Mixed Broadleaved Tree Species Increases Soil Phosphorus Availability but Decreases the Coniferous Tree Nutrient Concentration in Subtropical China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design and Investigation
2.3. Sampling
2.4. Chemical Analyses
2.5. Statistical Analyses
3. Results
3.1. Soil General Properties
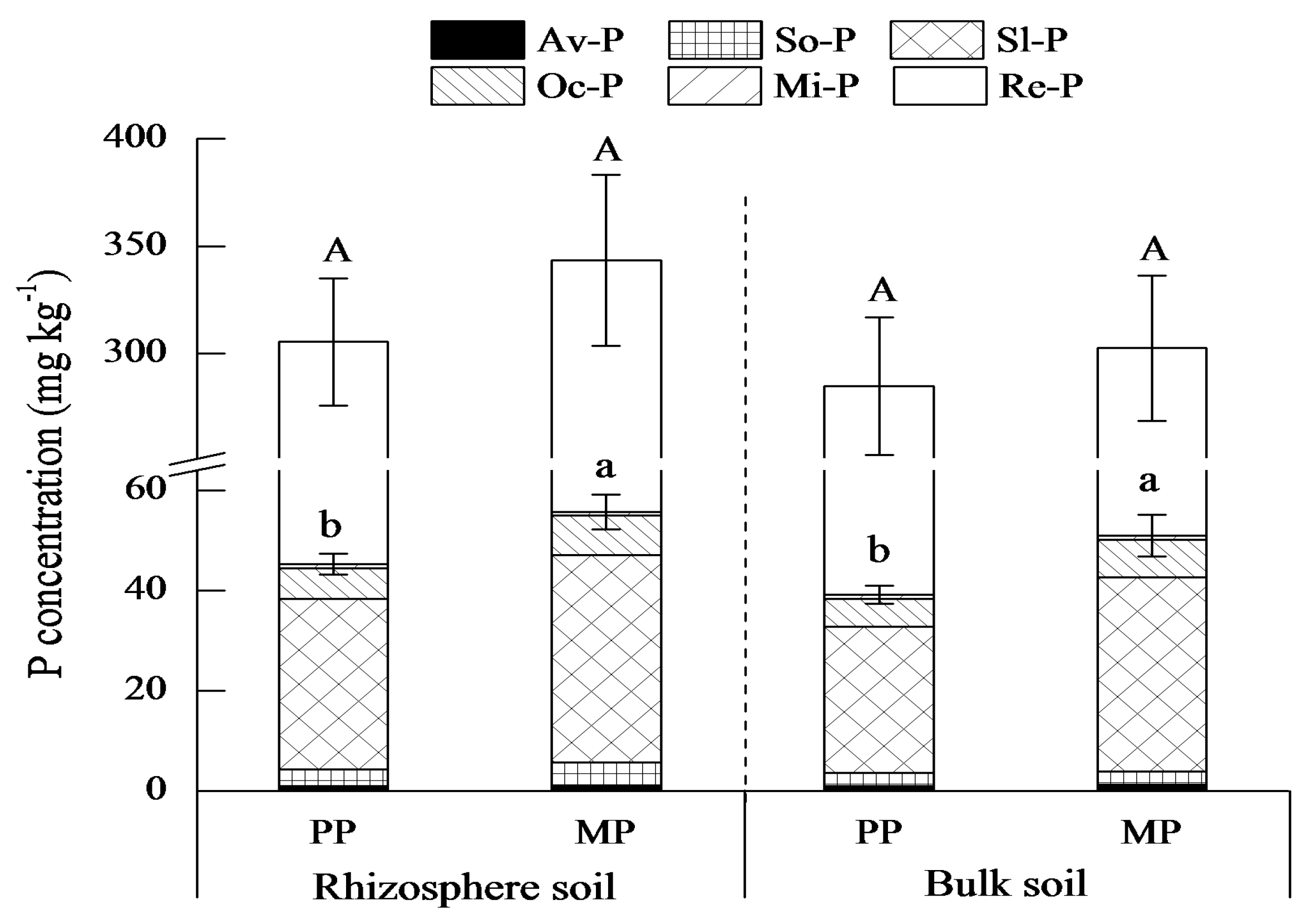
3.2. Phosphorus Fractions in Rhizosphere and Bulk Soils
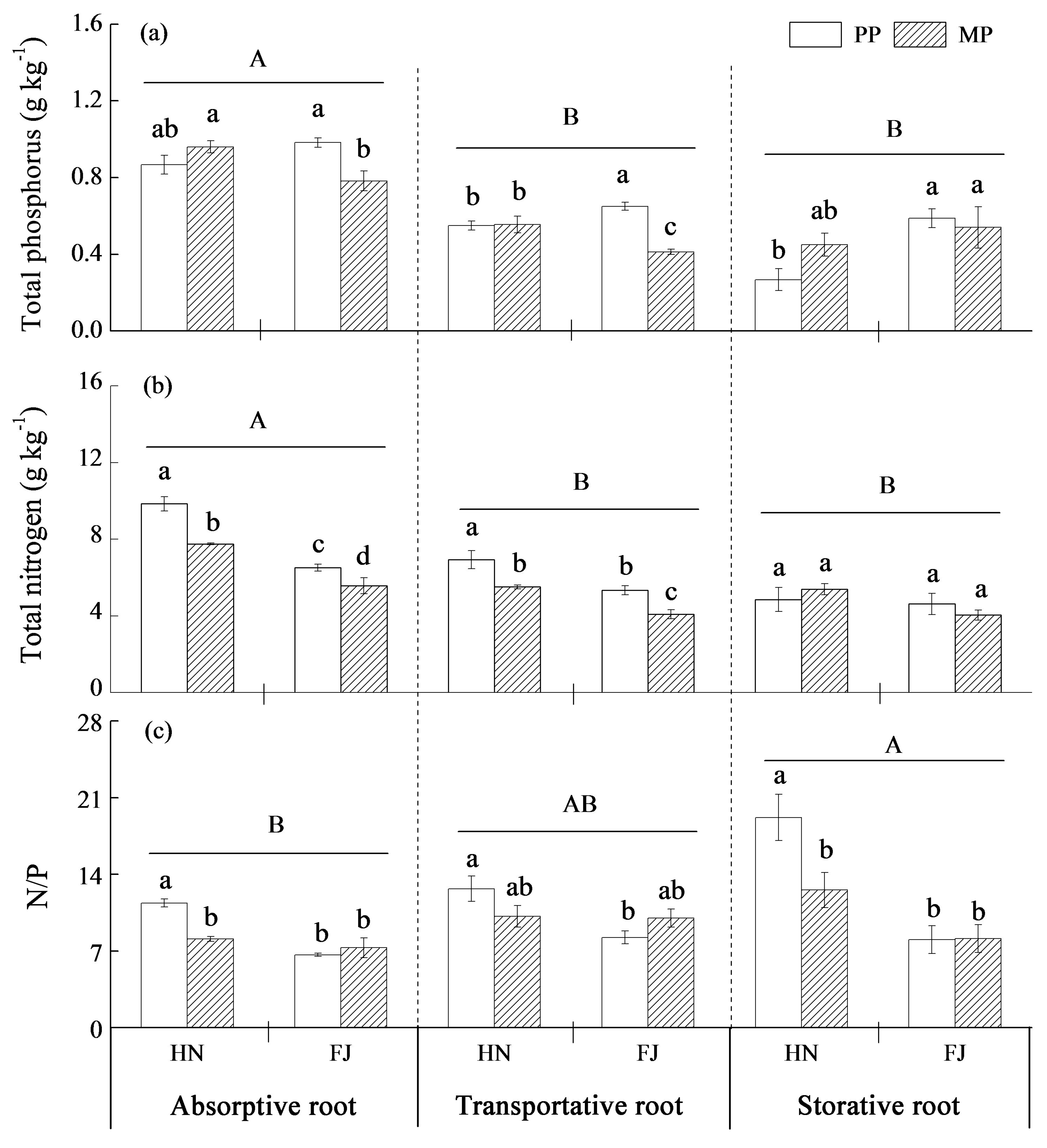
3.3. Nutrient Levels in Different Root Orders
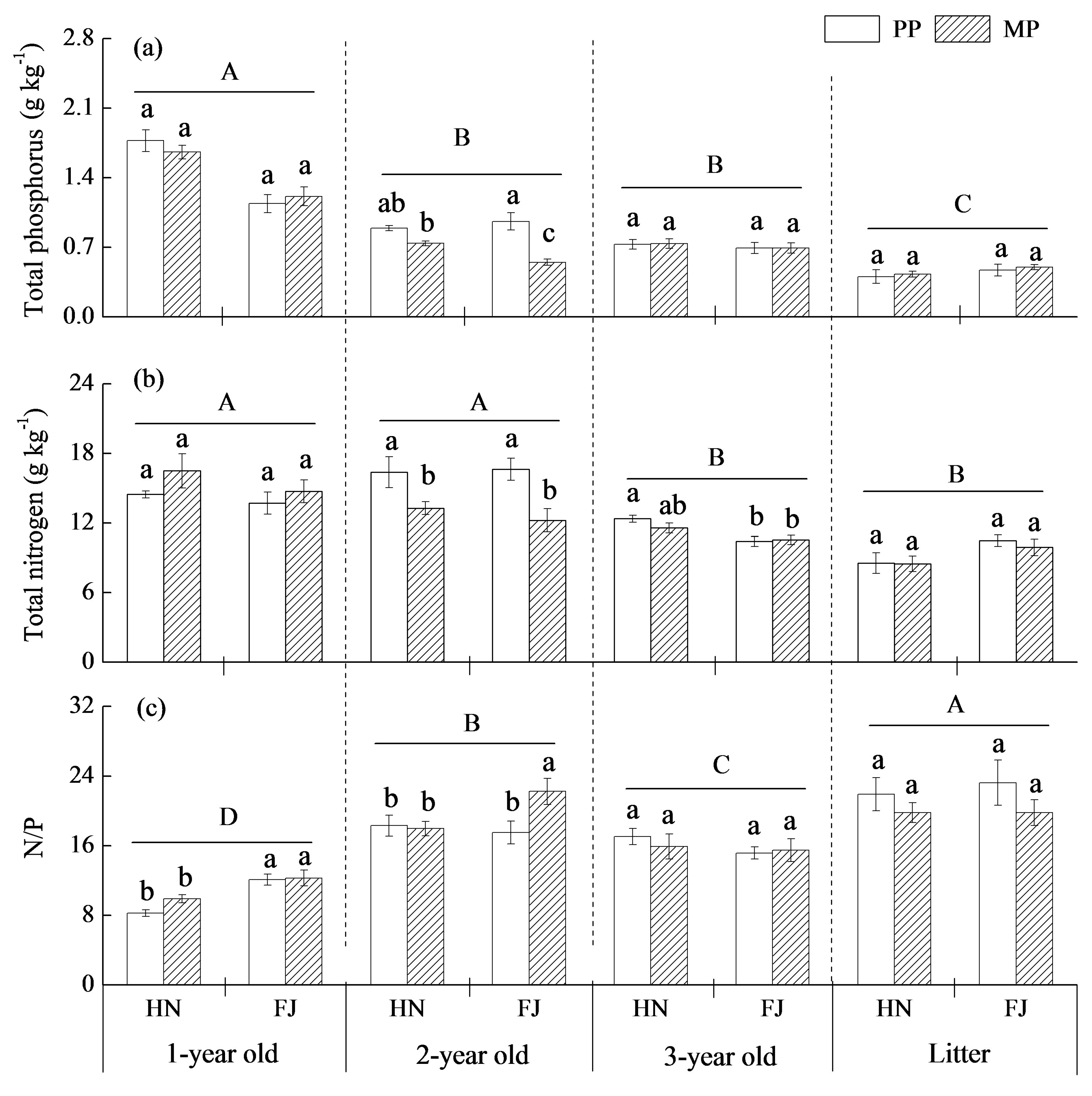
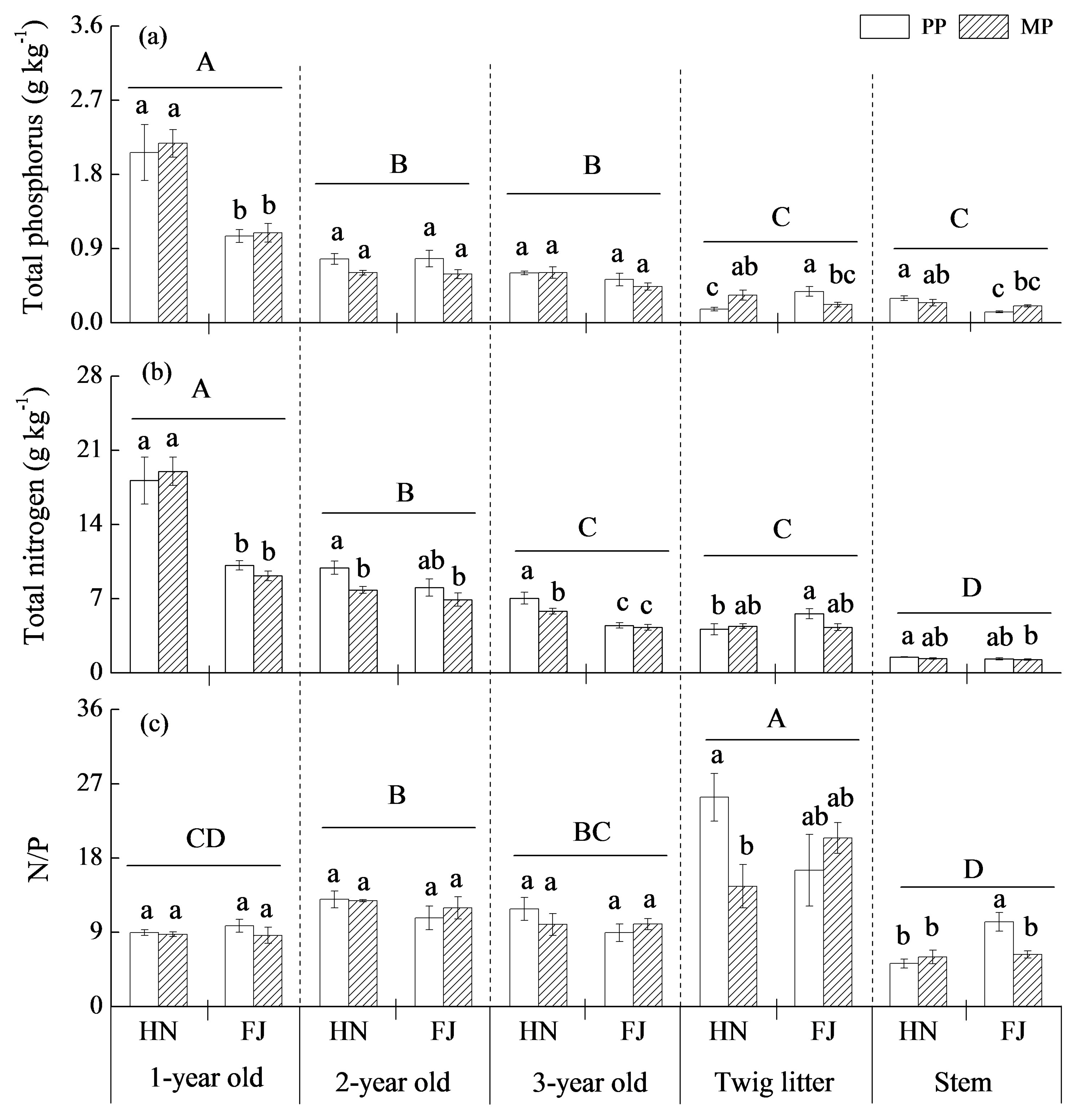
3.4. Nutrient Distributions in Aboveground Tissues
3.5. Linkages Among Soil, Root and Needle Nutrients
4. Discussion
4.1. Mixture Effect on Nutrient Concentrations in Rhizosphere and Bulk Soils
4.2. Mixture Effect Varies with Soil P Fraction
4.3. The Response of Chinese Fir to Mixture Effect Varies with Root Orders, Needle, and Twig Ages
4.4. Linkages between Soil, Root, and Needle Nutrients
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, J. The 8th forest resources inventory results and analysis in China. For. Econ. 2004, 24, 6–8. [Google Scholar]
- Wen, L.; Lei, P.; Xiang, W.; Yan, W.; Liu, S. Soil microbial biomass carbon and nitrogen in pure and mixed stands of Pinus massoniana and Cinnamomum camphora differing in stand age. Ecol. Manag. 2014, 328, 150–158. [Google Scholar] [CrossRef]
- Chen, H. Phosphatase activity and P fractions in soils of an 18−year−old Chinese fir (Cunninghamia lanceolata) plantation. For. Ecol. Manag. 2003, 178, 301–310. [Google Scholar] [CrossRef]
- Ma, X.; Heal, K.V.; Liu, A.; Jarvis, P.G. Nutrient cycling and distribution in different−aged plantations of Chinese fir in southern China. For. Ecol. Manag. 2007, 243, 61–74. [Google Scholar] [CrossRef]
