Abstract
Airborne bacteria play important roles in air pollution, human health and biogeochemical cycles. However, their spatial variation and determinant factors in forest environments are poorly understood. In this study, we selected five forest types in the Liuxihe National Park, South China, to analyze how near-surface bacterial community structure is related to the forest community structure and soil physicochemical properties. The results indicated that the dominant communities were mainly constituted by seven bacterial genera of the phyla Proteobacteria (49.7%–55.4%) and Firmicutes (44.2%–49.8%), including Exiguobacterium (42.0%–46.4%), Citrobacter (20.7%–25.8%), Acinetobacter (20.1%–22.1%), and Pseudomonas (7.8%–8.9%) etc. However, differences in the composition and diversity of the airborne bacterial communities were evident among the five forests, especially with respect to the dominant taxa. The relative abundance of Enterococcus and Bacillus in coniferous and broad-leaved mixed forest (MF), broad-leaved mixed forest (BF), and pure Cunninghamia lanceolata forest (CL) was significantly higher than that of the other forests, while the relative abundance of Citrobacter was significantly lower. The relative abundance of Citrobacter, Acinetobacter, and Pseudomonas in Proteobacteria were significantly negatively correlated with plant diversity and acid phosphatase activity but positively correlated with soil pH and soil available potassium. Contrastingly, the correlation between the relative abundance of most genera of Firmicutes and the above environmental factors is just the opposite of that for Proteobacteria. We provide direct evidence that native plant communities in the middle stage of succession, compared to planted forests and forest open space, generally had higher airborne bacterial diversity. Airborne bacterial diversity showed a significantly positive correlation with plant diversity (p < 0.05). Over all, soil pH, soil available potassium, and soil available phosphorus contributed to a high rate of the diversity of the airborne bacterial community in near-surface, followed by the plant diversity of the arbor layer and the near-surface air temperature. These results extended our understanding of the ecological patterns of airborne bacteria in forest ecosystems.
1. Introduction
Airborne bacteria are mainly derived from natural sources such as soil, plants and water through external forces [1,2,3] and are widely distributed in the atmosphere in a suspended form that is usually able to adapt to atmosphere environments [4,5]. Airborne bacterial communities are comprised of many taxa with different persistence and resilience [6,7]. These communities are not only associated with air pollution [8,9,10] and adverse effects on human health [11,12], but also have important ecological functions [5,13,14]. Traveling in the wind as bio-aerosols, airborne bacteria travel long-distances across geographical barriers, link distant ecosystems [15,16], and play an essential role in the development, evolution and dynamics of ecosystems [14,17]. They absorb and reflect atmospheric radiation, which in turn directly affect the energy budget in the atmosphere [18], and ice-nuclear active bacteria also act as efficient nuclei for cloud droplets, ice crystals and sediments to affect the hydrological cycle and climate [19,20,21]. Moreover, some opportunistic pathogens can spread through the air to human lungs and other organs, which can lead to infectious and allergic diseases [22,23].
The structure and diversity of airborne bacterial communities are related to many factors and may vary from site to site [24,25,26]. The heterogeneity of the environment and meteorological factors could be responsible for differences in the structure and diversity of airborne bacterial communities in different habitats. Air temperature and humidity [24,27], precipitation [5,28], plant and surface cover types [29,30], and anthropogenic activities [1,31,32] are among the known major factors affecting the composition and abundance of airborne bacterial communities. For example, there are significant geographical differences in airborne bacterial communities in the polluted atmosphere of urban areas. Proteobacteria were detected as the most abundant airborne bacteria in the three cities of Beijing, Seoul, and Nagasaki, accounting for approximately 44.5% of total airborne microorganisms [33]. However, at the genus level, bacteria were found to be significantly more abundant in the Beijing site than in the Seoul and Nagasaki sites. Similarly, the airborne bacteria in Milan and Venice were different and these differences vary with the seasons [27]. However, a different behavior was observed for airborne bacteria at Milan ad Venice sites: long-range transport significantly affected bacterial populations in Milan, whereas local ground wind had more influence in the Venice area.
The effect of local ground wind on airborne bacterial communities is caused by mechanical disturbance. Similarly, other meteorological factors, gaseous pollutants, and particulate matter chemical composition influence the bacterial community structure in the atmosphere [27,34,35]. Furthermore, the impact of meteorological changes on the diversity of airborne bacterial communities is greater than that of gaseous pollutants [35]. By contrast, the effect of environmental heterogeneity on airborne bacterial communities is due to the fact that environmental characteristics may affect the source of bacteria. Land management and bare soil are the major factors affecting airborne bacterial communities in agricultural fields, suburban areas, and forests [36]. The effects of land-use types on airborne bacteria is reported to be greater than that of microclimate environment and local terrestrial environments are a potential source for at least a portion of the near-surface atmospheric community [36]. Similarly, herbaceous vegetated landscapes have also been reported to be the local sources of terrestrial bacteria in the near-surface atmosphere [29]. Plant communities not only directly affect airborne microbes as a source and sink of airborne microorganisms [29], but also indirectly affect the source of airborne microbes by affecting soil physicochemical properties [14,37]. Plants are also sources of soil bacteria when rain droplets deposit epiphytes on the soil [38].
Soil and vegetation are major sources of airborne bacterial communities. However, little is known about how the structure and diversity of vegetation and the physicochemical properties of soil directly or indirectly affect airborne bacterial communities. Here, we selected five subtropical forests from the Liuxihe National Park in southeastern China to assess the effects of forest community structure and soil physiochemical properties on airborne bacterial communities in the near-surface environment. High-throughput sequencing based on different hypervariable regions of the 16S rDNA/rRNA gene was used to analyze the bacterial communities in aerosols [39]. The increase in the coverage and informativeness of DNA sequences has facilitated the elucidation of the species and diversity of airborne bacterial communities [40,41]. The main objectives of the present work were (1) to investigate the structure and biodiversity of airborne bacterial communities in different forest types using the high-throughput sequencing method; (2) to evaluate correlations among microclimatic environment, vegetation characteristics, soil physicochemical properties, and airborne bacterial communities. The results of this work will enhance our understanding of the composition of airborne bacterial community in different forest types and aid the identification of the relative importance of microclimatic environment, vegetation characteristics, and soil physicochemical properties on airborne bacterial community composition.
2. Materials and Methods
2.1. Sampling Sites
Samples were collected from the Liuxihe National Forest Park, Guangzhou City, China. The National Forest Park belongs to the evergreen broad-leaved forest zone and has a subtropical humid monsoon climate. The average annual rainfall is approximately 2148 mm, and the annual average temperature is 20.3 °C with a range from 11.8 to 31.9 °C.
Five types of forest were selected: pure Cunninghamia lanceolata forest (CL), coniferous and broad-leaved mixed forest (MF), broad-leaved mixed forest (BF), pure Litchi chinensis forest (LC), and pure Phyllostachys pubescens forest (PH). An open space without any vegetation was set as a control check group (CK). Three sampling sites (20 m × 20 m) were chosen from three separate locations per forest type. The location and distribution of the 18 sampling sites are shown in Figure 1. The distance between every two sampling sites is more than 50 meters to prevent interference during sampling [42,43]. CL is a pure coniferous forest at the early stage of forest community succession and Cunninghamia lanceolata is the dominant species. MF is a mixed forest in the middle stage of forest community succession with Cunninghamia lanceolata and local native broadleaf species as the dominant species. BF is the climax community of the region, and Castanopsis chinensis, Castanopsis faberi, Castanopsis fissa, and Schefflera octophylla are the dominant species. LC and PH are the main economic species of the region. The sampling sites in LC and PH were located in protected areas or within reserves without human disturbance.
