Ungulate Species and Abundance as well as Environmental Factors Determine the Probability of Terminal Shoot Browsing on Temperate Forest Trees
Abstract
1. Introduction
2. Materials and Methods
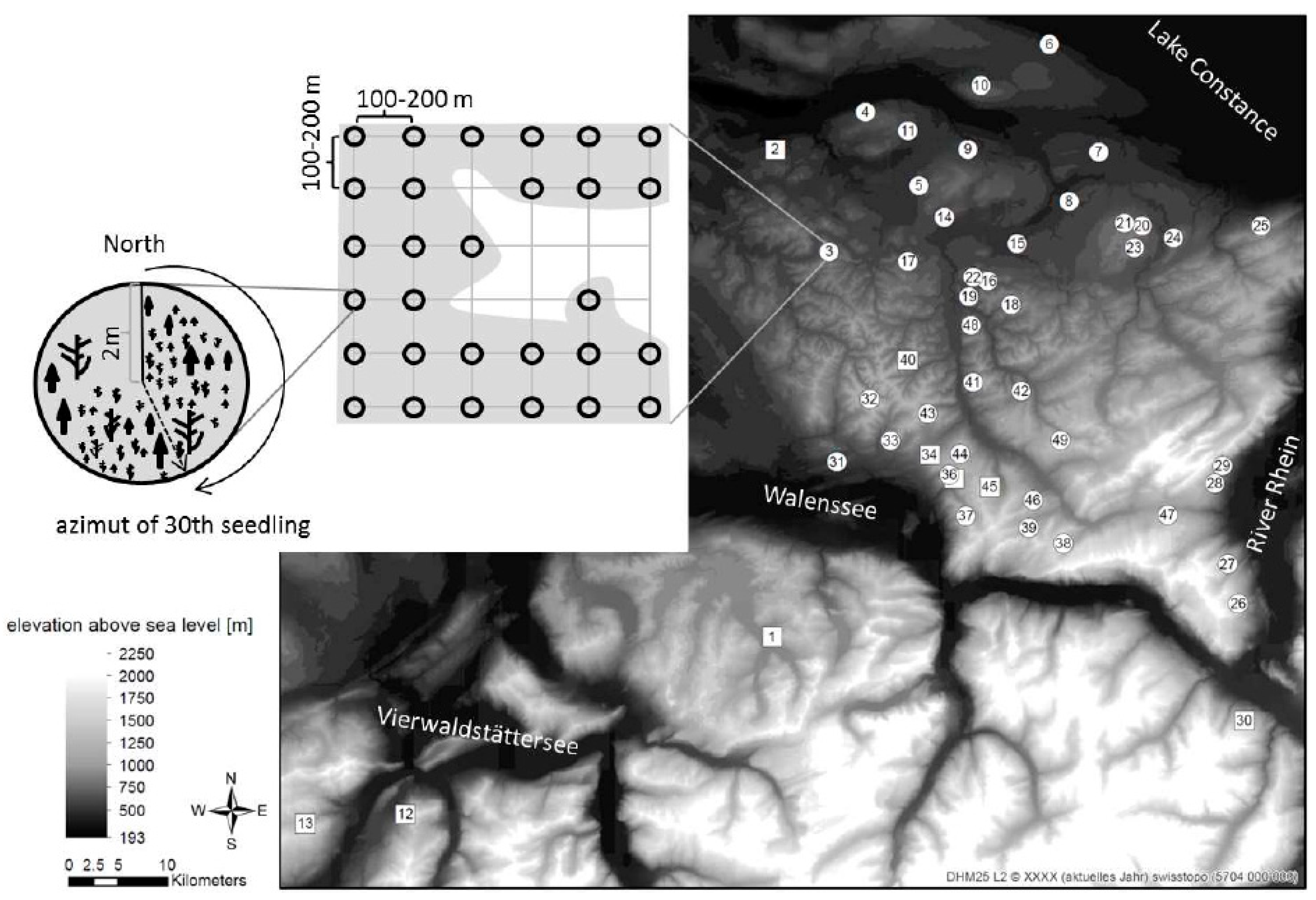
2.1. Study Sites and Sampling Design
2.2. Long-Term Field Data Collection
2.3. Additional Assessment of Variables
2.4. Supplementary Field Data Collection in 2012
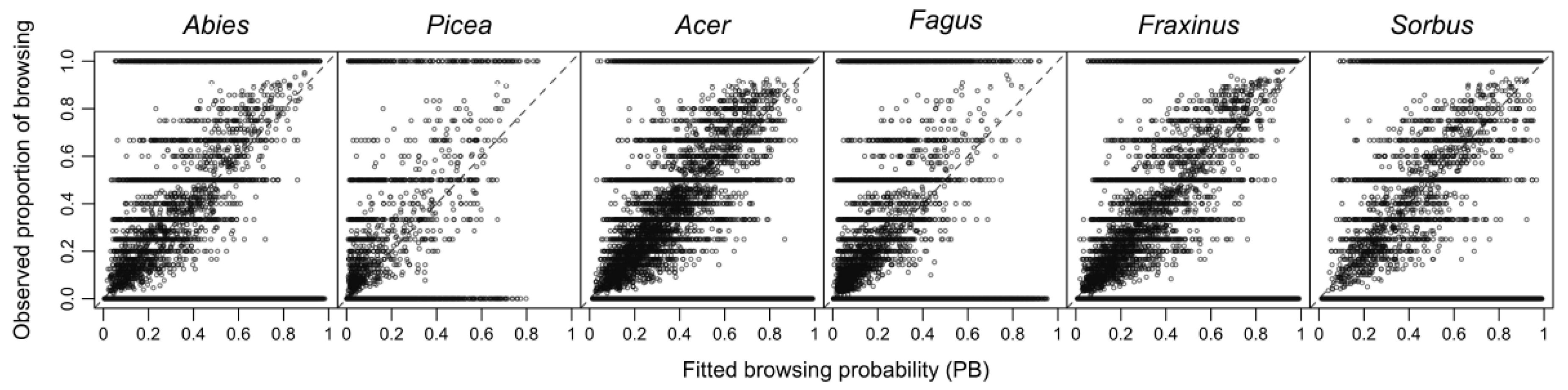
2.5. Data Analysis
3. Results
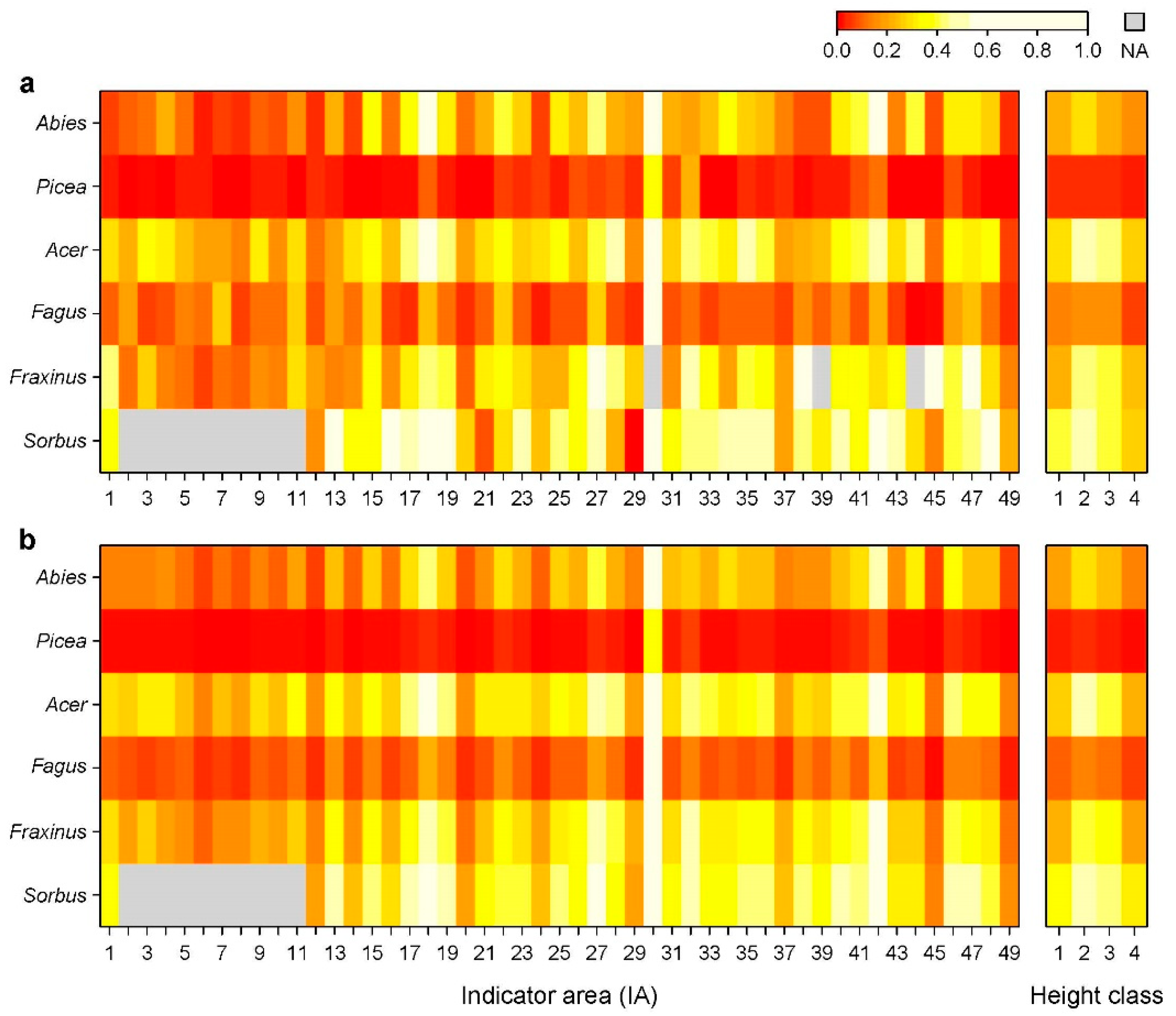
3.1. Distribution of Ungulates and Browsing Probability
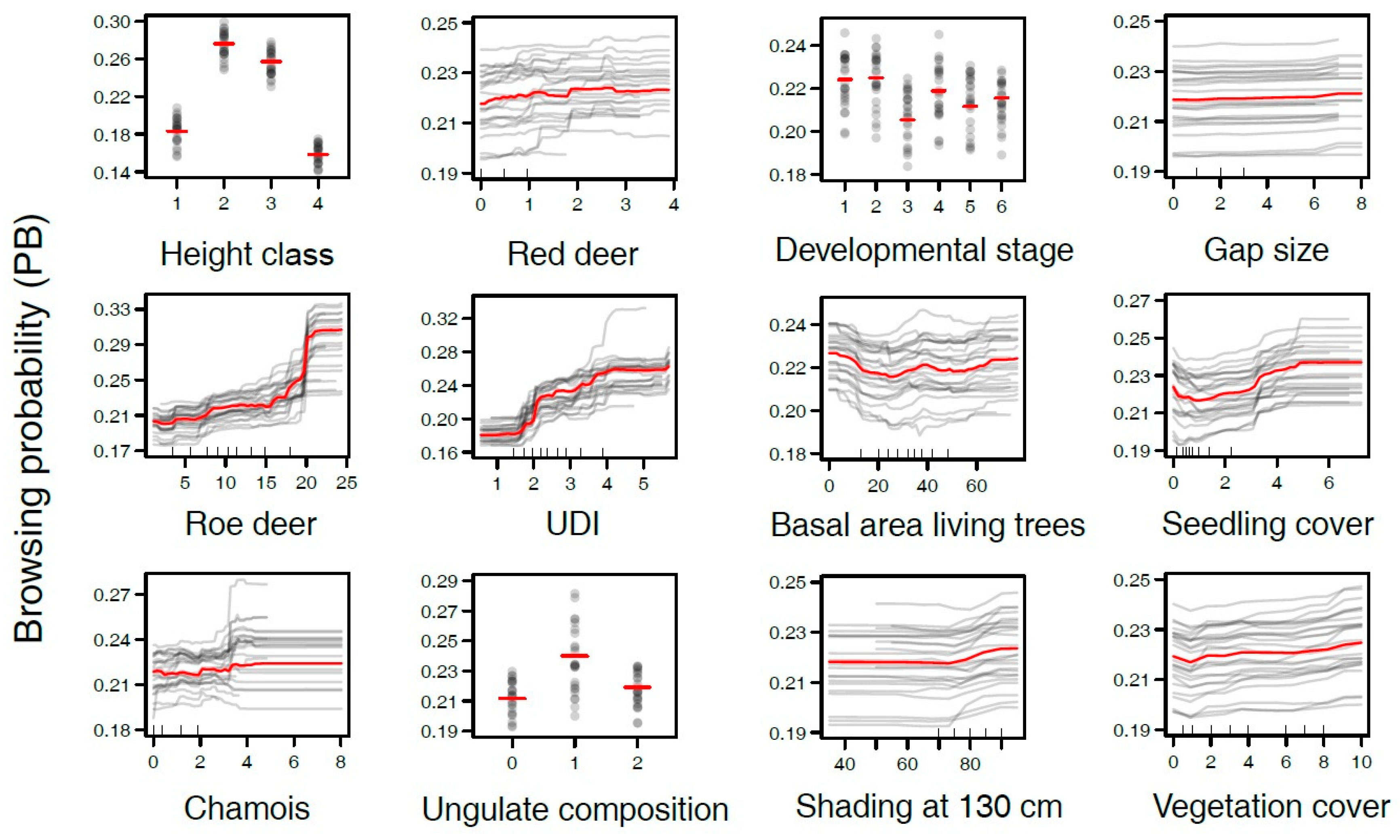
3.2. Variables Influencing Browsing Probability
3.3. Is Browsing on Tree Seedlings Positively Correlated with the Density of Ungulates?
3.4. Does Browsing on Tree Seedlings Depend on Ungulate Species?
3.5. Is Browsing on Tree Seedlings Increased in Early Developmental Stages?
3.6. Does Browsing on Tree Seedlings Differ between Gaps and Closed Canopy Stands?
3.7. Does the Presence of Stumps, Snags and Lying Logs Enhance the Probability of a Seedling to be Browsed?
3.8. Do the Cover of Tree Seedlings and Ground Vegetation Affect Browsing on Tree Seedlings?
4. Discussion
4.1. Browsing Is a Multifactorial Phenomenon
4.2. Is Browsing on Tree Seedlings Positively Correlated with the Density of Ungulates?
4.3. Does Browsing on Tree Seedlings Depend on Ungulate Species?
4.4. Is Browsing on Tree Seedlings Increased in Early Developmental Stages?
4.5. Does Browsing on Tree Seedlings Differ between Gaps and Closed Canopy Stands?
4.6. Does the Presence of Stumps, Snags and Lying Logs Enhance the Probability of a Seedling to Be Browsed?
4.7. Do the Cover of Seedlings and Ground Vegetation Affect Browsing on Tree Seedlings?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Category of Categorical Variable | n (%) or Median (min, lq, uq, max) |
|---|---|---|
| Location (N = 1704) | ||
| Canton | Nidwalden (NW) | 31 (2%) |
| Obwalden (OW) | 31 (2%) | |
| Schwyz (SZ) | 64 (4%) | |
| St. Gallen (SG) | 1264 (74%) | |
| Thurgau (TG) | 314 (18%) | |