- Meng, J.; Lu, Y.; Zeng, J. Transformation of a degraded Pinus massoniana plantation into a mixed-species irregular forest: Impacts on stand structure and growth in Southern China. Forests 2014, 5, 3199–3221. [Google Scholar] [CrossRef]
- Nunes, L.; Coutinho, J.; Nunes, L.F.; Rego, F.C.; Lopes, D. Growth, soil properties and foliage chemical analysis comparison between pure and mixed stands of Castanea saliva Mill.; Pseudotsuga menziesii (Mirb.) Franco, in Northern Portugal. System 2011, 20, 496–507. [Google Scholar]
- Yang, X.; Thornton, P.E.; Ricciuto, D.M.; Post, W.M. The role of phosphorus dynamics in tropical forests−A modeling study using CLM−CNP. Biogeosciences 2014, 11, 1667–1681. [Google Scholar] [CrossRef]
- Zou, X.; Wu, P.; Chen, N.; Wang, P.; Ma, X. Chinese fir root response to spatial and temporal heterogeneity of phosphorus availability in the soil. Can. J. For. Res. 2015, 45, 402–410. [Google Scholar] [CrossRef]
- Shenoy, V.; Kalagudi, G. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ota, A.T.; Kabeya, D.; Okamoto, T.; Saitoh, T.; Nishiyama, Y. The effects of mixed broad−leaved trees on the collembolan community in larch plantations of central Japan. Appl. Soil. Ecol. 2014, 83, 125–132. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth−promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fert. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root−induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Brzostek, E.R.; Greco, A.; Drake, J.E.; Finzi, A.C. Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 2013, 115, 65–76. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere effect on nutrient availability in soil and Its uptake by plants: A Review. Proc. Nat. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Hu, X.F.; Chen, F.S.; Nagle, G.; Fang, Y.T.; Yu, M.Q. Soil phosphorus fractions and tree phosphorus resorption in pine forests along an urban−to−rural gradient in Nanchang, China. Plant Soil 2011, 346, 97–106. [Google Scholar] [CrossRef]
