Abstract
Large-scale ash (Fraxinus spp.) dieback caused by the fungus Hymenoscyphus fraxineus has been a major concern throughout Europe for more than two decades. Most of the related research has been focused on Fraxinus excelsior L., and there is still little information on fungal involvement in the dieback of Fraxinus angustifolia Vahl, especially in roots and stem bases, which play an important role in decline progress and tree stability. The objectives of this study were to identify fungi present in visually healthy and symptomatic wood tissues in basal parts of narrow-leaved ash trees in different decline phases, in order to determine the possible role of these fungi and their importance in the dieback process. The stem bases and roots of 90 trees in three different health categories, determined based on crown defoliation, were sampled in natural stands affected by ash dieback. Isolated fungal cultures were identified based on the rDNA ITS (Internal transcribed spacer) region and their association with tree health status was analyzed. In total, 68 different fungal taxa were confirmed, including Hymenoscyphus fraxineus and Armillaria spp., which were mainly present in roots, although overall in lower frequencies than on common ash in other studies. Most frequently isolated fungal taxa, which encompassed 51% of all obtained isolates, were Trichoderma spp., Ilyonectria robusta, Fusarium solani, Cladosporium cladosporioides, and Diaporthe cotoneastri. Their associations with tree health categories and presence in both symptomatic and visually healthy wood indicate that they act as opportunistic pathogens and early colonizers of weakened ash tissues. Research also revealed that, although the extent of crown defoliation and presence of root and stem necroses were associated, basal symptoms occurred on a number of trees with healthy looking crowns, meaning they can develop independently.
1. Introduction
Narrow-leaved ash (Fraxinus angustifolia Vahl) is a species of great ecological and economical value in lowland and riparian forests of central Europe, the Pannonian Basin, and the Balkans [1], as well as in Croatia where it grows in single-species or mixed stands as the second most abundant floodplain tree species after pedunculate oak (Quercus robur L.), with growth stock of 17,619,000 m3 (3.19% of total stock), occupying 72,690 ha (3.06% of total forest area) [2]. In these habitats, this ash is significant as a pioneer tree species, being able to outstand longer flood periods and grow in areas unfavorable for pedunculate oak. It is thus concerning that narrow-leaved ash has been one of the most damaged forest tree species in Croatia since 2014, when the number of trees with crown defoliation greater than 25% increased rapidly in comparison with previous years and reached 75% of trees in 2017 [3].
The causative agent to which this phenomenon is mostly attributed is the known fungal pathogen of ash (Fraxinus spp.), Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz & Hosoya, responsible for large-scale dieback of common (Fraxinus excelsior L.) and narrow-leaved ash throughout Europe [4,5,6]. In Croatia, the fungus was first reported on common ash in 2009 [7], whereas on narrow-leaved ash it was not found until 2011, when typical symptoms of the disease (brown to orange bark necroses and cankers, foliage necroses, wilting, and premature shedding) were observed in trees’ crowns and the presence of the pathogen was confirmed in shoots and twigs. However, the pathogen was not found in all analyzed declining forest stands, although the same methods and materials were used [8,9,10]. A similar situation with Hymenoscyphus fraxineus being absent or not frequently isolated from symptomatic crown tissue of common ash was reported by several authors [11,12,13,14], and can be attributed to its slow growth and to antagonistic activity of other opportunistic fungi present in the same tissue [15], or sampling during high temperatures unfavorable for the pathogen [5,16,17,18]. In spite of possible explanations, the absence of H. fraxineus in parts of analyzed declining narrow-leaved ash trees in Croatia raised the question of the role of other fungi in the dieback process, as this theory had already been introduced in similar research conducted mainly on common ash, where various known pathogenic and opportunistic fungi were found in symptomatic tissues. Some of these species (Alternaria alternata (Fr.) Keissl., Cytospora pruinosa (Fr.) Sacc., Diaporthe eres Nitschke, Diplodia mutila (Fr.) Mont., Epicoccum nigrum Link, Fusarium avenaceum (Fr.) Sacc., F. lateritium Nees, F. oxysporum Schltdl., F. solani (Mart.) Sacc., Ilyonectria destructans (Zinssm.) Rossman, L. Lombard & Crous, Phomopsis sp.) may be considered as independent colonizers since they were able to cause wood and bark discolorations or necroses on stems of common ash seedlings in pathogenicity tests [11,12,19,20]. However, most of the research, in which parasitic fungi were found in symptomatic ash tissues, indicates that these might accelerate or contribute to ash dieback as accompanying or successive species to Hymenoscyphus fraxineus [13,15,21,22,23], or act opportunistically after tree weakening under the influence of abiotic factors [14].
In addition to crown symptoms, stem base and root rot were reported from multiple locations of narrow-leaved ash in Croatian lowland forests (unpublished data), which is in accordance with findings on declining common ash trees in several countries, where the occurrence of butt rot or collar necroses was frequently reported and attributed to Hymenoscyphus fraxineus and other opportunistic fungi [24,25,26,27,28], mostly Armillaria cepistipes Velen. and A. gallica Marxm. & Romagn. as important secondary pathogens [29,30,31,32]. The occurrence of collar rots associated with Hymenoscyphus fraxineus and Armillaria spp. was also confirmed on narrow-leaved ash in Slovenia by Hauptman et al. [33]. Some research suggested fungus-like organisms from the genus Phytophthora as causative agents of root and collar necroses on common ash [34] and as pathogenic to narrow-leaved ash [35], although many other studies have so far revealed no evidence of Phytophthora spp. involvement in ash dieback [23,28,36]. Regardless of fungal organisms causing them, root and stem base necroses and rot are considered to play a major role in the faster progression of ash dieback and loss of tree stability [24,25,32], obstructing forest management practices due to the higher risk of tree failure.
The majority of research on ash dieback has so far been conducted on Fraxinus excelsior as the most abundant ash species in Europe. Additionally, there is scarce information on fungal species involvement in narrow-leaved ash decline, especially regarding fungal communities in roots, collars, and stems, which seem to be crucial accelerating factors for tree dieback. The aim of this research was to identify fungi in roots and stem bases of narrow-leaved ash trees of different health status in order to do the following: (1) determine the presence and the frequency of confirmed causal agents of ash dieback, Hymenoscyphus fraxineus and Armillaria spp., in these tissues; and (2) determine the occurrence of other potentially pathogenic and opportunistic fungi in symptomatic and healthy tissues of trees in different decline phases and their possible role in the process of narrow-leaved ash dieback.