| Elevation above sea level (m) | 825 (466, 687, 1115, 1641) | |
| Slope (%) | 27 (0, 16, 40, 130) | |
| Aspect | North (N) | 835 (49%) |
| East (E) | 320 (19%) | |
| West (W) | 250 (15%) | |
| South (S) | 299 (18%) | |
| Climate (N = 1704) | ||
| Annual precipitation (cm) | 153 (95, 131, 192, 257) | |
| Spring precipitation (cm) | 41 (27, 38, 53, 72) | |
| Annual temperature (°C) | 8 (4, 6, 8, 10) | |
| Degree day sum ≥ 5.56 °C (°C day) | 1702 (869, 1364, 1856, 2130) | |
| Forest characteristics (N = 1704) | ||
| Developmental stage (from aerial images) | Young growth and thicket (1) | 350 (21%) |
| Polewood (2) | 228 (13%) | |
| Dominated by small timber trees (3) | 200 (12%) | |
| Dominated by medium timber trees (4) | 496 (29%) | |
| Dominated by large timber trees (5) | 311 (18%) | |
| Mixed stages (6) | 114 (7%) | |
| Others (o) | 5 (0%) | |
| Forest type 1 * | Broad-leaved forest (bf) | 984 (58%) |
| Coniferous forest (cf) | 718 (42%) | |
| Forest type 2 * | Acer-Fraxinus-Tilia (aft) | 133 (8%) |
| Fagus (f) | 851 (50%) | |
| Abies-Fagus (af) | 518 (30%) | |
| Abies-Picea (ap) | 200 (12%) | |
| Soil humidity * | Dry (1) | 34 (2%) |
| Semi-moist (2) | 799 (47%) | |
| Moist (3) | 656 (39%) | |
| Wet (4) | 152 (9%) | |
| Variable (v) | 61 (4%) | |
| Soil acidity * | Limestone debris (ld) | 89 (5%) |
| Basic (b) | 267 (16%) | |
| Neutral (n) | 1064 (63%) | |
| Acidic (a) | 282 (17%) | |
| Productivity (timber yield) * | Low (1) | 46 (3%) |
| Moderate (2) | 178 (10%) | |
| High (3) | 701 (41%) | |
| Very high (4) | 777 (46%) | |
| Ungulates (N = 379) | ||
| Roe deer (deaths per year and km2 of forested area) | 9 (0, 6, 14, 24) | |
| Red deer (deaths per year and km2 of forested area) | 0 (0, 0, 0, 4) | |
| Chamois (deaths per year and km2 of forested area) | 0 (0, 0, 1, 8) | |
| Ungulate density index (UDI) | 2 (0, 2, 3, 6) | |
| Ungulate composition † | No red deer + no chamois (0) | 17 (35%) |
| Red deer + chamois < 5% roe deer (1) | 11 (22%) | |
| Red deer + chamois ≥ 5% roe deer (2) | 21 (43%) | |
| Field data collected 2012 (N = 660) | ||
| Relief | Flat (1) | 147 (22%) |