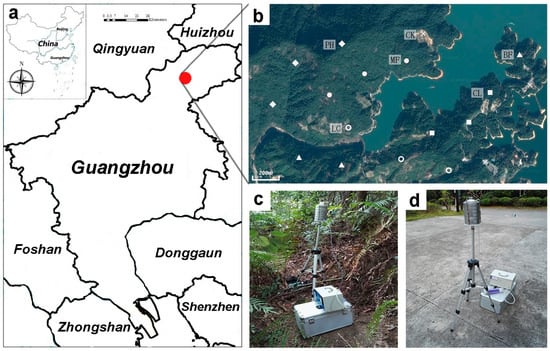
Figure 1.
(a): Location map of the Liuxihe Forest Park, South China; (b): Distribution of sample sites (CL = Cunninghamia lanceolata forest; MF = coniferous and broad-leaved mixed forest; BF = broad-leaved mixed forest; LC = litchi forest; PH = Phyllostachys pubescens forest; CK = control check group); (c,d): samplers.
2.2. Field Sampling
Meteorological data, including air temperature (AT), air relative humidity (ARH), dew point temperature (TD), were collected using a portable weather station (NK 5915, Nielson-Kellerman, Boothwyn, PA, USA). For each sampling site, five sampling points were selected according to the five-point sampling method (the diagonal was divided into four equal parts, the 1st, 2nd, and 3rd quantiles were selected as the five sampling points), and the hemispherical image was acquired using the hemispherical image technique [44]. The photos were processed and analyzed using Gap Light Analyzer software (version 2.0, Simon Fraser University, Burnaby, British Columbia, Canada) [45] to obtain the characteristics of the canopy structure [46], including leaf area index (LAI) and transmitted total solar radiation (TTot). Meanwhile, soil within the sampling sites at a depth of 0–10 cm was collected using a soil auger with a diameter of 4.5 cm. These soil samples were sent to the Testing Center of South China Agricultural University to determine the soil physicochemical properties, including soil pH (pH), soil organic matter (OM), soil alkali nitrogen (Olsen-N), soil available phosphorus (Olsen-P), soil available potassium (Olsen-K), catalase activity (CAT), acid phosphatase activity (ACP), and urease activity (URE). The soil temperature (ST) and soil relative humidity (SRH) were measured using a handheld monitor with an external soil temperature and humidity probe (TNHY-11, Top instrument co. LTD, China).
2.3. Aerosol Sampling
Aerosol sampling was carried out on three windless and sunny days, October 9th, 12, and 15, 2017. First, we standardized the spatial distance between replicate sampling sites in order to make it logistically feasible to travel to each replicate field site within the sampling time interval, while still accounting for habitat heterogeneity across replicate sites in our sampling design [47]. Similarly, three sampling sites (20 m × 20 m) were chosen from three separate locations per forest type. The location and distribution of the 18 sampling sites are shown in Figure 1. A sample site was randomly chosen at the same time each day, and the order of collection among different sites was then rotated to minimize effects of time on the assessment of airborne bacterial structure and diversity [36]. The order of sampling and a description of the sample collection are shown in Tables S1 and S2. Three replicate aerosol sampling points were selected within each sample site, and were arranged at the vertices of an equilateral triangle 10 m apart [47]. Aerosol samples were collected at a height of 1.5 m above the ground using samplers (PSW-6, Pusen Electronic Instrument, Changzhou, China) with glass fiber membranes (0.33 μm, autoclaved in advance) at a flow rate of 28.3 L/min for 35 min. The sampler was sterilized strictly with 75% ethanol before each sample collection. No one entered the sampling site within 20 meters after sampling started [48]. After the sampling was completed, the filter was quickly removed from the sampler with sterile forceps, placed in a sterile EP tube, stored in liquid nitrogen (−196 °C) and transported to the laboratory for further processing. A total 54 samples were collected from 18 sample sites.
2.4. DNA Extraction, PCR Amplification, and Sequencing
The three aerosol sampler filters from each site were processed separately during laboratory processing. Bacterial DNA was extracted directly from the filter using the Power Soil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. PCR amplification was performed with TaKaRa Premix Taq® (version 2.0, TaKaRa Biotechnology Co., Dalian, China). The negative controls were included for extraction and PCR to understand and control for potential laboratory/reagent contamination (no potential contamination was found). The V4-V5 region of the bacterial 16S rRNA gene was amplified using F515/R907 primers (5′-GTGCCAGCMGCCGCGGTAA-3′, 5′-CCGTCAATTCMTTTRAGTTT-3′) with a fragment size of 420 bp [49]. The amplification program was initiated by a 5 min denaturation step at 94 °C, followed by 31 cycles of 30 s at 4 °C, 30 s at 52 °C, and 45 s at 72 °C. The program was terminated with a 10-min elongation step at 72 °C before storage at 16 °C. The fragment length and concentration of the PCR product were detected by 1.0% agarose gel electrophoresis. The PCR mixed product was recovered using an EZNA Gel Extraction Kit (Omega, Doraville, GA, USA), and the target DNA fragments were eluted and recovered with TE buffer. The products were sequenced as paired-end reads at Guangdong Meg Gene Technology Co., Ltd., using the Illumina MiSeq platform (Illumina, San Diego, CA, USA), all the PCR products sequenced on the same MiSeq run. The sequencing data were subjected to bioinformatics analysis to determine the characteristics of the bacterial communities.
2.5. Bioinformatic Analysis and Phylogenetic Classification
Double-ended raw read data were filtered using Trimmomatic software version 0.33 [50] to filter reads containing N, mass values below 20, and filtered sequences with lengths less than 100 bp. The sequence was assigned to the corresponding sample using Mothur version 1.35.1 [51], followed by removal of the barcode and primers to obtain a paired-end clean read after quality control. The clean tags of each sample were clustered using Usearch version 8.0.1517 [52], and the UPARSE clustering method was used to cluster the sequences into OTUs (operational taxonomic units) with 97% identity. The first sequence in the OTU sequence was extracted as a representative sequence of different OTUs using Qiime version 1.9.1 software [53] and the RDP Classifier method. The representative sequence of each OTU was used to obtain species annotation information through comparison with the Greengenes (16S, chloroplasts, mitochondria). The singleton reads and nonspecific sequences (chloroplasts, mitochondria, unknown domain, chimeric sequences, etc.) were removed, and the number of valid tag sequences and the taxonomy summary information table of each OTU were obtained for the final analysis of each sample.
According to the species annotation and relative abundance information, the alpha diversity indices (Shannon, Simpson, Chao1, observed-species) and PD whole tree index were calculated using the alpha_diversity.py script in the Qiime. The sequencing depth between samples is not equal due to data filtering and the like. Therefore, the data needed to be resampled based on the number of sequences of the sample with the lowest number of sequences before calculating the diversity. Airborne bacterial communities (species and relative abundance) were analyzed from the phylum to genus level using both the total number of OTUs and abundant OTUs (Relative abundance >1%). Based on the species and relative abundance of the most important bacterial phyla and genera at the different types of sites, heatmaps were drawn using the heatmap function in R version 3.4.3 [54] and cluster analysis was performed. The phylogenetic tree was constructed according to the maximum likelihood method.