2. Materials and Methods
2.1. Field Sampling
Research was conducted in lowland forests at the Sava river basin, where narrow-leaved ash occurs naturally and occupies the largest continuous forest area (28,000 ha) in Croatia [37], and where H. fraxineus was confirmed in crowns of declining trees in 2015 [9,10]. Sampling was carried out in the period from April to September 2016 in narrow-leaved ash forest stands (both single-species stands and mixed with Quercus robur and Alnus glutinosa) exhibiting symptoms of ash dieback (increased crown transparency and tree mortality), located in the area of three forest management units, namely Črnovščak (45°45′ N, 16°18′ E), Josip Kozarac (45°23′ N, 16°48′ E), and Trstika (45°18′ N, 16°55′ E) (Figure 1). Trees selected for sampling were categorized in three health groups according to crown defoliation (H1 = crown defoliation ≤ 25%; H2 = crown defoliation 26–60%; H3 = crown defoliation 61–99%), which was assessed in accordance with the methodology of the International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests, known as ICP-Forests (Figure 2) [38]. Dead trees with 100% crown defoliation were not considered in this research. In each health category, 30 trees were randomly chosen for sampling. The average diameter at breast height (DBH) of selected trees was 21.29 cm (SD = 6.51 cm). Differences between average DBH of trees among categories were not statistically significant (F (2, 87) = 0.34, p = 0.71).
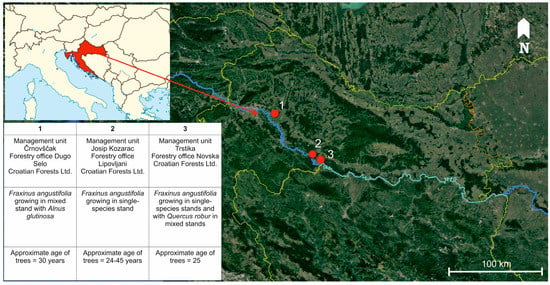
Figure 1.
Map of three sampling locations in lowland forests at the Sava river basin.

Figure 2.
Three health groups according to crown defoliation (H1 = crown defoliation ≤ 25%; H2 = crown defoliation 26–60%; H3 = crown defoliation 61–99%).
Root and stem base were sampled from a total of 90 selected trees. Two stem base samples (up to 20 cm height from the ground) were taken from each tree by extracting 10–15 cm long bore cores with an increment borer (5.15 mm diameter, Haglöf, Långsele, Sweden) at the edge of symptomatic tissue visible as discoloration or necrosis or at two opposite sites around the stem base circumference from visually healthy trees. The borer was sterilized between every sample. The root system of each tree was superficially excavated at approximately 1 m radius from a stem base, and root branches were checked for signs of discoloration, necrosis, or rot. One root branch was taken per tree, with or without the aforementioned symptoms, depending on the health status of the particular tree.
2.2. Isolation of Fungi from Tissue Samples
Roots were washed under running water and three cross-sections with bark, approximately 2–3 cm thick, exhibiting xylem discoloration or necrosis, were taken from each where possible. In visually healthy roots, cross-sections were taken at three randomly selected and mutually distant points along the root. These sections were surface sterilized by immersing in a solution of sodium hypochlorite (0.5% v/v) for three minutes and subsequently in ethanol (70% v/v) for one minute, followed by rinsing three times in sterile distilled water and drying on sterile filter paper in a laminar flow hood (Iskra Pio, Šentjernej, Slovenia) [39]. From each section, four xylem subsamples (wood chips) were aseptically taken at the edge of advancing discoloration or necrosis and plated on agar medium. Each of the two stem base samples taken per tree was debarked and cut in two or three sections that were 3–5 cm long (depending on the total length, which varied between 10 and 15 cm). Xylem tissue was surface sterilized using a different method for each of the two bore cores per tree. The first method included soaking samples for five seconds in a solution of hydrogen chloride (0.5% v/v) and washing in sterile distilled water, and second one included dipping the bore cores for two seconds in ethanol (70% v/v) and exposing them shortly to flame [40]. As the xylem in the stem samples was more exposed to the sterilizing agent due to its smaller size and lack of bark, the time of exposure was empirically reduced to two and five seconds, in order to obtain fungal growth. Both root and stem base subsamples were plated onto potato dextrose agar (PDA, Oxoid, Basingstoke, UK) supplemented with streptomycin sulphate (200 mg/L, Sigma-Aldrich, St. Louis, MO, USA) in 90 mm Petri dishes that were incubated in the dark at 19–20 °C for four weeks. Petri dishes were checked daily for fungal growth and emerging mycelia were subcultured on fresh PDA.
2.3. Identification of Isolated Fungal Taxa
The obtained pure cultures were grouped into morphotypes based on mycelium color, structure and growth speed, coloration of agar, and sporulation. At least one isolate of each morphotype group was used for molecular identification. Extraction of DNA was performed using the phenol–chloroform method according to Ježić et al. [41] with modifications [42]. PCR amplification was conducted with primers ITS 1 and ITS 4 [43] in 50 µl reactions containing 200 µM deoxyribonucleoside triphosphates, 0.4 µM of each primer, 1 U of Taq DNA polymerase with reaction buffer (Sigma-Aldrich, St. Louis, MO, USA), 1.5 mM MgCl2, and 1 µl of 100-fold diluted DNA template. Cycling conditions were as follows: an initial denaturation at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 45 s, extension at 72 °C for 90 s, and a final extension step at 72 °C for 5 min. The resulting PCR products were sequenced using primer ITS 4 at the DNA sequencing facility of Macrogen Europe (Amsterdam, Netherlands). After processing the raw data using the BioEdit Sequence Alignment Editor v.7.2.5 software (Raleigh, NC, USA) [44], sequences were identified by comparison with reference sequences in National Center for Biotechnology Information (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST) tool [45]. Sequences with 98–100% similarity were assigned to the species level and with 94–97% similarity to the genus level [30].
2.4. Statistical Analysis
Similarity between fungal communities in trees from different health categories was analyzed using qualitative Sorensen indices [46], which were calculated with EstimateS 9.1.0. software (Boulder, CO, USA) [47]. A Venn diagram of shared and unique fungal taxa among the health categories was constructed using eulerAPE [48]. Associations of fungal taxa with health categories were explored using correspondence analysis (CA), where the graphical display of data is based on Pearson’s chi-squared distance. In order to fulfill the conditions of a minimum number of observations required for the calculation of the chi-squared value, CA was performed on the 13 most frequent fungal taxa and Hymenoscyphus fraxineus. Analysis was performed in the Statistica 10 software package (StaSoft Inc. 2011, Tulsa, OK, USA).