| Crest (2) | 58 (9%) | |
| Hillside (3) | 407 (62%) | |
| Depression/hillside toe (4) | 39 (6%) | |
| other (5) | 9 (1%) | |
| Developmental stage | Young growth and thicket (1) | 99 (15%) |
| Polewood (2) | 69 (10%) | |
| Dominated by small timber trees (3) | 122 (18%) | |
| Dominated by medium timber trees (4) | 290 (44%) | |
| Dominated by large timber trees (5) | 65 (10%) | |
| Mixed stages (6) | 15 (2%) | |
| Shading at 40 cm above ground (%) | 80 (50, 75, 85, 95) | |
| Shading at 130 cm above ground (%) | 80 (35, 75, 85, 95) | |
| Gap size (approximated from treetop openings) | 0 (0, 0, 1, 8) | |
| Snow depth (approximated from treetop openings) | 0 (0, 0, 0, 6) | |
| Light transmittance (approximated from treetop openings) | 0 (0, 0, 2, 23) | |
| Basal area of living trees (m2 ha−1) | 32 (0, 20, 40, 77) | |
| Basal area of stumps (m2 ha−1) | 0 (0, 0, 4, 41) | |
| Basal area of snags and lying logs (m2 ha−1) | 0 (0, 0, 0, 13) | |
| Regeneration constraint (paths, rocks, stems) (Londo scale) | 0 (0, 0, 0, 6) | |
| Seedling cover (Londo scale) | 1 (0, 0, 1, 7) | |
| Vegetation cover (without trees, shrubs, mosses) (Londo scale) | 3 (0, 1, 6, 10) | |
| Cover by blackberry (Rubus fruticosus) (Londo scale) | 0 (0, 0, 2, 9) | |
| Cover by raspberry (Rubus idaeus) (Londo scale) | 0 (0, 0, 0, 5) | |
| Cover by ferns (Londo scale) | 0 (0, 0, 1, 9) | |
| Cover by graminoids (Londo scale) | 0 (0, 0, 1, 8) | |
| Cover by perennial herbs (Londo scale) | 0 (0, 0, 0, 4) | |
| Cover by blueberry (Vaccinium) (Londo scale) | 0 (0, 0, 0, 9) | |
| Cover by shrubs (Londo scale) | 0 (0, 0, 0, 8) | |
| 10–40 cm (Height Class 1) | 41–70 cm (Height Class 2) | 71–100 cm (Height Class 3) | 101–130 cm (Height Class 4) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Nbr | %br | N | Nbr | %br | N | Nbr | %br | N | Nbr | %br | |
| Abies | 31,282 | 6675 | 21% | 5042 | 1683 | 33% | 1592 | 356 | 22% | 933 | 103 | 11% |
| Picea | 30,271 | 1325 | 4% | 8650 | 616 | 7% | 4133 | 307 | 7% | 2344 | 163 | 7% |
| Acer | 44,484 | 11,838 | 27% | 9770 | 4961 | 51% | 2901 | 1251 | 43% | 1301 | 351 | 27% |