- Redondo-Brenes, A.; Montagnini, F. Growth, productivity, aboveground biomass, and carbon sequestration of pure and mixed native tree plantations in the Caribbean lowlands of Costa Rica. For. Ecol. Manag. 2006, 232, 168–178. [Google Scholar]
- Lemma, B. Soil chemical properties and nutritional status of trees in pure and mixed-species stands in south Ethiopia. J. Plant Nutr. Soil Sci. 2012, 175, 769–774. [Google Scholar] [CrossRef]
- Chen, F.S.; Niklas, K.J.; Liu, Y.; Fang, X.M.; Wan, S.Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Montagnini, F. Accumulation in above−ground biomass and soil storage of mineral nutrients in pure and mixed plantations in a humid tropical lowland. For. Ecol. Manag. 2000, 134, 257–270. [Google Scholar] [CrossRef]
- Rothe, A.; Binkley, D. Nutritional interactions in mixed species forests: A synthesis. Can. J. For. Res. 2001, 31, 1855–1870. [Google Scholar] [CrossRef]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: effects on nutrient proficiency. Glob. Chang. Biol. 2015, 21, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, J.; Di, Y.; Wang, H. Differences in Fine−Root Biomass of Trees and Understory Vegetation among Stand Types in Subtropical Forests. PLoS ONE 2015, 10, e0128894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S. Soil microbial properties and nutrients in pure and mixed Chinese fir plantations. J. Res. (Harbin) 2008, 19, 131–135. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty−three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- Chen, F.S.; Li, X.; Nagle, G.; Zhan, S.X. Topsoil phosphorus signature in five forest types along an urban−suburban−rural gradient in Nanchang, southern China. J. Res. 2010, 21, 39–44. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Spss, I. SPSS for Windows (16.0); SPSS Inc: Chicago, IL, USA, 2007. [Google Scholar]
- Richards, A.E.; Forrester, D.I.; Bauhus, J.; Scherer-Lorenzen, M. The influence of mixed tree plantations on the nutrition of individual species: a review. Tree Physiol. 2010, 30, 1192–1208. [Google Scholar] [CrossRef]
- Eshel, A.; Beeckman, T. Plant Roots: The Hidden Half; CRC Press: Bocaraton, FL, USA, 2013. [Google Scholar]
- Wang, Q.; Wang, S.; Fan, B.; Yu, X. Litter production, leaf litter decomposition and nutrient return in Cunninghamia lanceolata plantations in south China: effect of planting conifers with broadleaved species. Plant Soil 2007, 297, 201–211. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Huang, Y. Leaf litter decomposition in the pure and mixed plantations of Cunninghamia lanceolata and Michelia macclurei in subtropical China. Biol. Fert. Soils 2009, 45, 371–377. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Leffelaar, P.A.; Shen, J.; Zhang, F. Dynamics of phosphorus fractions in the rhizosphere of fababean (Vicia faba L.) and maize (Zea mays L.) grown in calcareous and acid soils. Crop Pasture Sci. 2017, 66, 1151. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 2006, 87, 1302–1313. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, Q.; Gao, H.; Yu, X. Decomposition and nitrogen and phosphorus release of leaf litters from main tree species in a mid−subtropical forest. Chin. J. Ecol. 2013, 32, 1653–1659. [Google Scholar]
- Gerke, J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178, 351–364. [Google Scholar] [CrossRef]