3. Results
Out of 270 root cross-sections (three sections per each of 90 roots) used for isolation, 231 (86%) resulted in growth of one to seven different mycelia. Out of 180 stem base samples (two per each of 90 trees), 153 (85%) showed growth of one to six different mycelia. In total, 907 pure mycelium cultures were obtained, belonging to 68 taxa (Figure 3, Table 1). Of those, 49 taxa were identified to species level, 17 to genus level, one to family level and one to order level. The majority of taxa belonged to the phylum Ascomycota (58), whereas Basidiomycota (8) and Zygomycota (2) were less abundant. At least one fungal taxon was obtained from each root and stem base of the analyzed trees, with an average number of six taxa per tree. The most frequently isolated taxa regarding both the number of obtained cultures and number of colonized trees were Trichoderma spp., Ilyonectria robusta, Fusarium solani, Cladosporium cladosporioides, and Diaporthe cotoneastri. These five taxa encompassed 51% of all isolates, while the remaining 63 taxa represented 49% of isolated cultures. Of the latter, 23 taxa were found just on one tree and most of them (22) were represented with only one obtained isolate (Figure 3).
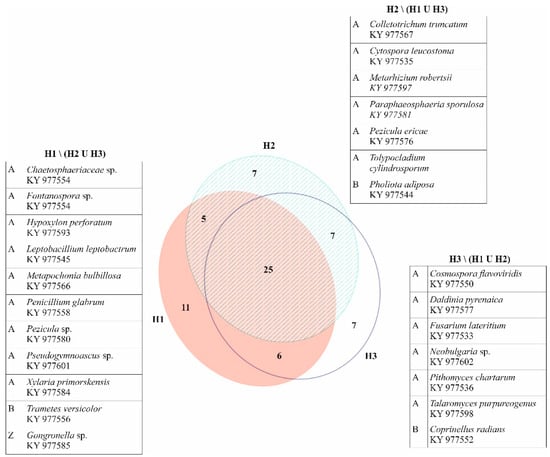
Figure 3.
Venn diagram showing numbers of common and unique fungal taxa among different health categories with list of fungal taxa (and their GenBank sequence accession numbers) present on Fraxinus angustifolia trees in only one health category, mainly found on one tree, and represented by one isolate, with the exception of Neobulgaria sp. (two isolates on one tree), Pholiota adiposa (two isolates on two trees), and Tolypocladium cylindrosporum (five isolates on two trees). A = Ascomycota; B = Basidiomycota; Z = Zygomycota.

Table 1.
Number of colonized Fraxinus angustifolia trees and obtained isolates (in brackets) in three health categories for each fungal taxon found in more than one category (H1 = crown defoliation ≤ 25%, H2 = crown defoliation 26–60%, H3 = crown defoliation 61–99%. A = Ascomycota, B = Basidiomycota, Z = Zygomycota).
Besides fungal taxa represented with one isolate, which were consequently present in only one sample type, two other taxa were present just in stem bases (Cordyceps confragosa and Talaromyces ohiensis) and four other taxa isolated only from roots (Cadophora sp., Dimorphospora foliicola, Neobulgaria sp., and Tolypocladium cylindrosporum), while the rest of the isolated fungi colonized both roots and stems of the analyzed trees.
Trees classified into health category H1 yielded 281 fungal cultures belonging to 47 different taxa (3–11 per tree), trees in category H2 gave growth to 312 isolates appointed to 44 taxa (2–9 per tree), and trees in category H3 resulted in growth of 314 fungal mycelia corresponding to 45 taxa (1–9 per tree). Trees from all health categories shared 25 common taxa, while another 25 taxa were found in just one (Figure 3). Sørensen similarity indices between categories were moderate to high (H1 vs. H2 = 0.659; H1 vs. H3 = 0.673; H2 vs. H3 = 0.719). The most frequent taxon on trees from category H1 was Ilyonectria robusta, followed by Trichoderma spp. and Cladosporium cladosporioides. Trees in categories H2 and H3 shared the three most frequent taxa, with the most present being Trichoderma spp., followed by Ilyonectria robusta and Fusarium solani.
Hymenoscyphus fraxineus was confirmed on only 8.9% of the analyzed trees, almost exclusively in health categories H2 and H3 with the exception of one isolate obtained from the symptomatic root tissue of a tree in category H1. It was mostly isolated from roots (87% of obtained cultures) and not found in both root and stem base of the same tree. Armillaria spp. colonized 14.4% of trees in all three health categories and were not detected on the same trees with Hymenoscyphus fraxineus in this research. They were isolated from trees in two management units, with isolates from Trstika being successfully identified to species level as Armillaria cepistipes, while isolates from Črnovščak could not be unambiguously identified because the ITS region sequences showed 99.66% similarity with three species, namely A. cepistipes, A. gallica, and A. sinapina. For this reason, all isolates belonging to genus Armillaria were grouped into one fungal taxon, Armillaria spp. They were also isolated mainly from roots (74% of obtained cultures). White mycelial fans characteristic for Armillaria spp. were observed only in roots, in 10 out of 13 trees where they were confirmed by isolation, and not on the other trees sampled in this research. The number of colonized trees and obtained isolates in different health categories for taxa present in more than one category is shown in Table 1.
Association of the 13 most frequent taxa and Hymenoscyphus fraxineus with different health categories was explored using correspondence analysis and is shown in Figure 4. Chi-squared testing revealed that differences between the observed and the expected frequencies were not statistically significant (total chi-squared = 21.49; df = 26; p = 0.7162). Dimensions 1 and 2 explained 76.42% and 23.58% of the inertia, respectively. The correspondence analysis map revealed that healthy trees in category H1 were most associated with Cladosporium cladosporioides, declining trees in category H2 with Fusarium solani, and most affected trees in category H3 with Coprinellus micaceus and Absidia sp. Out of 14 fungal taxa taken in consideration for correspondence analysis, only Hymenoscyphus fraxineus and Armillaria spp. were found exclusively in symptomatic wood tissues in both roots and stem bases, while other fungi colonized both visually healthy and symptomatic wood tissue.
