niemeyer, T.; von Oheimb, G. Effects of prescribed burning on plant available nutrients in dry heathland ecosystems. Plant Ecol. 2007, 189, 279–289. [Google Scholar] [CrossRef]
- White, E.M.; Thompson, W.W.; Gartner, F.R. Heat effects on nutrient release from soils under ponderosa pine. J. Range Manag. 1973, 26, 22–24. [Google Scholar] [CrossRef]
- López-Poma, R.; Bautista, S. Plant regeneration functional groups modulate the response to fire of soil enzyme activities in a Mediterranean shrubland. Soil Biol. Biochem. 2014, 79, 5–13. [Google Scholar] [CrossRef]
- Fontúrbel, M.T.; Barreiro, A.; Vega, J.A.; Martín, A.; Jiménez, E.; Carballas, T.; Fernández, C.; Díaz-Raviña, M. Effects of an experimental fire and post-fire stabilization treatments on soil microbial communities. Geoderma 2012, 191, 51–60. [Google Scholar] [CrossRef]
- Fontúrbel, M.T.; Fernández, C.; Vega, J.A. Prescribed burning versus mechanical treatments as shrubland management options in NW Spain: Mid-term soil microbial response. Appl. Soil Ecol. 2016, 107, 334–346. [Google Scholar] [CrossRef]
- Saa, A.; Trasar-Cepeda, C.; Gil-Sotres, F.; Carballas, T. Changes in soil phosphorus and acid phosphatase activity immediately following forest fires. Soil Biol. Biochem. 1993, 25, 1223–1230. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Factors affecting glucosidase and galactosidase activities in soils. Soil Biol. Biochem. 1990, 22, 891–897. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Vieira, D.C.S.; Fernández, C.; Vega, J.A.; Keizer, J.J. Does soil burn severity affect the post-fire runoff and interrill erosion response? A review based on meta-analysis of field rainfall simulation data. J. Hydrol. 2015, 523, 452–464. [Google Scholar] [CrossRef]
- MacDonald, L.H.; Larsen, I.J. Effects of forest fires and post-fire rehabilitation: A Colorado case study. In Fire Effects on Soils and Restoration Strategies; Cerda, A., Robichaud, P.R., Eds.; Science Publishers: Enfield, NH, USA, 2009; pp. 423–452. [Google Scholar]
- Moody, J.A.; Shakesby, R.A.; Robichaud, P.R.; Cannon, S.H.; Martin, D.A. Current research issues related to post-wildfire runoff and erosion processes. Earth Sci. Rev. 2013, 122, 10–37. [Google Scholar] [CrossRef]
- Inbar, A.; Lado, M.; Sternbergd, M.; Tenaua, H.; Ben-Hur, M. Forest fire effects on soil chemical and physicochemical properties, infiltration, runoff, and erosion in a semiarid Mediterranean region. Geoderma 2014, 221, 131–138. [Google Scholar] [CrossRef]
- Cerdà, A.; Doerr, S.H. The influence of vegetation recovery on soil hydrology and erodibility following fire: An eleven-year investigation. Int. J. Wildland Fire 2005, 14, 423–437. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H. Wildfire as a hydrological and geomorphological agent. Earth Sci. Rev. 2006, 74, 269–307. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire in Mediterranean climate ecosystems—A comparative overview. Isr. J. Ecol. Evol. 2012, 58, 123–135. [Google Scholar] [CrossRef]
- Duane, A.; Aquilué, N.; Canelles, Q.; Morán-Ordoñez, A.; De Cáceres, M.; Brotons, L. Adapting prescribed burns to future climate change in Mediterranean landscapes. Sci. Total Environ. 2019, 677, 68–83. [Google Scholar] [CrossRef]
- Fernandes, P.M. Fire-smart management of forest landscapes in the Mediterranean basin under global change. Landsc. Urban Plan. 2013, 110, 175–183. [Google Scholar] [CrossRef]

| Rating Factors | Burn Severity Scale | ||||||
|---|---|---|---|---|---|---|---|
| Unburned | Low | Moderate | High | ||||
| 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | |
| Substrate | |||||||
| Litter/light fuel consumed | None | <10% | 10–20% | 20–40% | 40–80% | 80–98% | 98% |