| Fagus | 37,102 | 3617 | 10% | 10,471 | 1529 | 15% | 5421 | 697 | 13% | 371 | 265 | 8% |
| Fraxinus | 30,978 | 7232 | 23% | 10,293 | 4520 | 44% | 3829 | 1592 | 42% | 1972 | 474 | 24% |
| Sorbus | 13,842 | 4768 | 34% | 5184 | 2694 | 52% | 2071 | 1136 | 55% | 1027 | 459 | 45% |
| Quercus | 441 | 163 | 37% | 92 | 62 | 67% | 24 | 16 | 67% | 23 | 7 | 30% |
| Other broad-leaved trees | 3176 | 1057 | 33% | 1574 | 675 | 43% | 778 | 284 | 37% | 552 | 142 | 26% |
| Other coniferous trees | 676 | 120 | 18% | 150 | 26 | 17% | 62 | 3 | 5% | 54 | 3 | 6% |
| Total | 192,252 | 36,795 | 19% | 51,226 | 16,766 | 33% | 20,811 | 5642 | 27% | 11,577 | 1967 | 17% |
| Fixed Effects | Odds Ratio (95% CI) | |
|---|---|---|
| Height class | ||
| 10–40 cm | reference | |
| 41–70 cm | 1.92 (1.63–2.25) | |
| 71–100 cm | 1.59 (1.34–1.88) | |
| 101–130 cm | 0.73 (0.61–0.87) | |
| Species | ||
| Acer | reference | |
| Abies | 0.52 (0.39–0.68) | |
| Picea | 0.04 (0.03–0.05) | |
| Fagus | 0.19 (0.15–0.25) | |
| Fraxinus | 1.06 (0.80–1.40) | |
| Quercus | 3.16 (2.02–4.95) | |
| Sorbus | 1.30 (0.98–1.73) | |
| Other broad-leaved trees | 1.33 (0.99–1.80) | |
| Other coniferous trees | 0.30 (0.20–0.46) | |
| Year | ||
| 1995 | 1.16 (0.79–1.71) | |
| 1996 | 0.94 (0.64–1.39) | |
| 1997 | 0.75 (0.51–1.11) | |
| 1998 | 0.95 (0.64–1.40) | |
| 1999 | 0.47 (0.32–0.69) | |
| 2000 | 0.24 (0.17–0.35) | |
| 2001 | 0.37 (0.25–0.53) | |
| 2002 | 0.29 (0.20–0.41) | |
| 2003 | 0.35 (0.24–0.51) | |
| 2004 | 0.32 (0.22–0.46) | |
| 2005 | 0.32 (0.22–0.47) | |
| 2006 | 0.24 (0.17–0.34) | |
| 2007 | 0.20 (0.13–0.29) | |
| 2008 | 0.25 (0.18–0.36) | |
| 2009 | 0.28 (0.19–0.41) | |
| 2010 | 0.34 (0.24–0.49) | |
| 2011 | 0.23 (0.15–0.35) | |
| Random effects | N | Variance |
| IA | 49 | 0.602 |
| IA:plot | 1704 | 0.057 |
| Species:IA:plot | 13,632 | 0.679 |
| Year:IA:plot | 13,536 | 0.635 |
| Height class:IA:plot | 6816 | 0.246 |
| Species:year | 146 | 0.053 |
| Species:height class | 36 | 0.020 |
| Year:height class | 68 | 0.005 |
References