2.6. Statistical Analyses
The unweighted UniFrac distance matrix between samples was obtained through Fast UniFrac analysis. The “vegan” package [55] was used for nonmetric multidimensional scaling (NMDS) analysis and plotting. Spearman linear correlations were conducted between each environmental factor and bacterial relative abundance. Aggregated-boosted tree (ABT) analysis was carried out using the “gbm” package [56] with 10,000 trees for the boosting and 10-fold cross-validation. Differences in community structure between the different forest types or between clusters were tested using paired analysis of similarity (ANOSIM). Redundancy analysis (RDA) was conducted to explore the effects of environmental factors on bacterial community structure. Before the variation partition analysis (VPA), forward selection was performed to eliminate multicollinearity in the two subsets and to detect the most representative environmental factors that shape the microbial communities. Mantel tests were performed between each environmental variable and community structure. ANOSIM, RDA, VPA, and the Mantel test were all performed using the “vegan” package; the Bonferroni correction was applied to correct multiple tests. Figures were drawn using the “ggplot 2” package [57].
3. Results
3.1. Environmental Characteristics across Different Forest Types
The environmental characteristics of the five forests are shown in Table S3. The LC forest had the highest LAI (4.26) and lowest TTot (4.92) index, while the PH forest had the lowest LAI (2.27) and highest TTot (20.49) index. TTot and LAI differed significantly among the five forests (p < 0.05) (Table S3). The Simpson index (Dt) of the arbor layer in the MF, BF, and CL forests was 0.79, 0.73, and 0.53, respectively. Moreover, the Pielou’s J index (Jt) of the arbor layer in the MF, BF and CL forests was 0.84, 0.86, and 0.81, respectively. However, the Simpson index and Pielou’s J index of the arbor layer in the PH and LC forests (only one type of tree in the arbor layer) were missing values. The Simpson and Pielou’s J index differed significantly among the five forest types (p < 0.05), while the plant diversity showed no significant difference in the shrub herb layer (p > 0.05). The Simpson and Pielou’s J indexes of the shrub herb layer varied from 0.92 to 0.94 and from 0.86 to 0.89, respectively. These results indicated that the structural characteristics of the five forests were quite different from each other, whereas the characteristics of shrub herb layer were similar among forest types.
By contrast, the microclimate environments of the five forest types were relatively similar. AT was highest (26.8 °C) in the LC forest and lowest (23.2 °C) in the PH forest, whereas ARH was lowest (74.46%) in the LC forest and highest (82.80%) in the PH forest. TD of the five forests was between 20.0 °C and 21.7 °C. AT in the CK is not highest (25.9 °C), but ARH is the lowest (74.2%).
Soil physicochemical properties were similar among the five forest types (p > 0.05), except for pH and ACP. The pH values of the different forests ranged from 4.13 (MF forest) to 4.87 (LC forest), and the ACP content was lowest in the LC and highest in the MF forest, with a range of 212.64 to 956.94 μg/g.
3.2. Diversity and Composition of Airborne Bacterial Communities across Different Forest Types
A total of 4,021,990 effective sequences were obtained from 54 samples with an average length of approximately 373.4 bp, representing 629 OTUs from 25 bacterial phyla based on 97% sequence similarity. Statistics on the data processing results and diversity index are shown in Table 1. The rarefaction curve of the observed-species index indicated that the amount of sequencing data was sufficient for further analysis (Figure S1). The resampling of different samples was conducted to eliminate errors caused by differences in sequencing depth between samples. A total of 46 OTUs were shared among the different sites in the forest, accounting for 15.18% of the total (303 OTUs based on the re-sampling). The Venn analysis is shown in Figure S2. The unique OTUs were mostly found in the CL and MF forests. Statistical analysis of the observed-species index and PD whole tree index showed that there were significant differences in the richness and diversity of the airborne bacterial communities between the six sampling sites (p < 0.01) (Figure 2a,b). The observed-species index (95) and PD whole tree index (11) of CL were the highest among the five forest types, followed by the MF (80, 10) and BF (74, 9); the corresponding values in the CK (66, 7) were lower than those in the forest. One-way ANOVA and multiple comparisons between the index groups indicated that there were no significant differences in the richness and diversity of the bacterial communities between CL and MF and between BF, LC, PH, and CK. The observed-species index and PD whole tree index of CL were significantly different from those of BF, LC, PH, and CK. The observed-species index of MF was also significantly different from those of BF, LC, PH, and CK. In addition, the PD whole tree index of MF was significantly different from those of LC, PH, and CK.

Table 1.
Statistics on the data processing results.
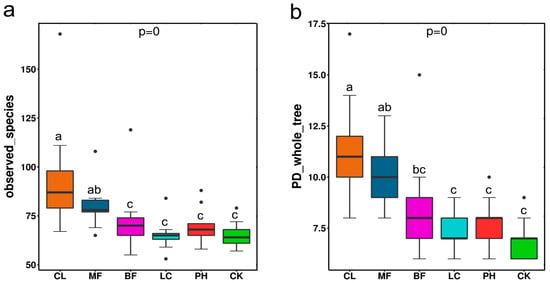
Figure 2.
Map of intergroup (a) observed species index and (b) PD whole tree index statistics of airborne bacterial community diversity. The solid lines represent the median values; the top and bottom of the boxes represented the first and third quartiles. Whiskers include 95% of data. Different letters represent statistically significant differences (p < 0.05).
Almost all sequences from the 54 aerosol samples belonged to Proteobacteria (53.67%) and Firmicutes (46.05%), and the remaining 23 bacterial phyla accounted for less than 1% (Figure 3a). Citrobacter (23.93%), Acinetobacter (20.91%), and Pseudomonas (8.42%) were the dominant genera in Proteobacteria, accounting for 53.27% of the total bacterial genera. Exiguobacterium (43.14%), Bacillus (1.10%), Enterococcus (1.00%), and Streptococcus (0.33%) were the dominant genera in Firmicutes, accounting for 45.59% of the total bacterial genera. The remaining bacterial genera accounted for only approximately 1.14% of the total (Figure 3b). The bacterial taxa from the five different forest types and the CK group were mainly distributed in the seven bacterial genera of the Proteobacteria and Firmicutes phyla. However, if the relative abundances of bacterial taxa were calculated, Proteobacteria (p = 0.0039), Firmicutes (p = 0.0073), Citrobacter (p = 3.04e−6), Exiguobacterium (p = 0.0455), Pseudomonas (p = 0.0124), and Streptococcus (p = 5.33e−6) differed significantly among the different forest types. A comparison of the relative abundances of the significantly different species in different groups of samples is shown in Figure S3.
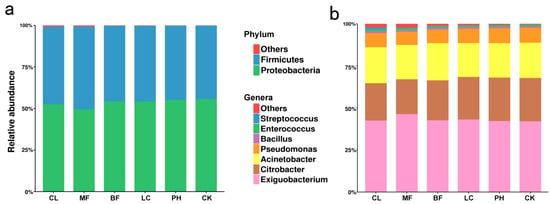
Figure 3.
Relative abundance of airborne bacteria in each vegetation-covered forest at the (a) phylum and (b) genus levels.
The airborne bacteria of different forest types were clustered based on the composition and abundance of the dominant bacterial genera (Figure 4). The relative abundances of the dominant genera in the CK group were smaller than those of the forests except for Citrobacter and Pseudomonas. The BF, LC, and PH forests and the CK group were clustered into one class, and the CL and MF forests were clustered into another class. Similarly, NMDS analysis of the airborne bacteria also indicated that the bacterial structures within the CL and MF forests were not easily distinguishable in an evolutionary lineage, while the bacterial structure in the BF, LC, and PH forests and the CK group was relatively close, within certain similarities (Figure 5).
