Diverging Responses of Two Subtropical Tree Species (Schima superba and Cunninghamia lanceolata) to Heat Waves
Abstract
1. Introduction
2. Materials and Methods
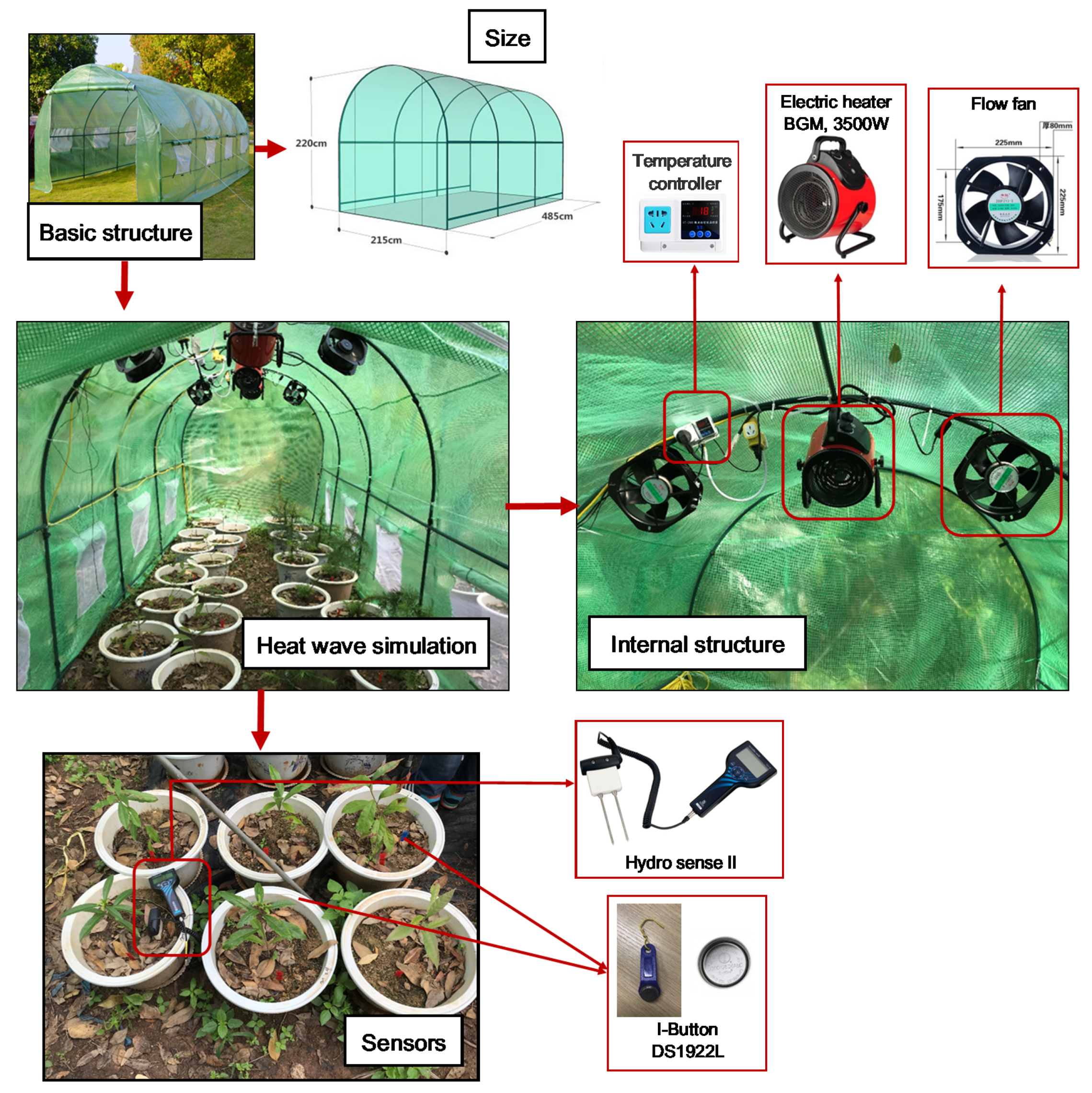
2.1. Experimental Platform
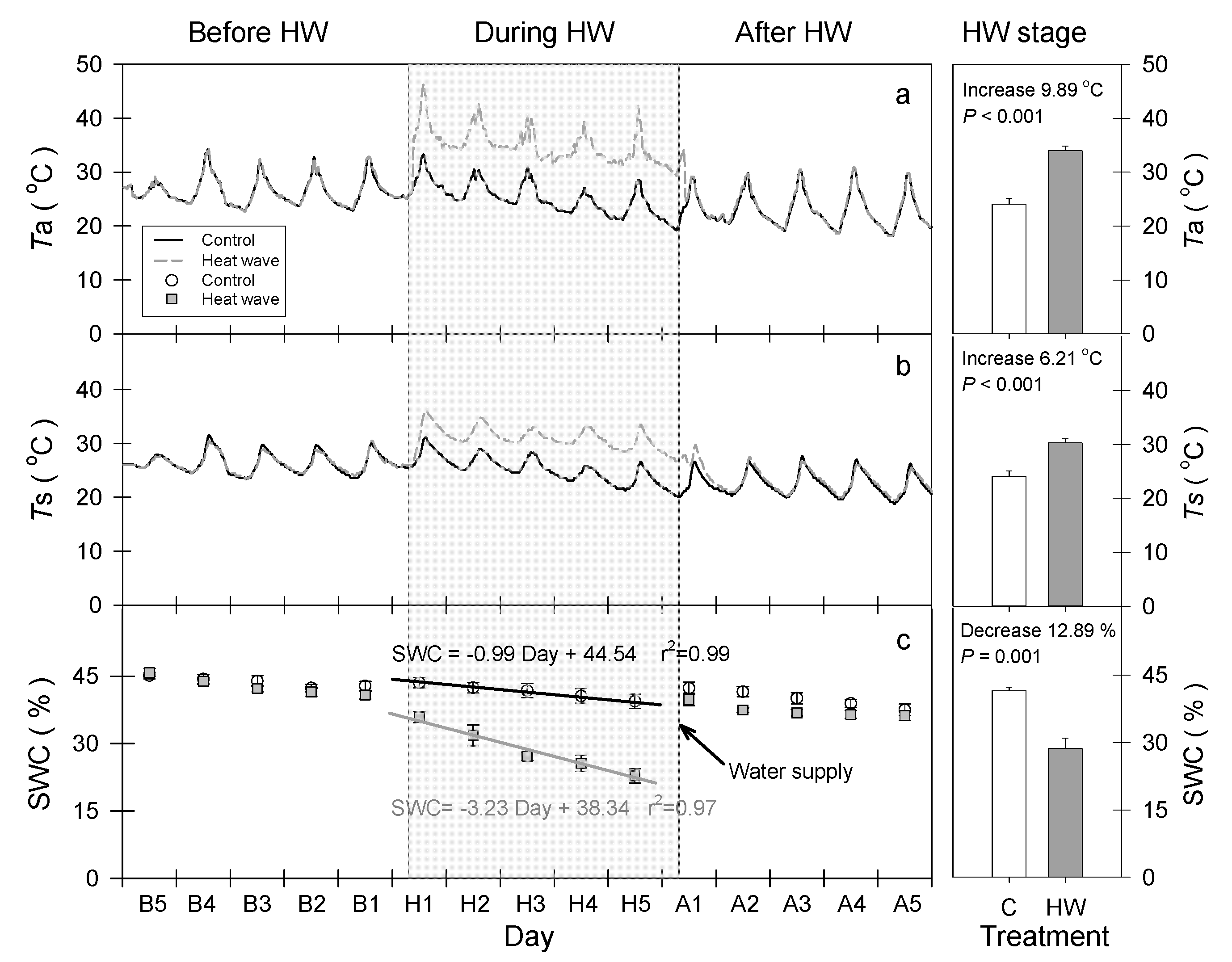
2.2. Heat Wave Simulation
2.3. Stem Hydraulic Conductivity
2.4. Leaf Gas Exchange
2.5. Final Harvest
2.6. Data Analysis and Statistics
3. Results
3.1. HW Effects on Plant Hydraulic Characteristics
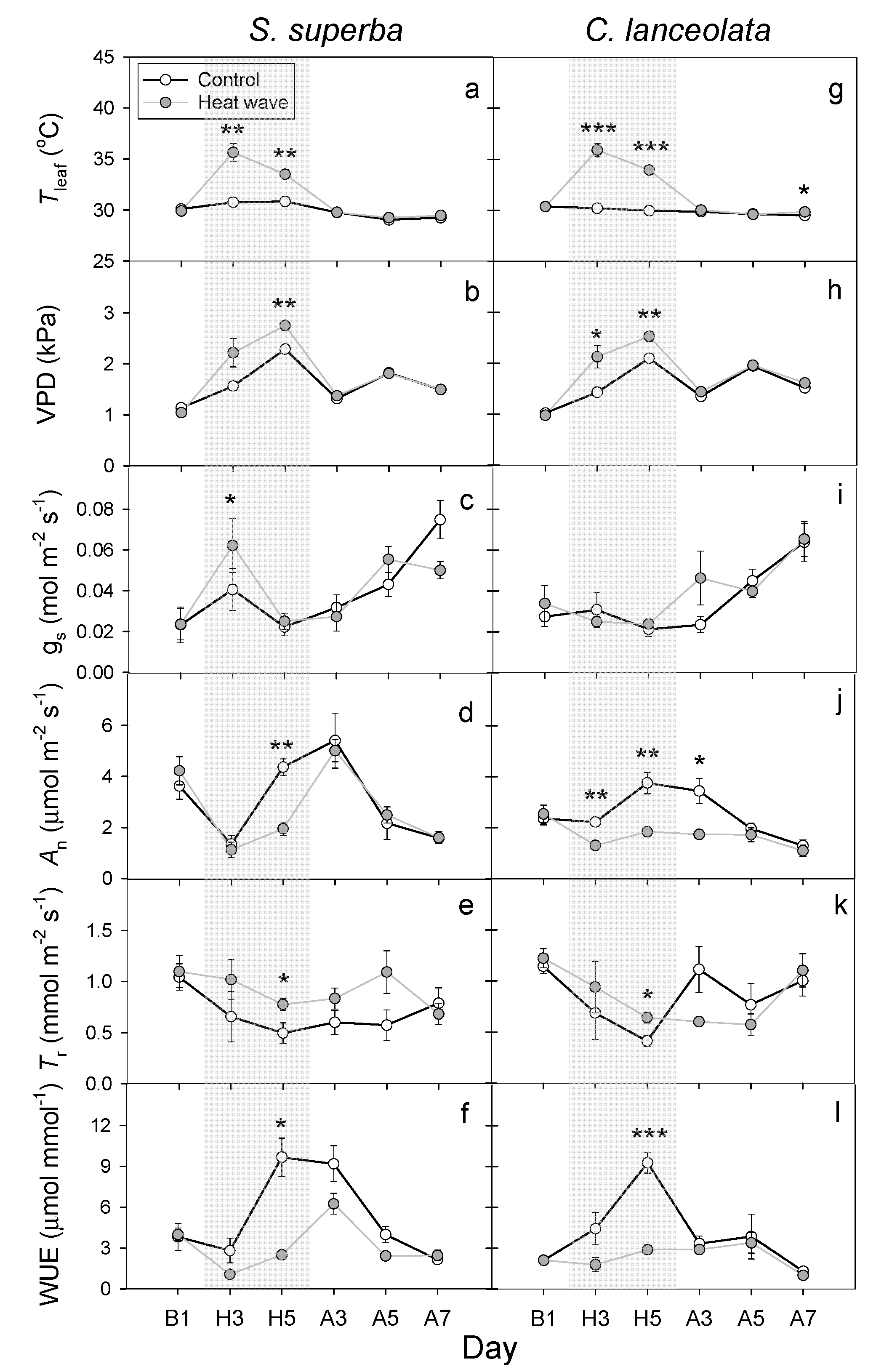
3.2. HW Effects on Leaf Gas Exchange and WUE
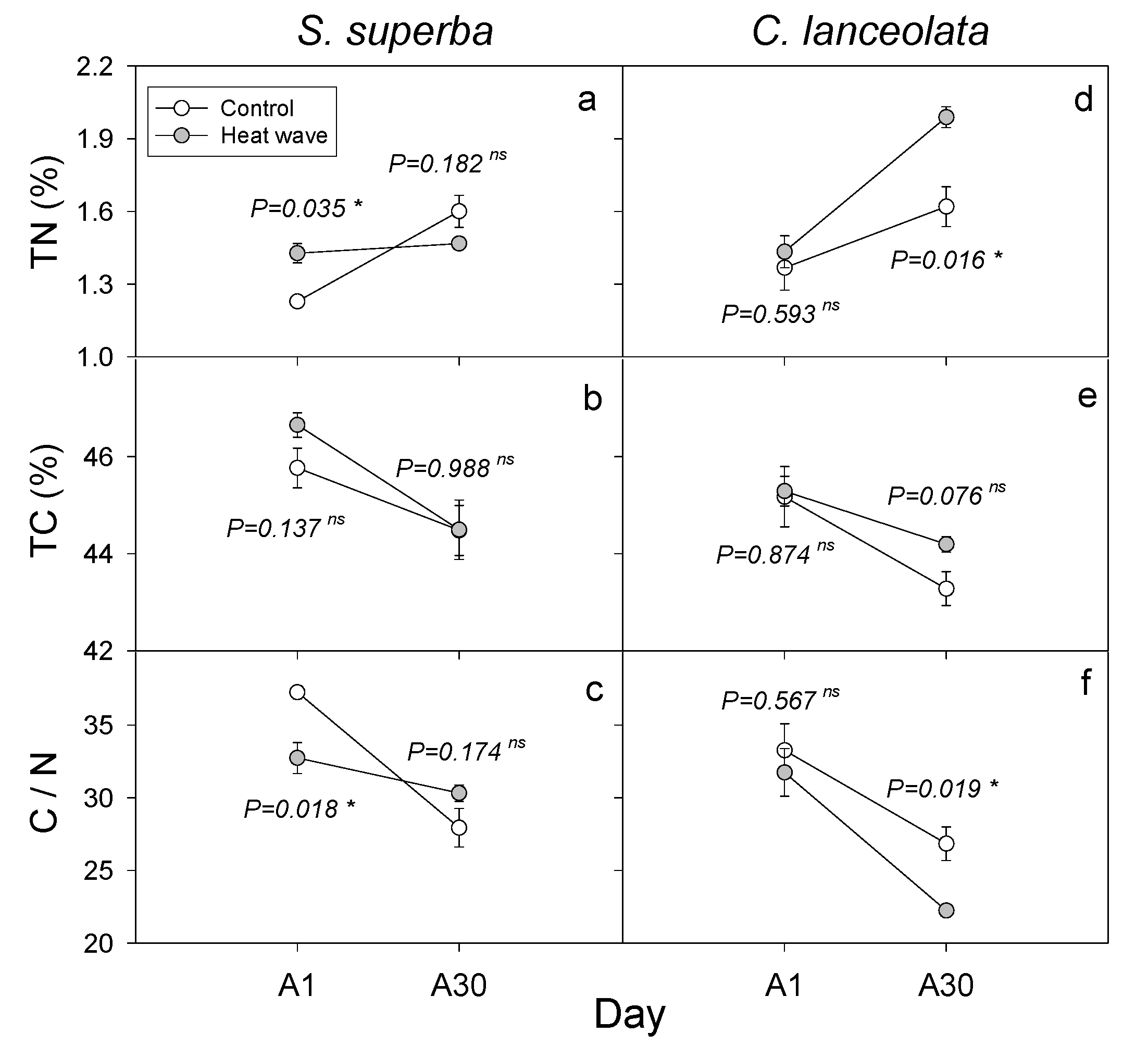
3.3. HW Effects on Plant Leaf Nutrients and Biomass
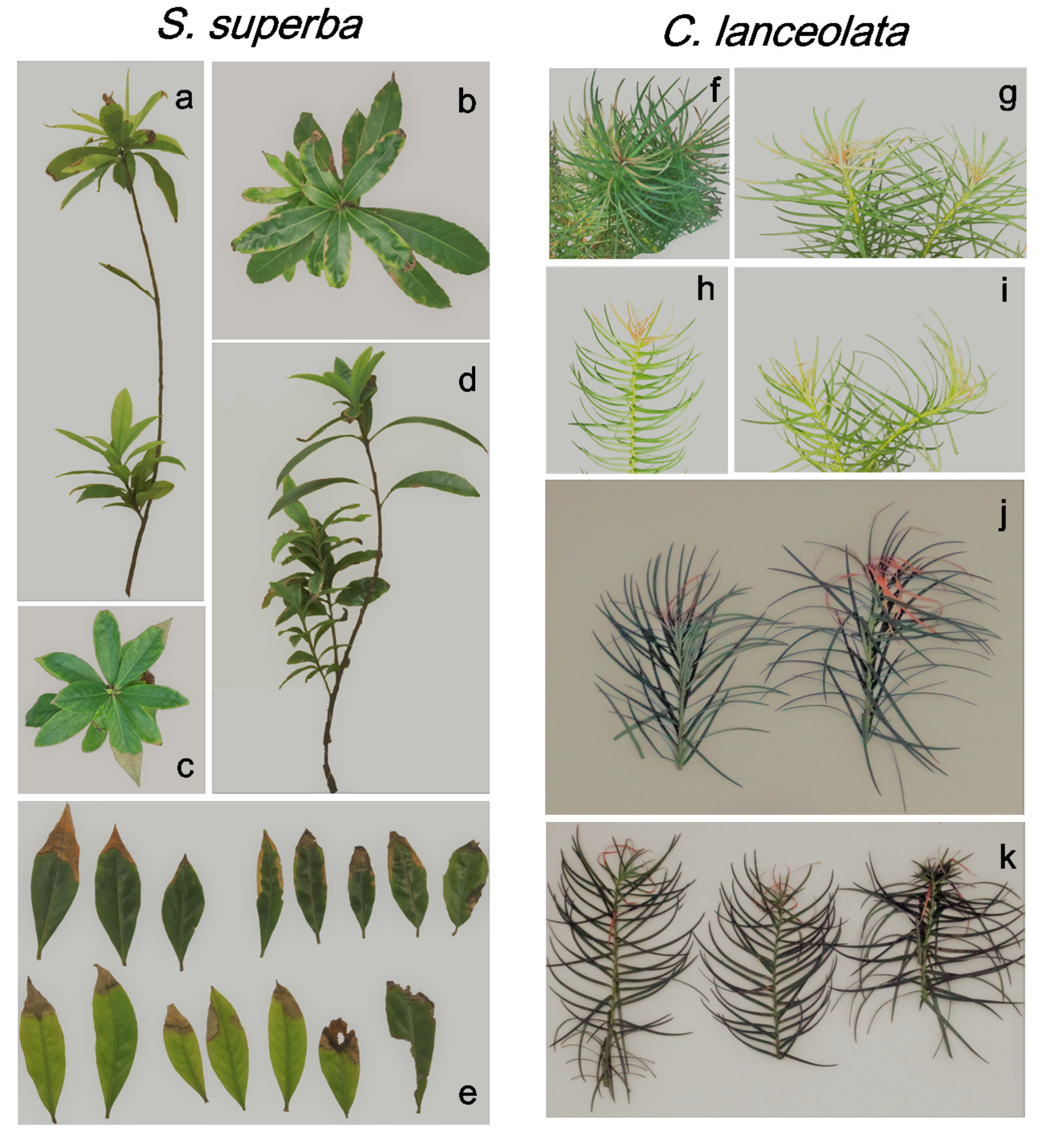
3.4. HW Effects on Plant Appearance
4. Discussion
4.1. HWs Caused a Negative Effect on Plant Water Transmission System by Xylem Embolism
4.2. HWs Decreased WUE Due to the Reduction in the Leaf An or Increase in the Tr
4.3. Differences in the Response of Two Subtropical Tree Species to HWs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. 2013: Climate change 2013: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University: Cambridge, UK, 2013. [Google Scholar]
- Meehl, G.A. More Intense, More Frequent, and Longer Lasting Heat Waves in the 21st Century. Science 2004, 305, 994–997. [Google Scholar] [CrossRef]
- De Boeck, H.; Dreesen, F.E.; Janssens, I.A.; Nijs, I. Climatic characteristics of heat waves and their simulation in plant experiments. Glob. Chang. Boil. 2010, 16, 1992–2000. [Google Scholar] [CrossRef]
- Sippel, S.; Reichstein, M.; Ma, X.; Mahecha, M.D.; Lange, H.; Flach, M.; Frank, D. Drought, Heat, and the Carbon Cycle: A Review. Curr. Clim. Chang. Rep. 2018, 4, 266–286. [Google Scholar] [CrossRef]
- Teskey, R.O.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2014, 38, 1699–1712. [Google Scholar] [CrossRef]
- Gutschick, V.P.; BassiriRad, H. Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. New Phytol. 2003, 160, 21–42. [Google Scholar] [CrossRef]
- Sanz-Lazaro, C. Climate extremes can drive biological assemblages to early successional stages compared to several mild disturbances. Sci. Rep. 2016, 6, 30607. [Google Scholar] [CrossRef]
- Bauweraerts, I.; Wertin, T.M.; Ameye, M.; McGuire, M.A.; Teskey, R.O.; Steppe, K. The effect of heat waves, elevated [CO2] and low soil water availability on northern red oak (Quercus rubra L.) seedlings. Glob. Chang. Boil. 2012, 19, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Chen, J.; Dong, G.; Shao, C. Heavy mowing enhances the effects of heat waves on grassland carbon and water fluxes. Sci. Total. Environ. 2018, 627, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tang, J.; Sun, X.; Wang, S.; Wu, J.; Dong, X.; Fang, J. Heat Waves in China: Definitions, Leading Patterns, and Connections to Large-Scale Atmospheric Circulation and SSTs. J. Geophys. Res. Atmos. 2017, 122, 10,679–10,699. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef]