| Char and colour | None | Blackened litter, no changes in soil | Charred remains, recognisable litter | Grey and white ash, grey soil | White ash, reddened soil | ||
| Soil Property | Shrubland | Heathland | Oak Forest |
|---|---|---|---|
| Physical | |||
| MWD (mm) | 1.39 (0.38) | 1.66 (0.48) | 1.50 (0.45) |
| Chemical | |||
| pH | 4.88 (0.17) | 4.79 (0.25) | 5.45 (0.29) |
| Total C (%) | 2.90 (1.40) | 6.79 (2.62) | 8.56 (1.81) |
| TOC (%) | 2.70 (1.35) | 6.38 (2.61) | 8.12 (1.67) |
| TIC (%) | 0.20 (0.29) | 0.42 (0.53) | 0.44 (0.35) |
| Total N (%) | 0.17 (0.06) | 0.35 (0.14) | 0.56 (0.11) |
| Available P (mg kg−1) | 4.54 (2.53) | 4.97 (2.17) | 11.15 (4.95) |
| Na+ (cmol kg−1) | 0.04 (0.04) | 0.05 (0.03) | 0.04 (0.02) |
| K+ (cmol kg−1) | 0.16 (0.04) | 0.29 (0.10) | 0.54 (0.16) |
| Mg2+ (cmol kg−1) | 0.23 (0.09) | 0.47 (0.32) | 2.60 (0.59) |
| Ca2+ (cmol kg−1) | 0.94 (0.58) | 1.03 (0.79) | 8.11 (2.26) |
| CEC (cmol kg−1) | 1.63 (0.63) | 2.77 (1.39) | 12.30 (2.88) |
| Biochemical | |||
| β-glucosidase (µmol p-NP g−1 dw soil h−1) | 2.11 (0.75) | 2.56 (1.10) | 4.35 (1.15) |
| Urease (µmol N-NH4+ g−1 dw soil h−1) | 4.83 (2.08) | 3.78 (1.71) | 12.20 (6.93) |
| Acid phosphatase (µmol p-NP g−1 dw soil h−1) | 6.16 (2.26) | 12.59 (5.56) | 18.98 (4.64) |
| Soil Property | Treatment | F Value | p Value |
|---|---|---|---|
| Physical | |||
| MWD | Ecosystem | 3.514 | 0.033 |
| CBI | 16.694 | 0.000 | |
| Ecosystem*CBI | 1.012 | NS | |
| Chemical | |||
| pH | Ecosystem | 3.379 | 0.037 |
| CBI | 27.607 | 0.000 | |
| Ecosystem*CBI | 4.558 | 0.002 | |
| Total C | Ecosystem | 1.739 | NS |
| CBI | 8.643 | 0.000 | |
| Ecosystem*CBI | 4.611 | 0.002 | |
| TOC | Ecosystem | 2.131 | NS |
| CBI | 7.932 | 0.001 | |
| Ecosystem*CBI | 5.085 | 0.001 | |
| TIC | Ecosystem | 4.196 | 0.017 |
| CBI | 2.972 | NS | |
| Ecosystem*CBI | 6.975 | 0.000 | |
| Total N | Ecosystem | 14.505 | 0.000 |
| CBI | 4.594 | 0.012 | |
| Ecosystem*CBI | 5.127 | 0.001 | |
| Available P | Ecosystem | 0.119 | NS |
| CBI | 3.614 | 0.030 | |
| Ecosystem*CBI | 3.626 | 0.008 | |
| Na+ | Ecosystem | 3.917 | 0.023 |
| CBI | 1.675 | NS | |
| Ecosystem*CBI | 0.161 | NS | |
| K+ | Ecosystem | 16.634 | 0.000 |
| CBI | 4.269 | 0.016 | |
| Ecosystem*CBI | 4.738 | 0.001 | |
| Mg2+ | Ecosystem | 21.649 | 0.000 |
| CBI | 1.750 | NS | |
| Ecosystem*CBI | 2.019 | NS | |
| Ca2+ | Ecosystem | 10.106 | 0.000 |
| CBI | 3.057 | NS | |
| Ecosystem*CBI | 2.838 | 0.027 | |
| CEC | Ecosystem | 11.636 | 0.000 |
| CBI | 2.807 | NS | |
| Ecosystem*CBI | 2.085 | NS | |
| Biochemical | |||
| β-glucosidase | Ecosystem | 6.018 | 0.003 |
| CBI | 30.664 | 0.000 | |
| Ecosystem*CBI | 2.398 | NS | |
| Urease | Ecosystem | 3.987 | 0.021 |
| CBI | 6.077 | 0.003 | |
| Ecosystem*CBI | 0.666 | NS | |
| Acid phosphatase | Ecosystem | 1.427 | NS |
| CBI | 15.533 | 0.000 | |
| Ecosystem*CBI | 5.202 | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta, S.; Fernández-García, V.; Calvo, L.; Marcos, E. Soil Resistance to Burn Severity in Different Forest Ecosystems in the Framework of a Wildfire. Forests 2020, 11, 773. https://doi.org/10.3390/f11070773
Huerta S, Fernández-García V, Calvo L, Marcos E. Soil Resistance to Burn Severity in Different Forest Ecosystems in the Framework of a Wildfire. Forests. 2020; 11(7):773. https://doi.org/10.3390/f11070773
Chicago/Turabian StyleHuerta, Sara, Víctor Fernández-García, Leonor Calvo, and Elena Marcos. 2020. "Soil Resistance to Burn Severity in Different Forest Ecosystems in the Framework of a Wildfire" Forests 11, no. 7: 773. https://doi.org/10.3390/f11070773
APA StyleHuerta, S., Fernández-García, V., Calvo, L., & Marcos, E. (2020). Soil Resistance to Burn Severity in Different Forest Ecosystems in the Framework of a Wildfire. Forests, 11(7), 773. https://doi.org/10.3390/f11070773