- Chen, L.; Kong, C. Autoinhibition and soil allelochemical (cyclic dipeptide) levels in replanted Chinese fir (Cunninghamia lanceolata) plantations. Plant Soil 2014, 374, 793–801. [Google Scholar] [CrossRef]
- Lambers, H.; Hayes, P.E.; Laliberte, E.; Oliveira, R.S.; Turner, B.L. Leaf manganese accumulation and phosphorus−acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef]
- Di, Y.; Fu, X.; Wang, H.; Li, W.; Wang, S. N concentration of old leaves and twigs is more sensitive to stand density than that of young ones in Chinese fir plantations: A case study in subtropical China. J. For. Res. 2018, 29, 163–169. [Google Scholar] [CrossRef]
- Wang, M.; Murphy, M.T.; Moore, T.R. Nutrient resorption of two evergreen shrubs in response to long−term fertilization in a bog. Oecologia 2014, 174, 365–377. [Google Scholar] [CrossRef]
- Zeugin, F.; Potvin, C.; Jansa, J.; Scherer-Lorenzen, M. Is tree diversity an important driver for phosphorus and nitrogen acquisition of a young tropical plantation? For. Ecol. Manag. 2010, 260, 1424–1433. [Google Scholar] [CrossRef]
- De Graaff, M.A.; Classen, A.T.; Castro, H.F.; Schadt, C.W. Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 2010, 188, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, S. Allelopathic effects of eucalyptus and the establishment of mixed stands of eucalyptus and native species. For. Ecol. Manag. 2009, 258, 1391–1396. [Google Scholar] [CrossRef]
- Bell, C.; Carrillo, Y.; Boot, C.M.; Rocca, J.D.; Pendall, E.; Wallenstein, M.D. Rhizosphere stoichiometry: are C: N: P ratios of plants, soils, and enzymes conserved at the plant species-level? New Phytol. 2014, 201, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Elgersma, K.J.; Yu, S.; Vor, T.; Ehrenfeld, J.G. Microbial−mediated feedbacks of leaf litter on invasive plant growth and interspecific competition. Plant Soil 2012, 35, 341–355. [Google Scholar] [CrossRef]