- Qu, L.; Dong, G.; De Boeck, H.J.; Tian, L.; Chen, J.; Tang, H.; Xin, X.; Chen, J.; Hu, Y.; Shao, C. Joint forcing by heat waves and mowing poses a threat to grassland ecosystems: Evidence from a manipulative experiment. Land Degrad. Dev. 2019. [Google Scholar] [CrossRef]
- Qu, L.; Chen, J.; Dong, G.; Jiang, S.; Li, L.; Guo, J.; Shao, C. Heat waves reduce ecosystem carbon sink strength in a Eurasian meadow steppe. Environ. Res. 2016, 144, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hao, Z.; Hao, F.; Li, C.; Zhang, X. Spatial and Temporal Variations of Compound Droughts and Hot Extremes in China. Atmosphere 2019, 10, 95. [Google Scholar] [CrossRef]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential Responses of Stomata and Photosynthesis to Elevated Temperature in Two Co-occurring Subtropical Forest Tree Species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef]
- Aspinwall, M.J.; Pfautsch, S.; Tjoelker, M.G.; Vårhammar, A.; Possell, M.; Drake, J.E.; Reich, P.B.; Tissue, D.; Atkin, O.K.; Rymer, P.D.; et al. Range size and growth temperature influence Eucalyptus species responses to an experimental heatwave. Glob. Chang. Boil. 2019, 25, 1665–1684. [Google Scholar] [CrossRef]
- O’Sullivan, O.S.; Heskel, M.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Chang. Boil. 2016, 23, 209–223. [Google Scholar] [CrossRef]
- Yuan, W.; Cai, W.; Chen, Y.; Liu, S.; Dong, W.; Zhang, H.; Yu, G.; Chen, Z.; He, H.; Guo, W.; et al. Severe summer heatwave and drought strongly reduced carbon uptake in Southern China. Sci. Rep. 2016, 6, 18813. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.M.; A Law, E.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Duan, H.; Wu, J.; Huang, G.; Zhou, S.; Liu, W.; Liao, Y.; Yang, X.; Xiao, Z.; Fan, H. Individual and interactive effects of drought and heat on leaf physiology of seedlings in an economically important crop. AoB Plants 2016, 9, 1–16. [Google Scholar] [CrossRef]