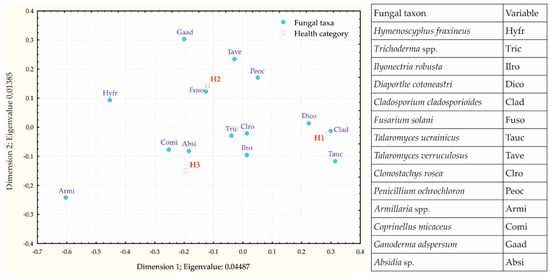
Figure 4.
Correspondence analysis map showing association of the 13 most frequent fungal taxa and Hymenoscyphus fraxineus with tree health categories (H1 = crown defoliation ≤ 25%, H2 = crown defoliation 26–60%, H3 = crown defoliation 61–99%).
Out of 30 trees classified in health category H1, 13 displayed some kind of symptoms (discoloration or necrosis) in the wood of root or stem base, whereas the remaining 17 trees showed no visible fungal disease symptoms in those tissues. In category H2, only three out of 30 trees had no visible symptoms in analyzed wood tissues of both root and stem base. There were no trees without symptomatic roots or stem bases in health category H3.
4. Discussion
Dieback of common and narrow-leaved ash has been a concerning issue throughout Europe for more than two decades [49,50,51,52], including Croatia where both tree species occur naturally. Most of the research indicating the important roles played by Hymenoscyphus fraxineus and other fungi in this phenomenon has been conducted on Fraxinus excelsior, whereas information on fungal species present on F. angustifolia are still less abundant. Out of 68 different fungal taxa found in this research in roots and stems of narrow-leaved ash, 20 were also found on declining common ash in similar studies, including already confirmed and well-known ash pathogens Hymenoscyphus fraxineus and Armillaria spp., but also Coprinellus micaceus, Diaporthe cotoneastri, Ilyonectria robusta, Metapochonia bulbillosa (Pochonia bulbillosa), Neonectria punicea, Psathyrella candolleana, Pseudeurotium bakeri, Trametes versicolor, and Xylaria polymorpha in roots and stem bases [25,30,31,53]; Cladosporium cladosporioides, Clonostachys rosea (Bionectria ochroleuca), Nemania serpens, Paraphaeosphaeria neglecta, and Penicillium glabrum in shoots or branches [11,12,13,14,23,54,55]; and Coprinellus disseminatus, Epicoccum nigrum, Fusarium lateritium, and Fusarium solani in both basal parts and crowns [11,12,13,14,15,19,25,30,31,53,54,55,56]. Considering only those taxa identified to species level, the majority (30 of 49) was not detected on common ash in previous studies and vice versa. Several species isolated from F. excelsior roots and collars considered as possible ash pathogens, such as Botryosphaeria stevensii, Cylindrocarpon destructans, and Diaporthe eres [19,20,21,25,30,31,53], were not found in this research, indicating that endophytic and opportunistic fungi contributing to dieback are not equal for common and narrow-leaved ash.
Although Hymenoscyphus fraxineus was confirmed in roots and stem bases of narrow-leaved ash in this research, it was not as frequently isolated (only 8 of 90 trees) as from root and stem collar lesions on common ash in several other studies [24,26,27,28,53,57]. The possibility that the pathogen was successively replaced by rapidly developing opportunistic fungi with the aging of necroses [5,15] is questionable in this case, because sampling was conducted only one year after H. fraxineus was detected in trees’ crowns in the researched area [9,10]. Although necroses were expanding deeper into the xylem, indicating they might be older, opinion is that a longer period of time is needed for the buildup of H. fraxineus inoculum pressure in leaf litter and the infection of trees’ basal parts [24,26]. This could mean that the pathogen was able to infect narrow-leaved ash roots and stems and progress rapidly through tissue even in the early stage of decline, where it was eventually replaced by other microorganisms. Knowing that increased soil moisture favors the development of basal lesions [27,28,32,58], and that narrow-leaved ash in Croatia occupies floodplain areas, this hypothesis should not be discarded. Other possibilities are that necroses were formed by other pathogenic fungi or that H. fraxineus was present in researched stands before official confirmation, which is rather unlikely since extensive studies were conducted during this period in the same area [59]. Applied stem tissue sampling with an increment borer could also have affected the lower H. fraxineus isolation frequency in this research given that, in some studies that report it to be more numerous, isolations were made from multiple points at the edge of stem necrosis [25,53]. Nevertheless, CA revealed the strongest association of the pathogen with trees in category H2, which are declining but still have not reached the final phase of dieback where secondary pathogens are expected to take over. An interesting finding was the prevalence of H. fraxineus in roots rather than in stem bases of affected trees, and never in both on the same tree. This could be again a consequence of applied methods, where a greater portion of roots was sampled and analyzed, or could point to the possibility that H. fraxineus infects trees via roots, as postulated by Meyn et al. [53], which should be determined in future studies with more detailed analysis of tree roots and stems.
Other confirmed pathogens in this study, Armillaria spp., were also more present in roots (74% of obtained isolates) than in stem bases of analyzed narrow-leaved ash, which is not surprising, since these species are known as root infecting pathogens [60,61]. Although Armillaria spp. were more frequent than Hymenoscyphus fraxineus, they were still isolated from a lower number of trees (13 of 90) in comparison with the 10 most frequent fungal taxa in this research. This finding was unexpected, as it is in disagreement with those on common ash and those of Hauptman et al. [33] on narrow-leaved ash, where authors found Armillaria spp. to be an important secondary damaging agent in roots and stem collars, often together with Hymenoscyphus fraxineus [24,26,27,28,30,31]. However, research in northwest Germany on F. excelsior also revealed quite low isolation frequency of these pathogens from stem collars [25]. One of the reasons for such a result could be the prevalence of other fungal species with reported abilities to cause bark and wood necroses (Diaporthe cotoneastri, Fusarium solani, Ilyonectria robusta), which might be more aggressive than originally thought. Another reason could be the sampling method used, where mostly necrotic and not rotten wood (xylem) from the edges of advancing discolorations/necroses was analyzed, from only two bore cores in stem samples, not encompassing bark samples, lesion centers, nor as much tissue as in some other studies which reported a high isolation frequency of Armillaria spp. [24,26,32]. Even so, CA showed that Armillaria spp. were most strongly associated with severely affected trees in category H3, indicating their important role in a final stage of tree dieback and failure once they colonize the xylem tissue.