- Coté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar]
- Putman, R.J. Ungulates in temperate forest ecosystems: Perspectives and recommendations for future research. For. Ecol. Manag. 1996, 88, 205–214. [Google Scholar] [CrossRef]
- Tixier, H.; Duncan, P. Are European roe deer browsers? A review of variations in the composition of their diets. Revue D’écologie 1996, 51, 3–17. [Google Scholar]
- Gebert, C.; Verheyden-Tixier, H. Variations of diet composition of red deer (Cervus elaphus L.) in Europe. Mammal. Rev. 2001, 31, 189–201. [Google Scholar] [CrossRef]
- Onderscheka, K.; Reimoser, F.; Völk, F.; Tataruch, F.; Steineck, T.; Klansek, E.; Zandl, J. Integrale Schalenwildhege im Rätikon unter besonderer Berücksichtigung der Walderhaltung; Forschungsinstitut für Wildtierkunde der Veterinärmedizinischen Universität Wien: Wien, Austria, 1990; p. 410. [Google Scholar]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and their Management in the 21th Century; Cambridge University Press: Cambridge, UK, 2010; p. 604. [Google Scholar]
- Hofmann, R.R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: A comparative view of their digestive system. Oecologica 1989, 78, 449–457. [Google Scholar] [CrossRef]
- Johnson, D.H. The comparison of usage and availability measurements for evaluating resource preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- Gill, R.M.A. A review of damage by mammals in north temperate forests: 1. Deer. For. 1992, 65, 145–169. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Heiri, C.; Huber, M.; Fehr, M.; Frei, M.; Gmür, P.; Imesch, N.; Zinggeler, J.; Brang, P.; Clivaz, J.-C.; et al. Einfluss wildlebender Huftiere auf die Waldverjüngung: Ein Überblick für die Schweiz. Schweiz. Z. Forstwes 2015, 166, 420–431. [Google Scholar] [CrossRef]
- Abegg, M.; Brändli, U.B.; Cioldi, F.; Fischer, C. Viertes Schweizerisches Landesforstinventar—Ergebnistabellen und Karten im Internet zum LFI 2009–2013 (LFI4b); Eidgenössische Forschungsanstalt WSL: Birmensdorf, Switzerland, 2014; Available online: http://www.lfi.ch/resultate/ (accessed on 15 July 2020).