- De Boeck, H.; Bassin, S.; Verlinden, M.; Zeiter, M.; Hiltbrunner, E. Simulated heat waves affected alpine grassland only in combination with drought. New Phytol. 2015, 209, 531–541. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Gast, A.; Weber, C.; Daub, B.; Arneth, A. Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiol. 2015, 36, 16. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Pockman, W.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Birami, B.; Gattmann, M.; Heyer, A.G.; Grote, R.; Arneth, A.; Ruehr, N.K. Heat Waves Alter Carbon Allocation and Increase Mortality of Aleppo Pine Under Dry Conditions. Front. For. Glob. Chang. 2018, 1, 8. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Klein, T.; Jansen, S.; Choat, B.; Sack, L. The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 13098–13103. [Google Scholar] [CrossRef] [PubMed]
- Trifilò, P.; Nardini, A.; Gullo, M.A.L.; Barbera, P.M.; Savi, T.; Raimondo, F. Diurnal changes in embolism rate in nine dry forest trees: Relationships with species-specific xylem vulnerability, hydraulic strategy and wood traits. Tree Physiol. 2015, 35, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Zeppel, M.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- St-Paul, N.M.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- De Dios, V.R.; E LoikiD, M.; Smith, R.A.; Tissue, D. Effects of a Heat Wave on Nocturnal Stomatal Conductance in Eucalyptus camaldulensis. Forests 2018, 9, 319. [Google Scholar] [CrossRef]
- Fauset, S.; Freitas, H.C.; Galbraith, D.R.; Sullivan, M.J.P.; Aidar, M.P.M.; Joly, C.A.; Phillips, O.L.; Vieira, S.A.; Gloor, E. Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. Plant Cell Environ. 2018, 41, 1618–1631. [Google Scholar] [CrossRef]
- Gleason, S.M.; Westoby, M.; Jansen, S.; Choat, B.; Hacke, U.; Pratt, R.B.; Bhaskar, R.; Brodribb, T.; Bucci, S.J.; Cao, K.; et al. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol. 2015, 209, 123–136. [Google Scholar] [CrossRef]
- Guha, A.; Han, J.; Cummings, C.; McLennan, D.; Warren, J. Differential ecophysiological responses and resilience to heat wave events in four co-occurring temperate tree species. Environ. Res. Lett. 2018, 13, 065008. [Google Scholar] [CrossRef]
- Ali, A.; Ma, W.; Yang, X.; Sun, B.; Shi, Q.; Xu, M. Biomass and carbon stocks in Schima superba dominated subtropical forests of eastern China. J. For. Sci. 2014, 60, 198–207. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Chen, G.; Xie, J.; Gao, R.; Li, Z.; Jin, Z. Carbon and nitrogen pools in Chinese fir and evergreen broadleaved forests and changes associated with felling and burning in mid-subtropical China. For. Ecol. Manag. 2005, 216, 216–226. [Google Scholar] [CrossRef]
- Fang, J.; Chen, A.; Peng, C.; Zhao, S.; Ci, L. Changes in Forest Biomass Carbon Storage in China Between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef]