| Parameters | Hunan | Fujian | ||
|---|---|---|---|---|
| PP | MP | PP | MP | |
| Basal area (m2 ha−1) | ||||
| Chinese fir | 35.31 ± 2.20 | 23.47 ± 2.26 | 69.75 ± 4.16 | 42.43 ± 2.02 |
| Broadleaved tree | — | 11.04 ± 1.21 | — | 11.97 ± 1.49 |
| Total | 35.31 ± 2.20 | 34.51 ± 2.23 | 69.75 ± 4.16 | 54.40 ± 2.96 |
| Stem density (trees ha−1) | ||||
| Chinese fir | 1410 ± 102 | 620 ± 86 | 2510 ± 129 | 2010 ± 76 |
| Broadleaved tree | — | 730 ± 68 | — | 710 ± 29 |
| Total | 1410 ± 102 | 1350 ± 47 | 2510 ± 129 | 2720 ± 85 |
| Shrub density (stems ha−1) | 8160 ± 1063 | 6320 ± 1038 | 14000 ± 2449 | 8500 ± 1000 |
| Herb cover (%) | 27.4 ± 12.4 | 14.1 ± 4.1 | 8.6 ± 4.0 | 2.6 ± 2.6 |
| Understory plant biomass (g m−2) | 154 ± 79 | 145 ± 42 | 214 ± 76 | 157 ± 63 |
| Parameter | Hunan | Fujian | ||
|---|---|---|---|---|
| PP | MP | PP | MP | |
| pH | ||||
| Rhizosphere soil | 4.36 ± 0.20 a | 4.39 ± 0.09 a | 4.35 ± 0.03 a | 4.29 ± 0.03 a |
| Bulk soil | 4.36 ± 0.21 a | 4.32 ± 0.08 a | 4.33 ± 0.01 a | 4.20 ± 0.03 a |
| Organic carbon (g kg−1) | ||||
| Rhizosphere soil | 16.0 ± 0.1 c | 18.1 ± 0.8 bc | 21.7 ± 1.1 b | 34.4 ± 2.7 a |
| Bulk soil | 11.3 ± 2.0 d | 15.6 ± 1.6 c | 18.9 ± 2.0 b | 23.3 ± 1.1 a |
| Total nitrogen (g kg−1) | ||||
| Rhizosphere soil | 0.6 ± 0.0 b | 1.0 ± 0.0 a | 0.8 ± 0.1 ab | 1.0 ± 0.1 a |
| Bulk soil | 0.5 ± 0.00 b | 0.8 ± 0.1 a | 0.6 ± 0.1 b | 0.7 ± 0.1 ab |
| C/N | ||||
| Rhizosphere soil | 27.6 ± 1.0 c | 18.0 ± 0.9 d | 30.7 ± 5.3 b | 36.0 ± 1.2 a |
| Bulk soil | 19.1 ± 1.5 b | 20.9 ± 2.6 b | 33.7 ± 2.0 a | 35.3 ± 4.4 a |
| Variables | Total P of Roots | Total P of Fresh Needles | Total P of Needle Litter | ||||
|---|---|---|---|---|---|---|---|