- Kupferschmid, A.D. Compensation capacity of Central European tree species in response to leader shoot browsing. In Ungulates: Evolution, Diversity and Ecology; Menendez, A., Sands, N., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–63. [Google Scholar]
- Gordon, I.J. Facilitation of red deer grazing by cattle and its impact on red deer performance. J. Appl. Ecol. 1998, 25, 1–10. [Google Scholar] [CrossRef]
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef]
- Welch, D.; Staines, B.W.; Scott, D.; French, D.D.; Catt, D.C. Leader browsing by red and roe deer on young Sitka spruce trees in Western Scotland (UK): I. Damage rates and the influence of habitat factors. Forestry 1991, 64, 61–82. [Google Scholar] [CrossRef]
- Baraza, E.; Zamora, R.; Hódar, J.A. Conditional outcomes in plant–herbivore interactions: Neighbours matter. Oikos 2006, 113, 148–156. [Google Scholar] [CrossRef]
- Heikkilä, R.; Härkönen, S. Moose browsing in young Scots pine stands in relation to forest management. For. Ecol. Manag. 1996, 88, 179–186. [Google Scholar] [CrossRef]
- Smit, C.; Vandenberghe, C.; Ouden, J.D.; Müller-Schärer, H. Nurse plants, tree saplings and grazing pressure: Changes in facilitation along a biotic environmental gradient. Oecologia 2007, 152, 265–273. [Google Scholar] [CrossRef]
- Partl, E.; Szinovatz, V.; Reimoser, F.; Schweiger-Adler, J. Forest restoration and browsing impact by roe deer. For. Ecol. Manag. 2002, 159, 87–100. [Google Scholar] [CrossRef]
- Moser, B.; Schütz, M.; Hindenlang, K. Importance of alternative food resources for browsing by roe deer on deciduous trees: The role of food availability and species quality. For. Ecol. Manag. 2006, 226, 248–255. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Zimmermann, S.; Bugmann, H. Browsing regime and growth response of naturally regenerated Abies alba saplings along light gradients. For. Ecol. Manag. 2013, 310, 393–404. [Google Scholar] [CrossRef]
- Cornelis, J.; Casaer, J.; Hermy, M. Impact of season, habitat and research techniques on diet composition of roe deer (Capreolus capreolus): A review. J. Zool. 1999, 248, 195–207. [Google Scholar] [CrossRef]
- Bergquist, J.; Örlander, G. Browsing damage by roe deer on Norway spruce seedlings planted on clearcuts of different ages: 1. Effect of slash removal, vegetation development, and roe deer density. For. Ecol. Manag. 1998, 105, 283–293. [Google Scholar] [CrossRef]
- Widmer, O.; Saïd, S.; Miroir, J.; Duncan, P.; Gaillard, J.M.; Klein, F. The effects of hurricane Lothar on habitat use of roe deer. For. Ecol. Manag. 2004, 195, 237–242. [Google Scholar] [CrossRef]
- Reyes, G.; Vasseur, L. Factors influencing deer browsing damage to red spruce (Picea rubens) seedlings in coastal red spruce-balsam fir stands of southwestern Nova Scotia. For. Ecol. Manag. 2003, 186, 349–357. [Google Scholar] [CrossRef]
- Moser, B.; Schütz, M.; Hindenlang, K. Resource selection by roe deer: Are windthrow gaps attractive feeding places? For. Ecol. Manag. 2008, 255, 1179–1185. [Google Scholar] [CrossRef]
- De Chantal, M.; Granström, A. Aggregations of dead wood after wildfire act as browsing refugia for seedlings of Populus tremula and Salix caprea. For. Ecol. Manag. 2007, 250, 3–8. [Google Scholar] [CrossRef]
- Pellerin, M.; Said, S.; Richard, E.; Hamann, J.-L.; Dubois-Coli, C.; Hum, P. Impact of deer on temperate forest vegetation and woody debris as protection of forest regeneration against browsing. For. Ecol. Manag. 2010, 260, 429–437. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Bugmann, H. Effect of microsites, logs and ungulate browsing on Picea abies regeneration in a mountain forest. For. Ecol. Manag. 2005, 205, 251–265. [Google Scholar] [CrossRef]
- Voser, P. Einflüsse hoher Rothirschbestände auf die Vegetation im Unterengadin und im Münstertal, Kanton Graubünden; Band 16 Ergebnisse der wissenschaftlichen Untersuchungen im Schweizerischen Nationalpark; Herausgegeben von der Kommission der Schweizerischen Naturforschenden Gesellschaft zur wissenschaftlichen Erforschung des Nationalparks; Lüdin AG: Liestal, Switzerland, 1987; p. 78. [Google Scholar]
- Motta, R. Impact of wild ungulates on forest regeneration and tree composition of mountain forests in Western Italian Alps. For. Ecol. Manag. 1996, 88, 93–98. [Google Scholar] [CrossRef]
- Tremblay, J.-P.; Huot, J.; Potvin, F. Density-related effects of deer browsing on the regeneration dynamics of boreal forests. J. Appl. Ecol. 2007, 44, 552–562. [Google Scholar] [CrossRef]
- Boulanger, V.; Dupouey, J.L.; Archaux, F.; Badeau, V.; Baltzinger, C.; Chevalier, R.; Corcket, E.; Dumas, Y.; Forgeard, F.; Mårell, A.; et al. Ungulates Increase forest plant species richness to the benefit of non-forest specialists. Glob. Chang. Biol. 2018, 24, e485–e495. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Wasem, U.; Bugmann, H. Light availability and ungulate browsing determine growth, height and mortality of Abies alba saplings. For. Ecol. Manag. 2014, 318, 359–369. [Google Scholar] [CrossRef]