- Yan, X.-L.; Wang, C.; Ma, X.; Wu, P. Root morphology and seedling growth of three tree species in southern China in response to homogeneous and heterogeneous phosphorus supplies. Trees 2019, 33, 1283–1297. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Kirschbaum, M.U.; Hou, Z.; Guo, Z. Carbon stock changes in successive rotations of Chinese fir (Cunninghamia lanceolata (lamb) hook) plantations. For. Ecol. Manag. 2004, 202, 131–147. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.-L.; Zhang, J. Assessing the effects of vegetation types on carbon storage fifteen years after reforestation on a Chinese fir site. For. Ecol. Manag. 2009, 258, 1437–1441. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Berninger, F.; Duan, B. Net primary production of Chinese fir plantation ecosystems and its relationship to climate. Biogeosciences 2014, 11, 5595–5606. [Google Scholar] [CrossRef]
- Lian, Y.; Zhang, Q. Conversion of a natural broad-leafed evergreen forest into pure and mixed plantation forests in a subtropical area: Effects on nutrient cycling. Can. J. For. Res. 1998, 28, 1518–1529. [Google Scholar] [CrossRef]
- Dai, E.; Zhu, J.; Wang, X.; Xi, W. Multiple ecosystem services of monoculture and mixed plantations: A case study of the Huitong experimental forest of Southern China. Land Use Policy 2018, 79, 717–724. [Google Scholar] [CrossRef]
- Bowman, D.; Williamson, G.J.; Keenan, R.; Prior, L.D. A warmer world will reduce tree growth in evergreen broadleaf forests: Evidence from Australian temperate and subtropical eucalypt forests. Glob. Ecol. Biogeogr. 2014, 23, 925–934. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D. Seasonal changes and daily courses of photosynthetic characteristics of 18-year-old Chinese fir shoots in relation to shoot ages and positions within the tree crown. Sci. Silvae Sin. 2000, 36, 19–26. (In Chinese) [Google Scholar]
- Liu, B.; Liu, Q.; Daryanto, S.; Guo, S.; Huang, Z.; Wang, Z.; Wang, L.; Ma, X. Responses of Chinese fir and Schima superba seedlings to light gradients: Implications for the restoration of mixed broadleaf-conifer forests from Chinese fir monocultures. For. Ecol. Manag. 2018, 51–57. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Secchi, F. Threats to xylem hydraulic function of trees under ‘new climate normal’ conditions. Plant, Cell Environ. 2014, 38, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.; Brodersen, C.R.; Duursma, R.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; O’Grady, J.G.; Tissue, D.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2012, 197, 862–872. [Google Scholar] [CrossRef]