Since two common ash pathogens, H. fraxineus and Armillaria spp., were not as numerous as some other taxa in necrotic narrow-leaved ash tissue, the question is raised as to whether some other fungi have the ability of causing symptoms in roots and stems of declining trees and whether their role has been underestimated. The most frequently isolated fungal genus in this research, Trichoderma spp., are usually reported as ubiquitous saprotrophs and endophytes in plants [62] and, in some cases, as antagonists of fungal pathogens [63]. In this study, they were abundant in all health categories, with no distinct association to a particular one according to CA. Without additional studies on exact species composition in trees of different health, it is difficult to conclude whether they could contribute to dieback or act antagonistically to other fungi involved in this process. Other more frequent fungal species isolated in this research, i.e., Cladosporium cladosporioides, Diaporthe cotoneastri, Fusarium solani, and Ilyonectria robusta, were also present in all tree health categories, in both symptomatic (discolored or necrotic) and visually healthy tissue. It is interesting that CA revealed they are more associated with trees in category H1, with the exception of Fusarium solani, which is most strongly linked to category H2. It supports the hypothesis that these fungal species act as endophytes and early colonizers of weakened root and stem tissue. Besides C. cladosporioides, which is a ubiquitous colonizer of both healthy and symptomatic tissue in different plant organs and species [12,13,14,15,23,64,65,66,67], the aforementioned fungi are reported as pathogenic on several wooden hosts. Fusarium solani, termed as the Fusarium solani species complex as it comprises at least 45 phylogenetically distinct species [68], is associated with box elder (Acer negundo L.) dieback [69] and stem cankers of English walnut (Juglans regia L.) [70]. Diaporthe cotoneastri is associated with the trunk canker of apple trees (Malus domestica Borkh.) [71] and Ilyonectria robusta with root diseases of kiwifruit (Actinidia chinensis Planch.), avocado (Persea Americana Mill.), and apple trees (Malus spp.) [72,73,74]. It is possible that these fungi contribute to enlarging the damage on ash roots and stem bases in interaction as a complex of several pathogens, as indicated by Marçais et al. [75] for pedunculate oak root rot.
Another species that was isolated less frequently, but which could have a more important role in contributing to dieback, is Neonectria punicea, identified on eight trees, five of them in category H3. This species was the second most frequently isolated fungus from stem collar lesions on common ash in Germany [25]. Several authors report that it is endophytic with the ability to become pathogenic and spread in weakened tissue [76,77,78]. In this research, it was isolated from both visually healthy and symptomatic tissue in all tree health categories confirming the aforementioned reports.
A number of fungal taxa did not differentiate much between different health categories in this study, likewise the composition of fungal communities as shown by the Venn diagram of common taxa and Sørensen indices values, which were moderate to high. This is in accordance with some of the previous research conducted on common ash [11,55] and it indicates that fungal species already present in the visually healthy trees are the ones which might contribute to narrow-leaved ash decline.
A number of trees displaying root or stem base discoloration or necrosis rapidly increased from category Hl to H2 and H3, revealing the association of crown defoliation and symptoms in basal parts of ash trees affected by dieback and confirming the results of previous research on common ash [24,28,29]. However, even 43% of trees in category H1 considered as healthy or in the initial stages of crown decline also revealed discolorations and necrosis in basal parts, indicating that these symptoms developed independently of crown infections as postulated by Husson et al. [28]. These findings also point out that good crown condition should not be used as a sole indicator for the absence of basal necroses, which can make forest management practices even more challenging, due to the fact that it is often difficult to observe these symptoms, especially on trees with more ridged bark.
5. Conclusions
This study revealed 68 different fungal taxa present in roots and stem bases of narrow-leaved ash trees in different phases of decline, including previously reported and well-known pathogens, Hymenoscyphus fraxineus and Armillaria spp. The majority of other isolated fungi was not found in similar research on common ash, revealing differences in communities of opportunistic pathogens contributing to dieback of these related tree species.
Although confirmed in symptomatic root and stem tissues, Hymenoscyphus fraxineus was not frequently isolated from basal parts of declining narrow-leaved ash trees, indicating that it was already replaced by faster developing opportunistic fungi at the time of sampling, or that necroses were formed by other pathogens triggered by a decrease in the trees’ vitality due to crown defoliation caused by H. fraxineus. In this sense, the most frequent opportunistic fungi in this research, Ilyonectria robusta, Fusarium solani, and Diaporthe cotoneastri, could be considered as endophytes that act as early colonizers of weakened root and stem tissue, while Armillaria spp., although less frequent than other fungal pathogens, plays the most important role in the loss of tree vitality, which leads to a fast crown decline.
Association of crown damage and root and stem necroses was confirmed in this study, although a relatively high number of trees with good crown condition (defoliation < 25%) and presence of root and stem necroses indicates that these symptoms can develop independently of crown decline. This implies that visual tree inspections conducted as part of usual forest management practices addressing the problem of ash dieback should be even more thorough, as there is no assurance that trees with a seemingly undamaged crown are not affected in their basal parts.
Because the enlargement of root and basal necroses and the acceleration of decline could be attributed to the interaction of several fungal species, opportunistic pathogens found in this study should be taken into consideration as factors contributing to narrow-leaved ash dieback. Further studies are needed to gain more insight into their pathogenicity towards narrow-leaved ash and the possibility of causing root and stem necroses individually or in combination.
The impaired health status of roots and stem bases determined in this research, as well as the presence of several opportunistic pathogens in these tissues, emphasize the need for focusing future forest management practices in narrow-leaved ash stands on mitigating the risks for disease development in basal parts of trees, as these usually accelerate the dieback process and enlarge the risk of tree failure.
Author Contributions
D.D. and J.K.O. conceived and designed the experiments; J.K.O. performed the experiments; M.M. analyzed the data; J.K.O. wrote the paper in consultation with D.D. and M.M.; D.D. supervised all phases of research, administrated the project, and acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was carried out with the financial support of the Croatian Science Foundation under the project “The role of biotic agents on vitality of narrow-leaved ash (Fraxinus angustifolia Vahl) in Croatian floodplain forests”–FRAXINPRO (IP-11-2013).