- Prietzel, J.; Ammer, C. Mixed mountain forests of the Bavarian Limestone Alps: Reduction of ungulate density results not only in increased regeneration success but also in improved soil fertility. Allg. Forst Jagdz. 2008, 179, 104–112. [Google Scholar]
- Kamler, J.; Homolka, M.; Barančeková, M.; Krojerová-Prokešová, J. Reduction of herbivore density as a tool for reduction of herbivore browsing on palatable tree species. Eur. J. For. Res. 2010, 129, 155–162. [Google Scholar] [CrossRef]
- Graff, P.; Aguiar, M.R.; Chaneton, E.J. Shifts in positive and negative plant interactions along a grazing intensity gradient. Ecology 2007, 88, 188–199. [Google Scholar] [CrossRef]
- Odermatt, O. Ergebnisse aus Untersuchungen zum Wildtiereinfluss auf die Waldverjüngung in der Schweiz. Schweiz. Z. Forstwes. 2009, 160, 294–302. [Google Scholar] [CrossRef][Green Version]
- Rüegg, D.; Nigg, H. Mehrstufige Verjüngungskontrollen und Grenzwerte für die Verbissintensität. Schweiz. Z. Forstwes. 2003, 154, 314–321. [Google Scholar] [CrossRef][Green Version]
- Rüegg, D. Verjüngungskontrolle: Methoden Stichproben in Indikatorflächen; Deutscher Forstwirtschaftsrat e. V.: Kaltbrunn, Switzerland, 2008. [Google Scholar]
- Federal Office of Topography swisstopo. Swisstopo, Digital Height Model DHM25 is a Set of Data Representing the 3D Form of the Earth’s Surface without Vegetation and Buildings; Federal Office of Topography swisstopo: Wabern, Switzerland, 2015. [Google Scholar]
- Thornton, P.E.; Running, S.W.; White, M.A. Generating surfaces of daily meteorological variables over large regions of complex terrain. J. Hydrol. 1997, 190, 214–251. [Google Scholar] [CrossRef]
- Allen, J.C. Modified sine wave method for calculating degree days. Environ. Entomol. 1976, 5, 388–396. [Google Scholar] [CrossRef]
- Frehner, M.; Wasser, B.; Schwitter, R. Nachhaltigkeit und Erfolgskontrolle im Schutzwald. In Wegleitung für Pflegemassnahmen in Wäldern mit Schutzfunktion; Bundesamt für Umwelt, Wald und Landschaft: Bern, Switzerland, 2005; p. 564. [Google Scholar]
- Keller, M. (Ed.) Schweizerisches Landesforstinventar, Anleitung für die Feldaufnahmen der Erhebung 2004–2007; Eidg. Forschungsanstalt WSL: Birmensdorf, Switzerland, 2005; p. 393. [Google Scholar]
- Cary Institute of Ecosystem Studies. Gap Light Analyzer (GLA); Simon Fraser University, Cary Institute of Ecosystem Studies: Millbrook, NY, USA, 1999. [Google Scholar]
- Bitterlich, W. The Relascope Idea; Commonwealth Agricultural Bureaux: Farnham, UK, 1984. [Google Scholar]
- Londo, G. The decimal scale for releves of permanent quadrats. Vegetatio 1976, 33, 61–64. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Neville, P.G. Controversy of variable Importance in random forests. J. Unified Stat. Tech. 2013, 1, 15–20. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org (accessed on 15 July 2013).
- Bates, D. Lme4: Linear Mixed-Effects Models Using Eigen and S4. R package, version 1.0-5. 2013. Available online: http://CRAN.R-project.org/package=lme4 (accessed on 15 July 2013).
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Brzeziecki, B.; Kienast, F. Classifying the life- history strategies of trees on the basis of the Grimian model. For. Ecol. Manag. 1994, 69, 167–187. [Google Scholar] [CrossRef]
- Ward, A.I.; White, P.C.L.; Walker, N.; Critchley, C.H. Conifer leader browsing by roe deer in English upland forests: Effects of deer density and understorey vegetation. For. Ecol. Manag. 2008, 256, 1333–1338. [Google Scholar] [CrossRef]
- Hothorn, T.; Brandl, R.; Müller, J. Large-scale model-based assessment of deer-vehicle collision risk. PLoS ONE 2012, 7, e29510. [Google Scholar] [CrossRef]
- Onderscheka, K.; Reimoser, F.; Tataruch, F.; Steineck, T.; Klansek, E.; Völk, F.; Willing, R.; Zandl, J. Integrale Schalenwildbewirtschaftung im Fürstentum Liechtenstein unter besonderer Berücksichtigung Landschaftsökologischer Zusammenhänge; Naturkundliche Forschung im Fürstentum Liechtenstein: Vaduz, Liechtenstein, 1989; Volume 11, p. 265. [Google Scholar]
- Kupferschmid, A.D.; Wasem, U.; Bugmann, H. Ungulate browsing leads to height and diameter growth reduction on Abies alba saplings planted along light gradients. Eur. J. For. Res. 2015, 134, 75–87. [Google Scholar] [CrossRef]
- Nichols, R.V.; Königsson, H.; Danell, K.; Spong, G. Browsed twig environmental DNA: Diagnostic PCR to identify ungulate species: Browsed twig Environmental DNA. Mol. Ecol. Resour. 2012, 12, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M. Verbissnachweis Mittels Kriminaltechnologischer Methoden; Institut für Wildbiologie und Jagdwirtschaft (IWJ), Department für Integrative Biologie und Biodiversitätsforschung, Universität für Bodenkultur: Wien, Austria, 2017; p. 46. [Google Scholar]
- Calkoen, S.T.V.B.; Leigh-Moy, K.; Cromsigt, J.P.; Spong, G.; Lebeau, L.C.; Heurich, M. The blame game: Using eDNA to identify species-specific tree browsing by red deer (Cervus elaphus) and roe deer (Capreolus capreolus) in a temperate forest. For. Ecol. Manag. 2019, 451, 117483. [Google Scholar] [CrossRef]