- Klein, T.; Zeppel, M.; Anderegg, W.R.L.; Bloemen, J.; De Kauwe, M.G.; Hudson, P.; Ruehr, N.K.; Powell, T.; Von Arx, G.; Nardini, A. Xylem embolism refilling and resilience against drought-induced mortality in woody plants: Processes and trade-offs. Ecol. Res. 2018, 1–17. [Google Scholar] [CrossRef]
- Li, X.; Blackman, C.J.; Choat, B.; Duursma, R.; Rymer, P.D.; Medlyn, B.E.; Tissue, D. Tree hydraulic traits are coordinated and strongly linked to climate-of-origin across a rainfall gradient. Plant, Cell Environ. 2018, 41, 646–660. [Google Scholar] [CrossRef]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Physiol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Sevanto, S.; McDowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2013, 37, 153–161. [Google Scholar] [CrossRef]
- Drake, J.E.; Tjoelker, M.G.; Varhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; López, R.; Aspinwall, M.J.; et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Chang. Boil. 2018, 24, 2390–2402. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Grote, R.; Mayr, S.; Arneth, A. Beyond the extreme: Recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiol. 2019, 39, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Cohen, S.; Yakir, D. Hydraulic adjustments underlying drought resistance of Pinus halepensis. Tree Physiol. 2011, 31, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.; Bowman, D.J.M.S.; Nichols, S.; Delzon, S.; Burlett, R. Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytol. 2010, 188, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Lovisolo, C.; Perrone, I.; Hartung, W.; Schubert, A. An abscisic acid-related reduced transpiration promotes gradual embolism repair when grapevines are rehydrated after drought. New Phytol. 2008, 180, 642–651. [Google Scholar] [CrossRef]
- Nardini, A.; Savi, T.; Trifilò, P.; Gullo, M.A.L. Drought Stress and the Recovery from Xylem Embolism in Woody Plants. In Progress in Botany; Springer Science and Business Media LLC: Cham, Switzerland, 2017; Volume 79, pp. 197–231. [Google Scholar]
- Meinzer, F.C.; McCulloh, K.A. Xylem recovery from drought-induced embolism: Where is the hydraulic point of no return? Tree Physiol. 2013, 33, 331–334. [Google Scholar] [CrossRef]