Acknowledgments
The authors thank Croatian Forests Ltd. for permission to carry out sampling in declining stands.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FRAXIGEN. Ash Species in Europe: Biological Characteristics and Practical Guidelines for Sustainable Use; Oxford Forestry Institute, University of Oxford: Oxford, UK, 2005; pp. 1–128. [Google Scholar]
- Čavlović, J. Prva nacionalna inventura šuma Republike Hrvatske; Ministarstvo Regionalnog Razvoja šumarstva i Vodnoga Gospodarstva i Šumarski Fakultet Sveučilišta u Zagrebu: Zagreb, Hrvatska, 2010; pp. 1–300.
- Potočić, N.; Seletković, I.; Jakovljević, T.; Marjanović, H.; Indir, K.; Medak, J.; Ognjenović, M.; Zorić, N. Oštećenost šumskih ekosustava Republike Hrvatske; Izvješće za 2019. godinu; Nacionalni Koordinacijski Centar za Procjenu i Motrenje Utjecaja Atmosferskog Onečišćenja i drugih čimbenika na šumske Ekosustave, Hrvatski šumarski institut: Jastrebarsko, Croatia, 2020; pp. 1–91. [Google Scholar]
- Kräutler, K.; Kirisits, T. The ash dieback pathogen Hymenoscyphus pseudoalbidus is asociated with leaf symptoms on ash species (Fraxinus spp.). J. Agric. Ext. Rural Dev. 2012, 9, 261–265. [Google Scholar]
- Gross, A.; Holdenrieder, O.; Pautasso, M.; Queloz, V.; Sieber, T.N. H ymenoscyphus pseudoalbidus, the causal agent of E uropean ash dieback. Mol. Plant Pathol. 2013, 15, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.; Holdenrieder, O. The teleomorph of Chalara fraxinea, the causal agent of ash dieback. For. Pathol. 2009, 39, 304–308. [Google Scholar] [CrossRef]
- Barić, L.; Diminić, D. Prvi nalaz patogene gljive Chalara fraxinea Kowalski na običnom jasenu (Fraxinus excelsior L.) u Gorskom kotaru. In Proceedings of the 54. Seminar Biljne Zaštite, Opatija, Hrvatska, 9–12 February 2010; p. 33. [Google Scholar]
- Barić, L.; Županić, M.; Pernek, M.; Diminić, D. First records of Chalara fraxinea in Croatia—A new agent of ash dieback (Fraxinus spp.). Šumarski List 2012, 461–469. [Google Scholar]
- Diminić, D. Nova bolest jasena (Fraxinus spp.) u Hrvatskoj. In Proceedings of the Proizvodnja Hrane i šumarstvo—Temelj Razvoja Istočne Hrvatske, Osijek, Hrvatska, 14–15 June 2015; pp. 363–373. [Google Scholar]
- Milotić, M.; Kranjec, J.; Diminić, D. Current status of ash dieback disease Hymenoscyphus fraxineus in Croatia. In Proceedings of the Natural Resources, Green Technology & Sustainable Development—GREEN/2, Zagreb, Hrvatska, 5–7 February 2016; p. 124. [Google Scholar]
- Bakys, R.; Vasaitis, R.; Barklund, P.; Thomsen, I.M.; Stenlid, J. Occurrence and pathogenicity of fungi in necrotic and non-symptomatic shoots of declining common ash (Fraxinus excelsior) in Sweden. Eur. J. For. Res. 2008, 128, 51–60. [Google Scholar] [CrossRef]
- Przybył, K. Fungi associated with necrotic apical parts of Fraxinus excelsior shoots. For. Pathol. 2002, 32, 387–394. [Google Scholar] [CrossRef]
- Davydenko, K.; Vasaitis, R.; Stenlid, J.; Menkis, A. Fungi in foliage and shoots of Fraxinus excelsior in eastern Ukraine: A first report on Hymenoscyphus pseudoalbidus. For. Pathol. 2013, 43, 462–467. [Google Scholar] [CrossRef]
- Pukacki, P.M.; Przybył, K. Frost Injury as a Possible Inciting Factor in Bud and Shoot Necroses of Fraxinus excelsior L. J. Phytopathol. 2005, 153, 512–516. [Google Scholar] [CrossRef]
- Kowalski, T.; Kraj, W.; Bednarz, B. Fungi on stems and twigs in initial and advanced stages of dieback of European ash (Fraxinus excelsior) in Poland. Eur. J. For. Res. 2016, 135, 565–579. [Google Scholar] [CrossRef]
- Hauptman, T.; Piškur, B.; De Groot, M.; Ogris, N.; Ferlan, M.; Jurc, D. Temperature effect on Chalara fraxinea: Heat treatment of saplings as a possible disease control method. For. Pathol. 2013, 43, 360–370. [Google Scholar] [CrossRef]
- Kowalski, T.; Bartnik, C. Morphologial variation in colonies of Chalara fraxinea isolated from ash (Fraxinus excelsior L.) stems with symptoms of dieback and effects of temperature on colony growth and structure. Acta Agrobot. 2012, 63, 99–106. [Google Scholar] [CrossRef]
- Grosdidier, M.; Ioos, R.; Marçais, B. Do higher summer temperatures restrict the dissemination of Hymenoscyphus fraxineus in France? For. Pathol. 2018, 48, e12426. [Google Scholar] [CrossRef]
- Przybył, K. Mycobiota of thin roots showing decay of Fraxinus excelsior L. young trees. Dendrobiology 2002, 48, 65–69. [Google Scholar]
- Kowalski, T.; Bilanski, P.; Kraj, W. Pathogenicity of fungi associated with ash dieback towards Fraxinus excelsior. Plant Pathol. 2017, 66, 1228–1238. [Google Scholar] [CrossRef]
- Kowalski, T.; Łukomska, A. The studies on ash dying (Fraxinus excelsior L.) in the Włoszczowa Forest Unit stands. Acta Agrobot. 2012, 58, 429–440. [Google Scholar] [CrossRef]
- Kowalski, T.; Holdenrieder, O. Pathogenicity of Chalara fraxinea. For. Pathol. 2009, 39, 1–7. [Google Scholar] [CrossRef]
- Bakys, R.; Vasaitis, R.; Barklund, P.; Ihrmark, K.; Stenlid, J. Investigations concerning the role of Chalara fraxineain declining Fraxinus excelsior. Plant Pathol. 2009, 58, 284–292. [Google Scholar] [CrossRef]
- Chandelier, A.; Gerarts, F.; Martin, G.S.; Herman, M.; Delahaye, L. Temporal evolution of collar lesions associated with ash dieback and the occurrence of Armillariain in Belgian forests. For. Pathol. 2016, 46, 289–297. [Google Scholar] [CrossRef]