| Absorp-tive | Transpor-tive | Storative | 1-yr old | 2-yr old | 3-yr old | ||
| P fractions in rhizosphere soil | |||||||
| Av-P | −0.04 ns | −0.16 ns | −0.11 ns | −0.34 ns | −0.12 ns | 0.05 ns | −0.21 ns |
| So-P | 0.08 ns | −0.26 ns | −0.50 ns | 0.19 ns | −0.26 ns | −0.16 ns | −0.35 ns |
| Sl-P | −0.58 * | −0.77 ** | −0.11 ns | −0.21 ns | −0.78 ** | −0.02 ns | −0.04 ns |
| Oc-P | −0.39 ns | −0.63 ** | 0.25 ns | −0.64 ** | −0.62 * | −0.10 ns | 0.21 ns |
| Mi-P | 0.34 ns | 0.25 ns | 0.25 ns | −0.38 ns | 0.16 ns | 0.17 ns | 0.24 ns |
| Ex-P | −0.18 ns | −0.31 ns | 0.47 ns | −0.86 ** | −0.40 ns | −0.06 ns | 0.39 ns |
| Re-P | −0.49 ns | −0.76 ** | −0.11 ns | −0.29 ns | −0.76 ** | −0.06 ns | −0.04 ns |
| Rhizosphere effect of phosphorus fractions | |||||||
| Av-P | 0.43 ns | 0.36 ns | −0.27 ns | −0.12 ns | 0.43 ns | 0.21 ns | −0.34 ns |
| So-P | 0.51 * | 0.00 ns | −0.17 ns | 0.08 ns | −0.08 ns | 0.10 ns | −0.27 ns |
| Sl-P | −0.02 ns | 0.02 ns | −0.51 * | 0.42 ns | 0.21 ns | −0.03 ns | −0.60 * |
| Oc-P | 0.07 ns | −0.12 ns | −0.39 ns | −0.04 ns | 0.05 ns | −0.18 ns | −0.54 * |
| Mi-P | −0.02 ns | 0.27 ns | 0.01 ns | 0.48 ns | 0.35 ns | −0.08 ns | 0.03 ns |
| Ex-P | 0.15 ns | −0.52 * | −0.31 ns | 0.06 ns | −0.39 ns | 0.38 ns | −0.03 ns |
| Re-P | 0.18 ns | 0.03 ns | −0.50 * | 0.34 ns | 0.19 ns | −0.06 ns | −0.69 ** |
| Variables | Total N of Roots | Total N of Fresh Needles | Total N of Needle Litter | ||||
|---|---|---|---|---|---|---|---|
| Absorp-tive | Transpor-tive | Storative | one-year old | two-year old | 3-year old | ||
| Phosphorus fractions in rhizosphere soil | |||||||
| Av−P | −0.18 ns | −0.31 ns | −0.14 ns | −0.24 ns | −0.09 ns | −0.12 ns | −0.14 ns |
| So−P | 0.51 * | −0.33 ns | 0.03 ns | −0.49 ns | 0.11 ns | −0.10 ns | 0.01 ns |