- Hammond, W.; Yu, K.; Wilson, L.; Will, R.E.; Anderegg, W.R.L.; Adams, H.D. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol. 2019, 223, 1834–1843. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; Downs, C.A.; Sharkey, T.D.; Coleman, J.S. The Small, Methionine-Rich Chloroplast Heat-Shock Protein Protects Photosystem II Electron Transport during Heat Stress. Plant Physiol. 1998, 116, 439–444. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of Photosynthesis in a C4 Plant, Maize, to Heat Stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef]
- Kuczyńska-Wiśnik, D.; Matuszewska, E.; Laskowska, E. Escherichia coli heat-shock proteins IbpA and IbpB affect biofilm formation by influencing the level of extracellular indole. Microbiology 2009, 156, 148–157. [Google Scholar] [CrossRef]
- Crous, K.Y.; Drake, J.E.; Aspinwall, M.J.; Sharwood, R.; Tjoelker, M.G.; Ghannoum, O. Photosynthetic capacity and leaf nitrogen decline along a controlled climate gradient in provenances of two widely distributed Eucalyptus species. Glob. Chang. Boil. 2018, 24, 4626–4644. [Google Scholar] [CrossRef]
- Wang, D.; Heckathorn, S.A.; Mainali, K.; Tripathee, R. Timing Effects of Heat-Stress on Plant Ecophysiological Characteristics and Growth. Front. Plant Sci. 2016, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Leigh, A.; Sevanto, S.; Close, J.; Nicotra, A. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-D.; Song, J.-J.; Li, R.-H.; Ye, Q. Plant hydraulics and photosynthesis of 34 woody species from different successional stages of subtropical forests. Plant Cell Environ. 2012, 36, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Velez, V.; Dalling, J.W. Growth dynamics of root and shoot hydraulic conductance in seedlings of five neotropical tree species: Scaling to show possible adaptation to differing light regimes. Oecologia 1998, 114, 293. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.L.; Wu, J.; You, W.H. And Recovery of saturated hydraulic conductivity along a forest successional series from abandoned land to mature, evergreen broad-leaved forest in eastern China. Soil Res. 2012, 50, 257. [Google Scholar] [CrossRef]
- Li, R.-S.; Yang, Q.-P.; Zhang, W.; Zheng, W.-H.; Chi, Y.; Xu, M.; Fang, Y.; Gessler, A.; Li, M.-H.; Wang, S.-L. Thinning effect on photosynthesis depends on needle ages in a Chinese fir (Cunninghamia lanceolata) plantation. Sci. Total. Environ. 2017, 580, 900–906. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, W.; Li, R.; Xu, M.; Wang, S. Different responses of non-structural carbohydrates in above-ground tissues/organs and root to extreme drought and re-watering in Chinese fir (Cunninghamia lanceolata) saplings. Trees 2016, 30, 1863–1871. [Google Scholar] [CrossRef]