- Langer, G. Collar rots in forests of Northwest Germany affected by ash dieback. Balt. For. 2017, 23, 4–19. [Google Scholar]
- Enderle, R.; Sander, F.; Metzler, B. Temporal development of collar necroses and butt rot in association with ash dieback. Iforest—Biogeosciences For. 2017, 10, 529–536. [Google Scholar] [CrossRef]
- Marçais, B.; Husson, C.; Godart, L.; Caël, O. Influence of site and stand factors on Hymenoscyphus fraxineus—induced basal lesions. Plant Pathol. 2016, 65, 1452–1461. [Google Scholar] [CrossRef]
- Husson, C.; Caël, O.; Grandjean, J.P.; Nageleisen, L.M.; Marçais, B. Occurrence of Hymenoscyphus pseudoalbidus on infected ash logs. Plant Pathol. 2012, 61, 889–895. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Thomsen, I.M.; Skovgaard, I.M.; Martinussen, T. Associations among symptoms of dieback in even-aged stands of ash (Fraxinus excelsiorL.). For. Pathol. 2010, 40, 7–18. [Google Scholar] [CrossRef]
- Bakys, R.; Vasiliauskas, A.; Ihrmark, K.; Stenlid, J.; Menkis, A.; Vasaitis, R. Root rot, associated fungi and their impact on health condition of declining Fraxinus excelsior stands in Lithuania. Scand. J. For. Res. 2010, 26, 128–135. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskas, R.; Larsson, K.-H.; Stenlid, J. Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 2005, 20, 337–346. [Google Scholar] [CrossRef]
- Enderle, R.; Peters, F.; Nakou, A.; Metzler, B. Temporal development of ash dieback symptoms and spatial distribution of collar rots in a provenance trial of Fraxinus excelsior. Eur. J. For. Res. 2013, 132, 865–876. [Google Scholar] [CrossRef]
- Hauptman, T.; Ogris, N.; De Groot, M.; Piškur, B.; Jurc, D. Individual resistance of Fraxinus angustifolia clones to ash dieback. For. Pathol. 2016, 46, 269–280. [Google Scholar] [CrossRef]
- Orlikowski, L.B.; Ptaszek, M.; Rodziewicz, A.; Nechwatal, J.; Thinggaard, K.; Jung, T. Phytophthora root and collar rot of mature Fraxinus excelsior in forest stands in Poland and Denmark. For. Pathol. 2011, 41, 510–519. [Google Scholar] [CrossRef]
- Akilli, S.; Serçe, Ç.U.; Katırcıoğlu, Y.Z.; Maden, S.; Katircioğlu, Y.Z. Phytophthora dieback on narrow leaved ash in the Black Sea region of Turkey. For. Pathol. 2013, 43, 252–256. [Google Scholar] [CrossRef]
- Schumacher, J.; Kehr, R.; Leonhard, S. Mycological and histological investigations of Fraxinus excelsior nursery saplings naturally infected by Chalara fraxinea. For. Pathol. 2009, 40, 419–429. [Google Scholar] [CrossRef]
- Anić, I. Uspijevanje i pomlađivanje sastojina poljskog jasena (Fraxinus angustifolia Vahl) u Posavini. Doctoral Thesis, University of Zagreb, Faculty of Forestry, Zagreb, Croatia, 2001. [Google Scholar]
- Eichhorn, J.; Roskams, P.; Potočić, N.; Timmermann, V.; Ferretti, M.; Mues, V.; Szepesi, A.; Durrant, D.; Seletković, I.; Schröck, H.-W.; et al. Part IV: Visual Assessment of Crown Condition and Damaging Agents. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE ICP Forests Programme Coordinating Centre, Ed.; Thünen Institute of Forest Ecosystems: Eberswalde, Germany, 2016; 49p. [Google Scholar]
- Greenfield, M.; Pareja, R.; Ortiz, V.; Jiménez, M.I.G.; Vega, F.E.; Parsa, S. A novel method to scale up fungal endophyte isolations. Biocontrol. Sci. Technol. 2015, 25, 1–6. [Google Scholar] [CrossRef]
- Hoff, J.A.; Klopfenstein, N.; McDonald, G.I.; Tonn, J.R.; Kim, M.-S.; Zambino, P.J.; Hessburg, P.F.; Rogers, J.D.; Peever, T.L.; Carris, L.M. Fungal endophytes in woody roots of Douglas-fir (Pseudotsuga menziesii) and ponderosa pine (Pinus ponderosa). For. Pathol. 2004, 34, 255–271. [Google Scholar] [CrossRef]
- Ježić, M.; Krstin, L.; Rigling, D.; Ćurković-Perica, M. High diversity in populations of the introduced plant pathogen, Cryphonectria parasitica, due to encounters between genetically divergent genotypes. Mol. Ecol. 2011, 21, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kranjec, J.; Diminić, D.; Hegol, M.; Milotić, M. Gljivama slični organizmi u tlu odumirućih sastojina poljskog jasena (Fraxinus angustifolia Vahl). Šumarski List 2017, 141, 122. [Google Scholar] [CrossRef][Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Philologenetics. In PCR Protocols; Elsevier BV: Amsterdam, The Netherlands, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biolog. Skri. 1948, 5, 1–34. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples; Version 9. User’s Guide and Application; University of Colorado Museum of Natural History : Boulder, CO, USA, 2013. [Google Scholar]
- Micallef, L.; Rodgers, P. eulerAPE: Drawing Area-Proportional 3-Venn Diagrams Using Ellipses. PLoS ONE 2014, 9, e101717. [Google Scholar] [CrossRef]
- Keča, N.; Kirisits, T.; Menkis, A. First report of the invasive ash dieback pathogen Hymenoscyphus fraxineus on Fraxinus excelsior and F. angustifolia in Serbia. Balt. For. 2017, 23, 56–59. [Google Scholar]
- Kirisits, T.; Matlakova, M.; Halmschlager, E.; Lakatos, F.; Mottinger-Kroupa, S. Chalara fraxineaassociated with dieback of narrow-leafed ash (Fraxinus angustifolia). Plant Pathol. 2010, 59, 411. [Google Scholar] [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European ash (Fraxinus excelsior) dieback—A conservation biology challenge. Boil. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Coker, T.L.R.; Rozsypálek, J.; Edwards, A.; Harwood, T.P.; Butfoy, L.; Buggs, R.J.A. Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants People Planet 2018, 1, 48–58. [Google Scholar] [CrossRef]