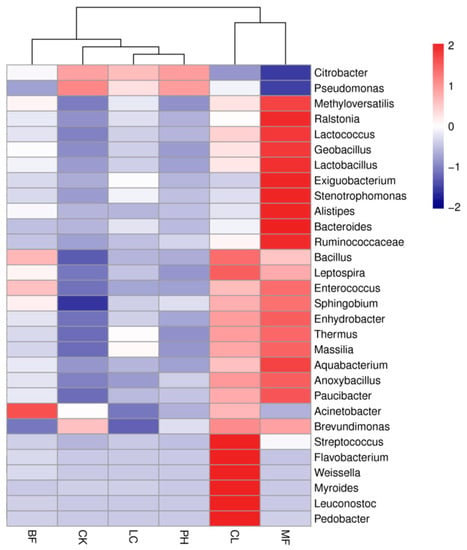
Figure 4.
Heatmap of relative abundances across a wide range of forest types. Forests are clustered based on the percent relative abundance of the genus-level classifications shown as rows. Each row was scaled so that the mean of each taxonomic group across sample types was calculated and colored by the corresponding z-score of each cell.
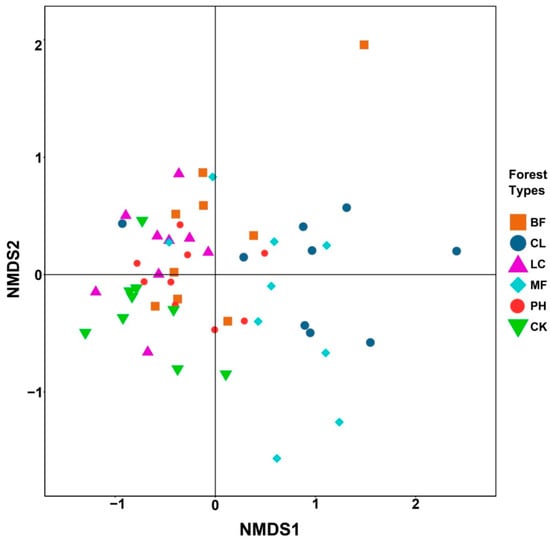
Figure 5.
Nonmetric multidimensional scaling of the pairwise distances between bacterial communities as calculated using the unweighted UniFrac values. The map represents the relationships among the bacterial communities of the different forest types.
3.3. Correlations between Environmental Factors and Diversity of Airborne Bacterial Communities
Correlations between the relative abundance of dominant airborne bacterial genera and environmental factors were analyzed (Figure 6). The relative abundance of Citrobacter, Acinetobacter, and Pseudomonas in Proteobacteria were significantly negatively correlated with plant diversities (Dt, Jt, Dsh, Jsh) and ACP but positively correlated with soil pH and Olsen-K. Conversely, the relative abundance of Exiguobacterium, Bacillus, Enterococcus, and Streptococcus in Firmicutes were significantly positively correlated with plant diversities (Dt, Jt, Dsh, Jsh) and ACP, but negatively correlated with soil pH and Olsen-K.
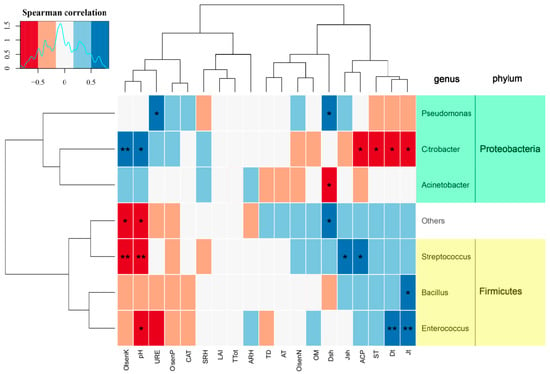
Figure 6.
Heatmap displaying the correlation between environmental factors and the relative abundance of airborne bacterial communities based on the Spearman correlation coefficient. The cluster tree on the left is a species clustering tree, and the cluster tree at the top is a clustering tree of environmental factors. Red represents a negative correlation, blue represents a positive correlation, and the darker the color, the higher the correlation. The “p” value is the correlation test result, where * indicates p < 0.05, and ** indicates p < 0.01.
Spearman’s rank correlation coefficient analysis indicated that both the observed-species and PD whole tree indexes were significantly negatively correlated with Olsen-K (p < 0.05) and significantly positively correlated with Jt (p < 0.05). The statistical results of the correlations between environmental factors and diversity indexes are shown in Table S4. The PD whole tree index was significantly positively correlated with Dt (p < 0.05). The diversities of airborne bacteria and plant diversities in the arbor layer showed a significant positive correlation (p < 0.05).
The ABT model was used to explain the relative importance of environmental factors to the diversity of airborne bacterial communities (Figure 7). Olsen-P was the major factor affecting the PD whole tree and observed-species indexes, and the relative impact ratios were 45.15% and 34.04%, respectively. Olsen-K and pH, which had the second highest relative impact ratios, with the relative impact ratios being 8.75%, 4.12%, 6.75%, and 6.54%, respectively. Among the three types of environmental factors (vegetation, climatic, and edaphic characteristics), the relative influence ratios affecting the bacterial diversity (PD whole tree and observed-species indexes) were as follows: soil physicochemical factors accounted for 82.46 and 76.30%, vegetation characteristics accounted for 10.55% and 15.04%, and microclimate environment accounted for 6.98% and 8.67%, respectively. Similarly, the analysis of the relative importance of environmental factors to airborne bacterial community structure based on UniFrac distance indicated that the relative influence ratio of soil physicochemical properties on airborne bacterial community structure was highest (approximately 5.03%–8.39% and 5.05%–7.70% for bacteria and dominant bacteria, respectively), while the vegetation characteristics had the lowest ratio (approximately 2.48%–4.33% and 2.61%–4.16%), regardless of whether OTU abundance was considered.
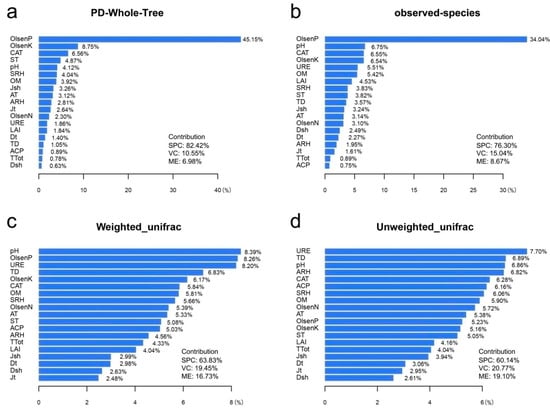
Figure 7.
Relative influence ratios (%) of soil physicochemical properties, vegetation characteristics and microclimatic environment on airborne bacterial abundance and structure based on the (a) PD whole tree index, (b) observed-species index, (c) weighted UniFrac distance, and (d) unweighted UniFrac distance.
To further determine the impact of environmental factors on the distribution of the airborne bacterial communities, VPA analysis based on the percent relative abundance of the all genus-level classifications was performed to find the relative impact ratio of each environmental factor group. The results indicated that pH, ACP, OM, Olsen-N, and SRH were the representative factors of soil physicochemical characteristics, Dsh, Jt, Jsh, and LAI were the representative factors of vegetation characteristics, and only AT was selected for the microclimate variable. The above ten selected environmental factors were used for the VPA analysis, and the results showed that the meteorological environment, soil physicochemical properties, and vegetation characteristics jointly shaped the airborne bacterial community (Figure 8). Permutation tests for unique contributions indicated that soil physicochemical characteristics contributed 19% to changes in bacterial communities and vegetation characteristics contributed approximately 16%. The contribution of the microclimate environment was minimal: approximately 10% provided by the AT index. The combination of vegetation characteristics and soil physicochemical characteristics and the combination of vegetation characteristics and microclimate environment each played roles of 6%. However, the combined effects of all three environmental factors were not detected. Subsets of the three environments together explained limited changes (57%) in the bacterial communities.