- Yang, F.; Feng, Z.; Wang, H.; Dai, X.; Fu, X. Deep soil water extraction helps to drought avoidance but shallow soil water uptake during dry season controls the inter-annual variation in tree growth in four subtropical plantations. Agric. For. Meteorol. 2017, v, 106–114. [Google Scholar] [CrossRef]
- Blackman, C.J.; Brodribb, T.; Jordan, G.J. Leaf hydraulics and drought stress: Response, recovery and survivorship in four woody temperate plant species. Plant, Cell Environ. 2009, 32, 1584–1595. [Google Scholar] [CrossRef]
- Dong, T.; Li, J.; Zhang, Y.; Korpelainen, H.; Ülo, N. Partial shading of lateral branches affects growth, and foliage nitrogen- and water-use efficiencies in the conifer Cunninghamia lanceolata growing in a warm monsoon climate. Tree Physiol. 2015, 35, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Tjoelker, M.G.; Aspinwall, M.J.; Battaglia, M.; Cano, F.J.; Carter, K.R.; Cavaleri, M.A.; Cernusak, L.A.; et al. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 2019, 222, 768–784. [Google Scholar] [CrossRef] [PubMed]


| Pre Heat Wave | Heat Wave Stage (5 Days) | Post Heat Wave (30 Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st Day (B1) | 3rd Day (H3) | 5th Day (H5) | 1st Day (A1) | 3rd Day (A3) | 5th Day (A5) | 7th Day (A7) | 30th Day (A30) | |
| Stem hydraulic conductivity | √ | |||||||
| Leaf gas exchange | √ | √ | √ | √ | √ | √ | ||
| Biomass | √ | |||||||
| Leaf C/N content | √ | √ | ||||||
| Plant appearance | √ | |||||||
| Factors | Hydraulic Characteristics | Biomass | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kh | Kmax | PLC | Ks | Kl | Hv | Leaf | Stem | Aboveground | Underground | Total | Root/Shoot | |
| HW | 0.003 | 0.008 | 0.003 | 0.007 | 0.002 | 0.726 | 0.381 | 0.951 | 0.559 | 0.470 | 0.717 | 0.740 |
| S | 0.628 | 0.307 | 0.083 | 0.001 | 0.005 | 0.320 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.020 |
| HW × S | 0.359 | 0.423 | 0.167 | 0.687 | 0.873 | 0.737 | 0.515 | 0.447 | 0.866 | 0.013 | 0.650 | 0.432 |
| Factors | df | Tleaf | VPD | gs | An | Tr | WUE |
|---|---|---|---|---|---|---|---|
| D | 5 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| D × HW | 5 | <0.001 | <0.001 | 0.455 | <0.001 | 0.421 | <0.001 |
| D × S | 5 | 0.306 | 0.039 | 0.244 | <0.001 | 0.341 | <0.001 |
| D × HW × S | 5 | 0.305 | 0.998 | 0.324 | 0.735 | 0.218 | 0.607 |
| HW | 1 | <0.001 | <0.001 | 0.501 | 0.004 | 0.012 | <0.001 |
| S | 1 | 0.310 | 0.526 | 0.183 | <0.001 | 0.234 | 0.005 |
| HW × S | 1 | 0.205 | 0.654 | 0.808 | 0.186 | 0.008 | 0.451 |
| Factors | df | Tleaf | VPD | gs | An | Tr | WUE |
|---|---|---|---|---|---|---|---|
| T | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| T × HW | 4 | 0.094 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 |