- Meyn, R.; Langer, G.J.; Gross, A.; Langer, E.J. Fungal colonization patterns in necrotic rootstocks and stem bases of dieback-affected Fraxinus excelsior L. For. Pathol. 2019, 49, e12520. [Google Scholar] [CrossRef]
- Żółciak, A.; Nowakowska, J.; Pacia, A.; Keča, N.; Oszako, T. Fungi isolated from shoots showing ash dieback in the Wolica Nature Reserve in Poland and artificially inoculated seedlings with Hymenoscyphus fraxineus. Folia For. Pol. 2019, 61, 42–50. [Google Scholar] [CrossRef]
- Haňáčková, Z.; Havrdová, L.; Černý, L.; Zahradník, D.; Koukol, O. Fungal Endophytes in Ash Shoots—Diversity and Inhibition of Hymenoscyphus fraxineus. Balt. For. 2017, 23, 89–106. [Google Scholar]
- Bakys, R.; Vasiliauskas, R.; Barklund, P.; Ihrmark, K.; Stenlid, J. Fungal attacks to root systems and crowns of declining Fraxinus excelsior. Aktuelt fra Skogforskningen 2006, 1-06, 71–72. [Google Scholar]
- Bengtsson, S.B.K.; Barklund, P.; Von Brömssen, C.; Stenlid, J. Seasonal Pattern of Lesion Development in Diseased Fraxinus excelsior Infected by Hymenoscyphus pseudoalbidus. PLoS ONE 2014, 9, e76429. [Google Scholar] [CrossRef]
- Muñoz, F.; Marçais, B.; Dufour, J.; Dowkiw, A. Rising Out of the Ashes: Additive Genetic Variation for Crown and Collar Resistance toHymenoscyphus fraxineusinFraxinus excelsior. Phytopathology 2016, 106, 1535–1543. [Google Scholar] [CrossRef]
- Milotić, M. The Role of Fungus Hymenoscyphus Fraxineus (T. Kowalski) Baral, Queloz & Hosoya in Ash Dieback (Fraxinus spp.) in the Republic of Croatia. Doctoral Thesis, University of Zagreb, Faculty of Forestry, Zagreb, Croatia, 2017. [Google Scholar]
- Thomas, H.E. Studies on Armillaria mellea (Vahl) Quel., infection, parasitism, and host resistance. J. Agric. Res. 1934, 48. [Google Scholar]
- Sipos, G.; Anderson, J.B.; Nagy, L.G. Armillaria. Curr. Boil. 2018, 28, R297–R298. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species–opportunistic, avirulent plant symbionts. Nat. Rev. Genet. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Chet, I.; Henis, Y. Degradation of plant pathogenic fungi by Trichoderma harzianum. Can. J. Microbiol. 1982, 28, 719–725. [Google Scholar] [CrossRef]
- Ragazzi, A.; Moricca, S.; Capretti, P.; Dellavalle, I.; Turco, E. Differences in composition of endophytic mycobiota in twigs and leaves of healthy and declining Quercus species in Italy. For. Pathol. 2003, 33, 31–38. [Google Scholar] [CrossRef]
- Gennaro, M.; Gonthier, P.; Nicolotti, G. Fungal Endophytic Communities in Healthy and Declining Quercus robur L. and Q. cerris L. Trees in Northern Italy. J. Phytopathol. 2003, 151, 529–534. [Google Scholar] [CrossRef]
- Giordano, L.; Gonthier, P.; Varese, G.C.; Miserere, L.; Nicolotti, G. Mycobiota inhabiting sapwood of healthy and declining Scots pine (Pinus sylvestris L.) trees in the Alps. Fungal Divers. 2009, 38, 69–83. [Google Scholar]
- Moricca, S.; Ginetti, B.; Ragazzi, A. Species- and organ-specificity in endophytes colonizing healthy and declining Mediterranean oaks. Phytopathol. Mediterr. 2012, 51, 587–598. [Google Scholar]
- O’Donnell, K. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Demirci, F.; Maden, S. A severe dieback of box elder (Acer negundo) caused byFusarium solani(Mart.) Sacc. in Turkey. Australas. Plant Dis. Notes 2006, 1, 13. [Google Scholar] [CrossRef]
- Chen, W.; Swart, W.J. First Report of Stem Canker of English Walnut Caused by Fusarium solani in South Africa. Plant Dis. 2000, 84, 592. [Google Scholar] [CrossRef]
- Abreo, E.; Sessa, L.; Bettucci, L.; Lupo, S.; Kopp, S.M. Phomopsis cotoneastri as a Pathogen Associated with Trunk Cankers and Death of Young Apple Trees cv. Cripps Pink. J. Phytopathol. 2012, 160, 434–436. [Google Scholar] [CrossRef]
- Herder, M.C. Identification of Ilyonectria Species Associated with and Determining Their Role in Avocado Decline. Doctoral Thesis, Lincoln University, Lincoln, New Zealand, 2014. [Google Scholar]
- Manici, L.M.; Kelderer, M.; Caputo, F.; Saccà, M.L.; Nicoletti, F.; Topp, A.R.; Mazzola, M. Involvement of Dactylonectria and Ilyonectria spp. in tree decline affecting multi-generation apple orchards. Plant Soil 2018, 425, 217–230. [Google Scholar] [CrossRef]
- Erper, I.; Agustí-Brisach, C.; Tunali, B.; Armengol, J. Characterization of root rot disease of kiwifruit in the Black Sea region of Turkey. Eur. J. Plant Pathol. 2013, 136, 291–300. [Google Scholar] [CrossRef]
- Marçais, B.; Caël, O.; Delatour, C. Interaction between root rot basidiomycetes and Phytophthora species on pedunculate oak. Plant Pathol. 2010, 60, 296–303. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Salgado-Salazar, C.; Crouch, J.A. Genome resources for the stem and bark canker pathogens Corinectria fuckeliana, Neonectria hederae and N. punicea. Plant Dis. 2019, 103, 389–391. [Google Scholar] [CrossRef]
- Castlebury, L.A.; Rossman, A.Y.; Hyten, A.S. Phylogenetic relationships of Neonectria/Cylindrocarpon on Fagus in North America. Botany 2006, 84, 1417–1433. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).