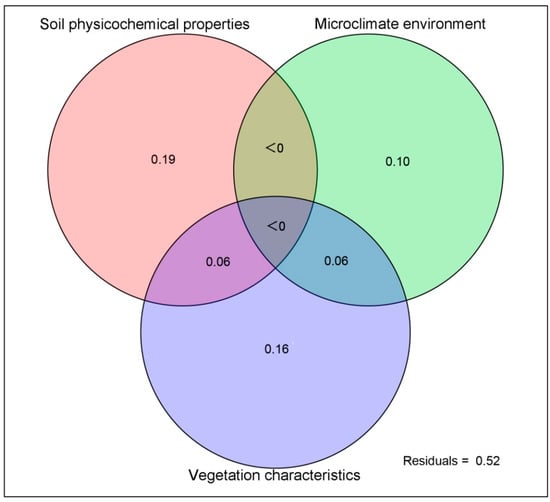
Figure 8.
Variance partitioning analysis based on the percent relative abundance of the all genus-level classifications. Soil physicochemical properties, vegetation characteristics, and microclimate environment are represented by the variance explained by soil physicochemical properties alone, vegetation characteristics alone, microclimate environment alone, or soil physicochemical properties, vegetation characteristics, and microclimate environment together. Residuals represent the variance that could not be explained by either pollutants or meteorological factors. Forward selection was performed before VPA to select significant factors.
4. Discussion
4.1. The Variation of Airborne Bacterial Communities in Different Forest Types
In the present work, Proteobacteria (53.67%) and Firmicutes (46.05%) were identified as the two dominant airborne bacterial phyla in forests, consistent with other studies on forest and urban areas [25]. Proteobacteria is the largest phylum of bacteria and is widely found in air [58]. The endospores produced by Firmicutes make them more suitable for dehydrated and extreme environments [59]. These most prevalent taxa were highly abundant at all sites [36,48]. Similarly, the results of this work indicated that all the dominant taxa of airborne bacteria in the five forest types and the CK group were Citrobacter, Acinetobacter, Pseudomonas, Exiguobacterium, and Bacillus. These dominant airborne bacterial genera were widespread in the near-surface atmospheric environment of the local forest area and were often comprised of plant- and soil-associated bacteria [29,60]. For example, Pseudomonadaceae and Microbacteriaceae were the dominant airborne bacterial families, comprising 9.5% and 3.6% of the reads in downwind air (downwind air samples were collected from the eastern boundary of the vegetated sites), respectively, were about 3- and 4-fold higher, respectively, than the number of reads in upwind air parcels [29].
In contrast, the airborne bacterial taxa in the CK group were almost the same as those common taxa in the forest, and were more like a subset of the airborne bacteria communities in the forests. Forests play a significant role as a reliable source of bacteria to form airborne bacterial communities in open spaces [29,30]. In particular, it seems likely that vegetation serves both as a source emitting microbe and as a modifier of airflow, which could tend to retain locally emitted microbes in some situations [2,48,61]. Therefore, compared with the CK group without vegetation, the dense forest areas were more suitable for the survival of certain specific types of dominant bacteria and non-dominant bacteria. The dominant bacteria in open space without vegetation and in urban areas are frequently communities that are able to adapt to high temperature, dry environments, and ultraviolet sunlight [4,5].
Although the taxa-level identity of dominant airborne bacteria was highly consistent in different forest types, the compositions of the dominant bacterial communities were significantly different. Similarly, the results of the statistical comparison between the index groups indicated that the relative abundance and diversity of airborne bacterial communities varied significantly among different forest types. This means that, within a certain landscape scale, changes of forest types did not change the taxa identity of airborne dominant bacteria, but changed the composition of bacterial community by affecting the relative abundance of dominant bacterial communities and the taxa-level identity of non-dominant bacteria. Although forest types have significant influence on the composition of airborne bacterial community, this influence is not as obvious as that of land-use types [36] or urban greenness [48]. This is due partly to the relatively low environmental differences between forests. It is possible that these effects will become apparent for a new spatial scale, such as the distribution of typical vegetation zones of different latitudes.
Results of one-way ANOVA and multiple comparisons of the diversity index were consistent with the cluster analysis and NMDS analysis: there were no significant differences in the community structure and diversity of the airborne bacterial communities between CL and MF and between BF, LC, PH, and CK. One possible reason is that the dominant species in both CL and MF is Cunninghamia lanceolata (Lamb.) Hook [62]. Second, there were no significant differences in environmental characteristics between CL and MF except pH, ACP, and Dt. The similarity of species composition and environmental characteristics between CL and MF results in a similar composition of airborne bacterial communities. Although the vegetation composition varies greatly between BF, LC, PH, and CK, the high similarity of the bacterial communities indicates that some special bacterial taxa spread all over the forest space. The differences of vegetation characteristics in different forest types incompletely explained the composition of the airborne bacterial communities. The potential source (soil bacteria) of airborne bacteria, which is affected by soil physicochemical properties, may be another important factor.
4.2. Relationship between Airborne Bacterial Communities and the Environmental Factors
Plant diversity usually increases with the progressive succession of plant communities and eventually stabilizes and develops into the climax community of the local area in the middle and late stages of plant community succession [63,64,65]. A notable result of this study is that the trend of changes in airborne bacterial communities and plant communities was similar during the succession of forest communities (progressive succession from CL to MF, BF); namely, plant communities often have high plant community diversity and airborne bacterial community diversity in the middle and late stage of forest community succession. This phenomenon might be due to the fact that vegetation serves as both a source and sink of airborne microorganisms and has more niches, thus providing more unique taxa of airborne bacteria [66,67]. The CK group had the lowest bacterial diversity, to some extent, consistent with the lack of vegetation coverage and the resultant lack of reliable sources and niches of bacteria. This study thus provides the first evidence for understanding how changes of vegetation characteristics and the succession of forest communities affect airborne bacterial communities. However, we cannot predict how further changes of the airborne bacterial community caused by vegetation characteristics would affect human health and forest ecological functions, nor do we know enough about the mechanisms through which vegetation-dominated airborne bacteria affects human well-being, to design forest space to maximize health benefits. Plant-associated bacteria may act as counterparts against pathogens within the microbial ecosystems, which can stabilize the ecosystem, enhance biodiversity, and prevent the outbreaks of pathogens [30].
Specific airborne fungal and bacterial species were strongly correlated with meteorological factors, such as temperature, humidity, and CO2 concentration [68]. In this study, AT is the important microclimate factor affecting the diversity of airborne bacterial communities in the absence of seasonal changes and violent meteorological events. A suitable temperature will accelerate air convection and increase the dispersion of bacteria in the atmosphere, or even directly bringing in exogenous bacteria to alter bacterial release, transportation, and deposition [5]. Conversely, a higher temperature will exert selective pressure on bacteria, thereby reducing the diversity of airborne bacterial communities [69,70]. The negative relationships of AT and TD with the airborne bacterial diversity index may confirm this. However, a higher ARH is more conducive to the survival of airborne bacterial communities, and a higher SRH will prevent dust from rising from the moist soil surface, thereby reducing the diversity of airborne bacteria in the near-surface atmosphere [71]. Similar results were found in our research: the diversity of airborne bacteria is negatively correlated with SRH and positively correlated with ARH. In addition, compared with the instability of the climate environment in the open space, the stability and buffering of the forest microclimate environment also provides a higher possibility for maintain a higher diversity of airborne bacterial community, although we do not know its potential mechanisms, especially when violent meteorological events occur frequently in forests.