- Bobek, B.; Boyce, M.S.; Kosobucka, m. Factors affecting red deer (Cervus elaphus) population density in southeastern Poland. J. Appl. Ecol. 1984, 21, 881–890. [Google Scholar] [CrossRef]
- Reimoser, F.; Gossow, H. Impact of ungulates on forest vegetation and its dependence on the silvicultural system. For. Ecol. Manag. 1996, 88, 107–119. [Google Scholar] [CrossRef]
- Brang, P.; Kupferschmid, A.D. Praxisrelevante Grundlagen: Walddynamik, in Wald und Wild—Grundlagen für die Praxis. Wissenschaftliche und methodische Grundlagen zum integralen Management von Reh, Gämse, Rothirsch und ihrem Lebensraum; Bundesamt für Umwelt BAFU: Bern, Switzerland, 2010; pp. 41–56. [Google Scholar]
- Bischof, T.; Thormann, J.J.; Zbinden, K.G.; Kupferschmid, A.D. Positive Beispiele der Wald-Wild-Situation bei der Weisstannenverjüngung. Schweiz. Z. Forstwesen. 2019, 170, 142–152. [Google Scholar] [CrossRef]
- Angst, C.; Bürgi, A.; Duelli, P.; Egli, S.; Heiniger, U.; Hindenlang, K.; Lässig, R. Waldentwicklung nach Windwurf in tieferen Lagen der Schweiz. Schlussbericht eines Projektes im Rahmen des Programms. In LOTHAR Evaluations-und Grundlagenprojekte; Eidg. Forschungsanstalt WSL: Birmensdorf, 2004; p. 98. [Google Scholar]
- Häsler, H. Impact of Browsing by Wild Ungulates on Growth and Regeneration of European Silver Fir. Ph.D. Thesis, Diss. ETH No. 179112008. University of Zürich, Zürich, Switzerland, 2008; p. 113. [Google Scholar]
- Jehl, H. Die Waldentwicklung auf Windwurfflächen im Nationalpark Bayerischer Wald. In 25 Jahre auf dem Weg zum Naturwald; Nationalparkverwaltung Bayerischer Wald, Ed.; Nationalparkverwaltung Bayerischer Wald: Neuschönau, Deutschland, 1995; pp. 112–146. [Google Scholar]
- Kuijper, D.P.J.; Cromsigt, J.; Churski, M.; Adam, B.; Jędrzejewska, B.; Jedrzejewski, W. Do ungulates preferentially feed in forest gaps in European temperate forest? For. Ecol. Manag. 2009, 258, 1528–1535. [Google Scholar] [CrossRef]
- Putman, R.J.; Moore, N.P. Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mammal. Rev. 1998, 28, 141–164. [Google Scholar] [CrossRef]
- Stöckli, V. Die Bedeutung der winterlichen Schneedecke für junge Fichten im Gebirgswald: Ergebnisse aus dem Lusiwald bei Davos. Bauhinia 2002, 16, 23–32. [Google Scholar]
- Kuijper, D.P.J.; Cromsigt, J.P.G.M.; Jędrzejewska, B.; Miścicki, S.; Churski, M.; Jedrzejewski, W.; Kweczlich, I. Bottom-up versus top-down control of tree regeneration in the Białowieża Primeval Forest, Poland. J. Ecol. 2010, 98, 888–889. [Google Scholar] [CrossRef]
- Kuijper, D.P.J.; De Kleine, C.; Churski, M.V.; Van Hooft, P.; Bubnicki, J.; Jędrzejewska, B. Landscape of fear in Europe: Wolves affect spatial patterns of ungulate browsing in Białowieża Primeval Forest, Poland. Ecography 2013, 36, 1–13. [Google Scholar] [CrossRef]
- Kuijper, D.P.J.; De Kleine, C.; Churski, M.; Van Hooft, P.; Bubnicki, J.W.; Jędrzejewska, B. Liability of saplings to browsing on a red deer range in the Scottish Highlands. J. Appl. Eclol. 1982, 19, 941–951. [Google Scholar]
- Schwyzer, A.; Lanz, A. Verjüngungserhebung im schweizerischen Landesforstinventar. In Proceedings of the Internationale Biometrische Gesellschaft—Deutsche Region AG Ökologie und Umwelt. 22 meeting, Göttingen, Germany, 20–21 September 2010; pp. 42–67. [Google Scholar]
- Schweiger, J.; Sterba, H. A model describing natural regeneration recruitment of Norway spruce (Picea abies (L.) Karst.) in Austria. For. Ecol. Manag. 1997, 97, 107–118. [Google Scholar] [CrossRef]
- Didion, M.; Kupferschmid, A.D.; Bugmann, H. Long-term effects of ungulate browsing on forest composition and structure. For. Eco. Manag. 2009, 258, 44–55. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupferschmid, A.D.; Bütikofer, L.; Hothorn, T.; Schwyzer, A.; Brang, P. Ungulate Species and Abundance as well as Environmental Factors Determine the Probability of Terminal Shoot Browsing on Temperate Forest Trees. Forests 2020, 11, 764. https://doi.org/10.3390/f11070764
Kupferschmid AD, Bütikofer L, Hothorn T, Schwyzer A, Brang P. Ungulate Species and Abundance as well as Environmental Factors Determine the Probability of Terminal Shoot Browsing on Temperate Forest Trees. Forests. 2020; 11(7):764. https://doi.org/10.3390/f11070764
Chicago/Turabian StyleKupferschmid, Andrea Doris, Lukas Bütikofer, Torsten Hothorn, Andreas Schwyzer, and Peter Brang. 2020. "Ungulate Species and Abundance as well as Environmental Factors Determine the Probability of Terminal Shoot Browsing on Temperate Forest Trees" Forests 11, no. 7: 764. https://doi.org/10.3390/f11070764
APA StyleKupferschmid, A. D., Bütikofer, L., Hothorn, T., Schwyzer, A., & Brang, P. (2020). Ungulate Species and Abundance as well as Environmental Factors Determine the Probability of Terminal Shoot Browsing on Temperate Forest Trees. Forests, 11(7), 764. https://doi.org/10.3390/f11070764