| T × S | 4 | 0.081 | 0.004 | 0.027 | 0.616 | 0.388 | 0.775 |
| T × HW × S | 4 | 0.057 | 0.006 | 0.269 | 0.072 | 0.008 | 0.253 |
| HW | 1 | <0.001 | <0.001 | 0.579 | 0.001 | <0.001 | <0.001 |
| S | 1 | 0.865 | 0.729 | 0.378 | 0.864 | 0.097 | 0.193 |
| HW × S | 1 | 0.006 | 0.002 | 0.062 | 0.076 | 0.260 | 0.079 |
| Factors | Leaf Nutrient | |||
|---|---|---|---|---|
| df | TN | TC | C/N | |
| D | 1 | <0.001 | 0.001 | <0.001 |
| D × HW | 1 | 0.831 | 0.957 | 0.231 |
| D × S | 1 | 0.016 | 0.739 | 0.194 |
| D × HW × S | 1 | 0.001 | 0.243 | 0.010 |
| HW | 1 | 0.034 | 0.121 | 0.052 |
| S | 1 | 0.008 | 0.014 | 0.004 |
| HW × S | 1 | 0.097 | 0.906 | 0.303 |
| Schima superba | Cunninghamia lanceolata | |||||
|---|---|---|---|---|---|---|
| Control | Heat Wave | p-Value | Control | Heat Wave | p-Value | |
| Leaf (g) | 1.64 ± 0.31 | 2.45 ± 0.94 | 0.186 | 24.47 ± 1.95 | 29.77 ± 3.25 | 0.484 |
| Stem (g) | 1.75 ± 0.89 | 3.05 ± 1.34 | 0.158 | 15.85 ± 3.26 | 14.33 ± 6.18 | 0.678 |
| Aboveground (g) | 3.39 ± 1.06 | 5.50 ± 1.73 | 0.083 | 40.32 ± 4.86 | 44.10 ± 8.84 | 0.711 |
| Underground (g) | 2.41 ± 1.31 | 4.97 ± 2.09 | 0.083 | 20.17 ± 2.28 | 15.86 ± 3.27 | 0.074 |
| Total (g) | 5.80 ± 2.32 | 10.46 ± 0.50 | 0.008 | 60.49 ± 6.43 | 59.96 ± 9.32 | 0.964 |
| Root/shoot | 0.71 ± 0.04 | 0.90 ± 0.03 | 0.206 | 0.50 ± 0.05 | 0.36 ± 0.03 | 0.203 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.; De Boeck, H.J.; Fan, H.; Dong, G.; Chen, J.; Xu, W.; Ge, Z.; Huang, Z.; Shao, C.; Hu, Y. Diverging Responses of Two Subtropical Tree Species (Schima superba and Cunninghamia lanceolata) to Heat Waves. Forests 2020, 11, 513. https://doi.org/10.3390/f11050513
Qu L, De Boeck HJ, Fan H, Dong G, Chen J, Xu W, Ge Z, Huang Z, Shao C, Hu Y. Diverging Responses of Two Subtropical Tree Species (Schima superba and Cunninghamia lanceolata) to Heat Waves. Forests. 2020; 11(5):513. https://doi.org/10.3390/f11050513
Chicago/Turabian StyleQu, Luping, Hans J. De Boeck, Huihua Fan, Gang Dong, Jiquan Chen, Wenbin Xu, Zhiqiang Ge, Zijing Huang, Changliang Shao, and Yalin Hu. 2020. "Diverging Responses of Two Subtropical Tree Species (Schima superba and Cunninghamia lanceolata) to Heat Waves" Forests 11, no. 5: 513. https://doi.org/10.3390/f11050513
APA StyleQu, L., De Boeck, H. J., Fan, H., Dong, G., Chen, J., Xu, W., Ge, Z., Huang, Z., Shao, C., & Hu, Y. (2020). Diverging Responses of Two Subtropical Tree Species (Schima superba and Cunninghamia lanceolata) to Heat Waves. Forests, 11(5), 513. https://doi.org/10.3390/f11050513