Soil is a large and reliable source of airborne microorganisms, which has an important impact on the airborne microbial communities [3,72]. In theory, all biological materials can be released either directly or resuspended into the air upon various disturbances from the soil surfaces [73]. For example, biological aerosol emission caused by wind [74], raindrop impingement [38], or other external disturbances can cause soil bacteria to suspend in the air. On the other hand, the composition and abundance of these soil microorganisms will change with variations in soil physicochemical properties [75,76]. Soil is rich in microorganisms, including phosphate-dissolving microorganisms and potassium-dissolving microorganisms that contribute to the conversion of phosphorus and potassium to effective states [77]. For example, in phosphorus-deficient soil, bacterial communities increase their relative abundance and the abundance of phytase genes to catalyze the most recalcitrant phosphorus-containing compounds (phytate) and release phosphatases in response to soil phosphorus limitation [76,77,78]. Our study area is characterized by lateritic red soil with severe phosphorus limitation, which explains why soil Olsen-P is the most important factor affecting the diversity of near-surface airborne bacterial communities [79,80]. This subtle, yet significant, effect of soil physicochemical properties on the airborne bacterial community composition indirectly proved that soil bacteria are a potential source of the near-surface atmospheric bacterial community. At the same time, the soil bacteria may interact with airborne bacteria to various degrees. However, we do not know to what extent the effects of soil physicochemical properties on the source of airborne bacteria (i.e. soil bacteria) will affect the airborne bacterial community, and we do not know which factor predominantly affect the emission process of microorganisms from the soil into the air. The bacterial surface concentration, the emission ability of soil microorganism, the soil composition, the porosity of surface soils, the degree of soil exposure, air or soil temperature and humidity, and wind speed and direction may all influence this emission process. Therefore, future research needs to understand the microbial community by determining the major factors affecting the emission process of microorganism between air and soil.
Soil physicochemical properties were the main factors that determine the structure and diversity of near-surface airborne bacterial communities, followed by vegetation characteristics and microclimate environment. This is probably because soil is the main source of bacterial communities, followed by vegetation. The response of dominant airborne bacterial communities to soil pH, Olsen-P, and Olsen-K is the strong evidence that the dominant airborne bacterial communities may be primarily come from the soil. Studies on the main influencing factors of airborne bacterial community structure in different land-use types found similar results: the characteristics of the local terrestrial surfaces (such as vegetation cover, land management, and amount of bare soil) have a greater influence on the composition of airborne bacterial community than local atmospheric conditions [36]. Interestingly, similar environmental factors, especially soil available phosphorus, were correlated with most genera of both two dominant bacterial phyla (Proteobacteria and Firmicutes), but with nearly opposite effects on the two domains. This may be the result of airborne bacterial communities being a mixture of bacteria from soils and plants [36]. As we know, Proteobacteria include many bacteria responsible for nitrogen fixation [78], which may be the main reason for the high positive correlation between most genera of Proteobacteria and the soil physicochemical properties. Many Firmicutes produce endospores, which are resistant to desiccation and can better survive on the surface of plant leaves [59]. This is probably why most genera of Firmicutes are significantly positively correlated with plant diversity. Therefore, it is necessary to further compare the structure and diversity of bacterial communities between airborne bacteria and other possible sources in different forest types. Future research may be able to use bacterial taxa indicative of possible source environments to identify and compare inputs of bacteria in the atmosphere, and analyze the contribution of the source of bacteria to the composition of airborne bacteria.
5. Conclusions
In the five different forest types of Liuxihe Forest Park, the airborne bacterial communities were dominated by the genera Exiguobacterium (42.03%–46.35%), Citrobacter (20.7%–25.84%), Acinetobacter (20.13%–22.12%), and Pseudomonas (7.81%–8.89%). Differences in the community structure and diversity of dominant airborne bacteria were evident in the different forest types. This study is the first to show a positive correlation between plant diversity and airborne bacterial diversity in forest. Interestingly, different types of environmental factors were correlated with most genera of both two dominant bacterial phyla (Proteobacteria and Firmicutes), but with opposite effects, of which soil available phosphorus was the most important driving factor. Soil physicochemical properties were identified as the major environmental factors that shape the airborne bacterial communities, followed by vegetation characteristics and microclimate environment. The results of this work will help us further understand the impact of forests on airborne bacterial communities, which could provide forest managers the ability to better design, manage, and protect forest to promote human health and forest ecological functions.
Supplementary Materials
The following are available online at https://www.mdpi.com/1999-4907/11/5/561/s1, Figure S1: The rarefaction curve of the observed-species index, Figure S2: The Venn analysis of OUTs, Figure S3: The significantly different species, Table S1: The actual order and description of samples collection, Table S2: Description of samples collection and the corresponding meteorological factors, Table S3: Environmental characteristics of the five forests, Table S4: Correlation between environmental factors and airborne bacterial community diversity.
Author Contributions
Conceptualization, J.F. and H.C.; Data curation, J.F., Q.D. and H.C.; Formal analysis, J.F.; Funding acquisition, H.C.; Investigation, J.F., Q.D., X.L., N.D. and L.X.; Methodology, J.F. and H.C.; Supervision, H.C.; Validation, H.C.; Visualization, J.F.; Writing—original draft, J.F.; Writing—review and editing, J.F., Q.D., W.S. and H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangdong Forestry Science and Technology Innovation Project (2017KJCX010).
Acknowledgments
We thank Rongyin Huang, Zhichao Guo, and Yuemou Shi for their assistance with the field works.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miletto, M.; Lindow, S.E. Relative and contextual contribution of different sources to the composition and abundance of indoor air bacteria in residences. Microbiome 2015, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.I.; Marr, L.C. Sources of airborne microorganisms in the built environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Meola, M.; Lazzaro, A.; Zeyer, J. Bacterial composition and survival on Sahara dust particles transported to the European Alps. Front. Microbiol. 2015, 6, 1454. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kang, Y.; Hwang, Y.; Yoo, S.; Jang, H.; Oh, H.; Kang, J.; Chang, D.; Na, K.; Kim, G. Evaluation of airborne bacteria and fungi in surgical areas at the animal hospital. J. Vet. Clin. 2017, 34, 76–81. [Google Scholar] [CrossRef]
- Murata, K.; Zhang, D. Concentration of bacterial aerosols in response to synoptic weather and land-sea breeze at a seaside site downwind of the Asian continent. J. Geophys. Res.-Atmos. 2016, 121, 11636–11647. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal variability in bacterial and fungal diversity of the near-surface atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Liu, Z.; Rodriguez-Hernandez, M.; Knight, R.; Henn, M.; Hernandez, M.T. Short-term temporal variability in airborne bacterial and fungal populations. Appl. Environ. Microb. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, D.; Shi, Y.; Li, B.; Yang, J.; Yu, X.; Chen, N.; Kakikawa, M. Cell concentration, viability and culture composition of airborne bacteria during a dust event in Beijing. J. Environ. Sci. China 2017, 55, 33–40. [Google Scholar] [CrossRef]
- Gou, H.; Lu, J.; Li, S.; Tong, Y.; Xie, C.; Zheng, X. Assessment of microbial communities in PM1 and PM10 of Urumqi during winter. Environ. Pollut. 2016, 214, 202–210. [Google Scholar] [CrossRef]
- Li, Y.; Fu, H.; Wang, W.; Liu, J.; Meng, Q.; Wang, W. Characteristics of bacterial and fungal aerosols during the autumn haze days in Xi’an, China. Atmos. Environ. 2015, 122, 439–447. [Google Scholar] [CrossRef]
- Duchaine, C. Assessing microbial decontamination of indoor air with particular focus on human pathogenic viruses. Am. J. Infect. Control. 2016, 44, S121–S126. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Park, W.M.; Ahn, J.K.; Lee, K.J.; Min, K.B.; Park, J.B. Relationship between culturable airborne bacteria concentrations and ventilation systems in underground subway stations in Seoul, South Korea. Air Qual. Atmos. Health 2016, 9, 173–178. [Google Scholar] [CrossRef]
- Mayol, E.; Arrieta, J.M.; Jimenez, M.A.; Martinez-Asensio, A.; Garcias-Bonet, N.; Dachs, J.; Gonzalez-Gaya, B.; Royer, S.; Benitez-Barrios, V.M.; Fraile-Nuez, E.; et al. Long-range transport of airborne microbes over the global tropical and subtropical ocean. Nat. Commun. 2017, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Froehlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Poehlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Amores-Arrocha, H.; Malard, L.; Els, N.; Sattler, B.; Pearce, D. Characterisation of arctic bacterial communities in the air above Svalbard. Biology 2017, 6, 29. [Google Scholar] [CrossRef]
- Iwata, K.; Watanabe, M.; Kurai, J.; Burioka, N.; Nakamoto, S.; Hantan, D.; Shimizu, E. Association between transported Asian dust and outdoor fungal concentration during winter in a rural area of western Japan. Genes Environ. 2017, 39, 19. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef]
- Malavelle, F.F.; Haywood, J.M.; Ones, A.J.; Gettelman, A.; Larisse, L.C.; Bauduin, S.; Allan, R.P.; Karset, I.H.H.; Kristjansson, J.E.; Oreopoulos, L.; et al. Strong constraints on aerosol-cloud interactions from volcanic eruptions. Nature 2017, 546, 485–491. [Google Scholar] [CrossRef]
- Bauer, H.; Giebl, H.; Hitzenberger, R.; Kasper-Giebl, A.; Reischl, G.; Zibuschka, F.; Puxbaum, H. Airborne bacteria as cloud condensation nuclei. J. Geophys. Res. Atmos. 2003, 108 (D21), 4658. [Google Scholar] [CrossRef]
- Šantl-Temkiv, T.; Sahyoun, M.; Finster, K.; Hartmann, S.; Augustin-Bauditz, S.; Stratmann, F.; Wex, H.; Clauss, T.; Nielsen, N.W.; Sørensen, J.H.; et al. Characterization of airborne ice-nucleation-active bacteria and bacterial fragments. Atmos. Environ. 2015, 109, 105–117. [Google Scholar] [CrossRef]
- Huffman, J.A.; Prenni, A.J.; DeMott, P.J.; Poehlker, C.; Mason, R.H.; Robinson, N.H.; Froehlich-Nowoisky, J.; Tobo, Y.; Despres, V.R.; Garcia, E.; et al. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 2013, 13, 6151–6164. [Google Scholar] [CrossRef]
- Sorrell, E.M.; Schrauwen, E.; Linster, M.; De Graaf, M.; Herfst, S.; Fouchier, R. Predicting ’airborne’ influenza viruses: (trans-) mission impossible? Curr. Opin. Virol. 2011, 1, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367. [Google Scholar] [CrossRef] [PubMed]
- Genitsaris, S.; Stefanidou, N.; Katsiapi, M.; Kormas, K.A.; Sommer, U.; Moustaka-Gouni, M. Variability of airborne bacteria in an urban Mediterranean area (Thessaloniki, Greece). Atmos. Environ. 2017, 157, 101–110. [Google Scholar] [CrossRef]
- Garcia-Mena, J.; Murugesan, S.; Perez-Munoz, A.A.; Garcia-Espitia, M.; Maya, O.; Jacinto-Montiel, M.; Monsalvo-Ponce, G.; Pina-Escobedo, A.; Dominguez-Malfavon, L.; Gomez-Ramirez, M.; et al. Airborne bacterial diversity from the low atmosphere of greater Mexico city. Microb. Ecol. 2016, 72, 70–84. [Google Scholar] [CrossRef]
- Vokou, D.; Vareli, K.; Zarali, E.; Karamanoli, K.; Constantinidou, H.A.; Monokrousos, N.; Halley, J.M.; Sainis, I. Exploring biodiversity in the bacterial community of the mediterranean phyllosphere and its relationship with airborne bacteria. Microb. Ecol. 2012, 64, 714–724. [Google Scholar] [CrossRef]
- Innocente, E.; Squizzato, S.; Visin, F.; Facca, C.; Rampazzo, G.; Bertolini, V.; Gandolfi, I.; Franzetti, A.; Ambrosini, R.; Bestetti, G. Influence of seasonality, air mass origin and particulate matter chemical composition on airborne bacterial community structure in the Po Valley, Italy. Sci. Total Environ. 2017, 593, 677–687. [Google Scholar] [CrossRef]
- Itani, G.N.; Smith, C.A. Dust rains deliver diverse assemblages of microorganisms to the Eastern Mediterranean. Sci. Rep. 2016, 6, 22657. [Google Scholar] [CrossRef]
- Lymperopoulou, D.S.; Adams, R.I.; Lindow, S.E. Contribution of vegetation to the microbial composition of nearby outdoor air. Appl. Environ. Microb. 2016, 82, 3822–3833. [Google Scholar] [CrossRef]
- Berg, G.; Mahnert, A.; Moissl-Eichinger, C. Beneficial effects of plant-associated microbes on indoor microbiomes and human health? Front. Microbiol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Adams, R.I.; Bhangar, S.; Pasut, W.; Arens, E.A.; Taylor, J.W.; Lindow, S.E.; Nazaroff, W.W.; Bruns, T.D. Chamber Bioaerosol Study: Outdoor air and human occupants as sources of indoor airborne microbes. PLoS ONE 2015, 10, e128022. [Google Scholar] [CrossRef]
- Heo, K.J.; Lim, C.E.; Kim, H.B.; Lee, B.U. Effects of human activities on concentrations of culturable bioaerosols in indoor air environments. J. Aerosol Sci. 2017, 104, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, E.H.; Lee, S.; Ko, G.; Honda, Y.; Hashizume, M.; Deng, F.; Yi, S.; Kim, H. Airborne bacterial communities in three East Asian cities of China, South Korea, and Japan. Sci. Rep. 2017, 7, 5545. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Du, R.; Ren, W.; Lu, Z.; Fu, P. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 2018, 610, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Q.; Deng, Y.; Wang, Y.; Wang, X.; Zhang, H.; Sun, X.; Ouyang, Z. Meteorological factors had more impact on airborne bacterial communities than air pollutants. Sci. Total Environ. 2017, 601, 703–712. [Google Scholar] [CrossRef]
- Bowers, R.M.; McLetchie, S.; Knight, R.; Fierer, N. Spatial variability in airborne bacterial communities across land-use types and their relationship to the bacterial communities of potential source environments. ISME J. 2011, 5, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.D.B.Z. Long-term effect of re-vegetation on the microbial community of a severely eroded soil in sub-tropical China. Plant Soil 2010, 328, 447–458. [Google Scholar]
- Joung, Y.S.; Ge, Z.; Buie, C.R. Bioaerosol generation by raindrops on soil. Nat. Commun. 2017, 8, 14668. [Google Scholar] [CrossRef]
- Cha, S.; Srinivasan, S.; Jang, J.H.; Lee, D.; Lim, S.; Kim, K.S.; Jheong, W.; Lee, D.; Park, E.; Chung, H.; et al. Metagenomic analysis of airborne bacterial community and diversity in Seoul, Korea, during december 2014, Asian dust event. PLoS ONE 2017, 12, e1706931. [Google Scholar] [CrossRef]
- Griffin, D.W.; Gonzalez, C.; Teigell, N.; Petrosky, T.; Northup, D.E.; Lyles, M. Observations on the use of membrane filtration and liquid impingement to collect airborne microorganisms in various atmospheric environments. Aerobiologia 2011, 27, 25–35. [Google Scholar] [CrossRef]
- Meheust, D.; Gangneux, J.; Cann, P.L. Comparative evaluation of three impactor samplers for measuring airborne bacteria and fungi concentrations. J. Occup. Environ. Hyg. 2013, 10, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Jaffal, A.A.; Nsanze, H.; Bener, A.; Ameen, A.S.; Banat, I.M.; Mogheth, A.A.E. Hospital airborne microbial pollution in a desert country. Environ. Int. 1997, 23, 167–172. [Google Scholar] [CrossRef]
- Otano, N.N.; Pasquo, M.D.; Munoz, N. Airborne fungal richness: Proxies for floral composition and local climate in three sites at the El Palmar National Park (Coln, Entre Rios, Argentina). Aerobiologia 2015, 31, 537–547. [Google Scholar] [CrossRef]
- Frazer, G.W.; Fournier, R.A.; Trofymow, J.A.; Hall, R.J. A comparison of digital and film fisheye photography for analysis of forest canopy structure and gap light transmission. Agric. For. Meteorol. 2001, 109, 249–263. [Google Scholar] [CrossRef]
- Frazer, G.W.; Canham, C.D.; Lertzman, K.P. Gap Light Analyzer (GLA),Version 2.0: Imaging Software to Extract Canopy Structure and Gap Light Transmission from True-Colour Fisheye Photographs, Users Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada; Institute of Ecosystem Studies: Millbrook, NY, USA, 1999. [Google Scholar]
- Blennow, K. Sky view factors from high-resolution scanned fish-eye lens photographic negatives. J. Atmos. Ocean. Tech. 1995, 12, 1357–1362. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Mhuireach, G.; Johnson, B.R.; Altrichter, A.E.; Ladau, J.; Meadow, J.F.; Pollard, K.S.; Green, J.L. Urban greenness influences airborne bacterial community composition. Sci. Total Environ. 2016, 571, 680–687. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microb. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Oksanen, J.; Blanchet, F.F.; Kindt, R.; Legendre, P.; Minchin, P.R. Vegan: Community Ecology Package, R Package Version 2.3-3; Github Inc.: San Francisco, CA, USA, 2016. [Google Scholar]
- Greenwell, B.; Boehmke, B.; Cunningha, J.; Developers, G.B.M. Gbm: Generalized Boosted Regression Models, R Package Version 2.1.5; Github Inc.: San Francisco, CA, USA, 2019. [Google Scholar]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R Stat. Soc. A Stat. 2011, 174, 245. [Google Scholar] [CrossRef]
- Madigan, M.A.J.M. Brock Biology of Microorganisms, 11th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Filippidou, S.; Wunderlin, T.; Junier, T.; Jeanneret, N.; Dorador, C.; Molina, V.; Johnson, D.R.; Junier, P. A combination of extreme environmental conditions favor the prevalence of endospore-forming Firmicutes. Front. Microbiol. 2016, 7, 2101. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Striluk, M.L.; Aho, K.; Weber, C.F. The effect of season and terrestrial biome on the abundance of bacteria with plant growth-promoting traits in the lower atmosphere. Aerobiologia 2017, 33, 137–149. [Google Scholar] [CrossRef]
- Anding, L.; Chunyan, G.; Lifei, Y. The composition and structural feature of plant community in different karst stony desertification areas. Appl. Ecol. Environ. Res. 2017, 15, 1167–1183. [Google Scholar] [CrossRef]
- Wang, K.; Shao, R.; Shangguan, Z. Changes in species richness and community productivity during succession on the Loess Plateau (China). Pol. J. Ecol. 2010, 58, 501–510. [Google Scholar]
- Anderson, K.J. Temporal patterns in rates of community change during succession. Am. Nat. 2007, 169, 780–793. [Google Scholar] [CrossRef]
- Martirosyan, V.; Unc, A.; Miller, G.; Doniger, T.; Wachtel, C.; Steinberger, Y. Desert perennial shrubs shape the microbial-community miscellany in laimosphere and phyllosphere space. Microb. Ecol. 2016, 72, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, I.; Canedoli, C.; Imperato, V.; Tagliaferri, I.; Gkorezis, P.; Vangronsveld, J.; Padoa Schioppa, E.; Papacchini, M.; Bestetti, G.; Franzetti, A. Diversity and hydrocarbon-degrading potential of epiphytic microbial communities on Platanus x acerifolia leaves in an urban area. Environ. Pollut. 2017, 220, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Gusareva, E.S.; Acerbi, E.; Lau, K.; Luhung, I.; Premkrishnan, B.; Kolundzija, S.; Purbojati, R.W.; Wong, A.; Houghton, J.; Miller, D.; et al. Microbial communities in the tropical air ecosystem follow a precise diel cycle. Proc. Natl. Acad. Sci. USA 2019, 116, 23299–23308. [Google Scholar] [CrossRef]
- Barcenas-Moreno, G.; Gomez-Brandon, M.; Rousk, J.; Baath, E. Adaptation of soil microbial communities to temperature: Comparison of fungi and bacteria in a laboratory experiment. Glob. Chang. Biol. 2009, 15, 2950–2957. [Google Scholar] [CrossRef]
- Pietikainen, J.; Pettersson, M.; Baath, E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol. Ecol. 2005, 52, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air 2017, 27, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Layton, D.W.; Beamer, P.I. Migration of contaminated soil and airborne particulates to indoor dust. Environ. Sci. Technol. 2009, 43, 8199–8205. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, X.; Gerlein-Safdi, C.; Mu, Y.; Wang, D.; Lu, Q. Global sources, emissions, transport and deposition of dust and sand and their effects on the climate and environment: A review. Front. Environ. Sci. Eng. 2017, 11, 11. [Google Scholar] [CrossRef]
- Kanakidou, M.; Myriokefalitakis, S.; Tsigaridis, A.K. Aerosols in atmospheric chemistry and biogeochemical cycles of nutrients. Environ. Res. Lett. 2018, 13, 063004. [Google Scholar] [CrossRef]
- Puglisi, E.; Hamon, R.; Vasileiadis, S.; Coppolecchia, D.; Trevisan, M. Adaptation of soil microorganisms to trace element contamination: A review of mechanisms, methodologies, and consequences for risk assessment and remediation. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2435–2470. [Google Scholar] [CrossRef]
- Bershova, O.I. Effect of trace elements on the synthesis of hetero-auxins by soil microorganisms. Mikrobiolohichnyi Zhurnal 1959, 21, 3–10. [Google Scholar] [PubMed]
- Yao, Q.; Li, Z.; Song, Y.; Wright, S.J.; Guo, X.; Tringe, S.G.; Tfaily, M.M.; Pasa-Tolic, L.; Hazen, T.C.; Turner, B.L.; et al. Community proteogenomics reveals the systemic impact of phosphorus availability on microbial functions in tropical soil. Nat. Ecol. Evol. 2018, 2, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Sun, P.; Kang, D.; Zhao, F.; Feng, Y.; Ren, G.; Han, X.; Yang, G. Responsiveness of soil nitrogen fractions and bacterial communities to afforestation in the Loess Hilly Region (LHR) of China. Sci. Rep. 2016, 6, 28469. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; An, S.; Sun, H.; Bhople, P.; Chen, Z. Soil physicochemical and microbial characteristics of contrasting land-use types along soil depth gradients. Catena 2018, 162, 345–353. [Google Scholar] [CrossRef]
- Jia, R.; Zhao, X.; Huang, Z. Correlative analyses between quantities of microbial populations and soil physicochemical property under different accumulation of soil plastic film. Adv. Mater. Res. 2013, 610, 3091–3095. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).