| Sl−P | −0.05 ns | −0.55 * | −0.13 ns | −0.02 ns | −0.38 ns | −0.46 ns | −0.32 ns |
| Oc−P | −0.11 ns | −0.46 ns | −0.60 * | 0.34 ns | −0.71 ** | −0.73 ** | −0.39 ns |
| Mi−P | −0.07 ns | 0.16 ns | −0.33 ns | 0.69 ** | −0.51 * | −0.28 ns | −0.33 ns |
| Ex−P | −0.31 ns | −0.36 ns | −0.70 ** | 0.50* | −0.76 ** | −0.72 ** | −0.43 ns |
| Re−P | 0.03 ns | −0.57 * | −0.25 ns | −0.01 ns | −0.44 ns | −0.54 * | −0.34 ns |
| Rhizosphere effect of phosphorus fractions | |||||||
| Av−P | −0.12 ns | 0.27 ns | 0.11 ns | −0.12 ns | 0.12 ns | 0.17 ns | −0.14 ns |
| So−P | 0.46 ns | −0.14 ns | −0.03 ns | −0.35 ns | −0.02 ns | −0.03 ns | 0.11 ns |
| Sl−P | −0.09 ns | 0.27 ns | 0.55 * | −0.58* | 0.61 * | 0.72 ** | 0.24 ns |
| Oc−P | −0.06 ns | 0.02 ns | −0.10 ns | −0.49 ns | 0.31 ns | 0.28 ns | 0.01 ns |
| Mi−P | 0.04 ns | 0.47 ns | 0.25 ns | 0.07 ns | 0.30 ns | 0.56 * | 0.26 ns |
| Ex−P | 0.38 ns | −0.47 ns | 0.14 ns | −0.21 ns | 0.12 ns | −0.24 ns | −0.28 ns |
| Re−P | 0.05 ns | 0.20 ns | 0.40 ns | −0.68 ** | 0.54 * | 0.62 * | 0.23 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, W.-S.; Gu, H.-J.; Zhang, C.-c.; Zhang, Y.; Singh, A.N.; Fang, X.-M.; Fan, J.; Wang, H.-M.; Chen, F.-S. Mixed Broadleaved Tree Species Increases Soil Phosphorus Availability but Decreases the Coniferous Tree Nutrient Concentration in Subtropical China. Forests 2020, 11, 461. https://doi.org/10.3390/f11040461
Bu W-S, Gu H-J, Zhang C-c, Zhang Y, Singh AN, Fang X-M, Fan J, Wang H-M, Chen F-S. Mixed Broadleaved Tree Species Increases Soil Phosphorus Availability but Decreases the Coniferous Tree Nutrient Concentration in Subtropical China. Forests. 2020; 11(4):461. https://doi.org/10.3390/f11040461
Chicago/Turabian StyleBu, Wen-Sheng, Han-Jiao Gu, Can-can Zhang, Yang Zhang, Anand Narain Singh, Xiang-Min Fang, Jing Fan, Hui-Min Wang, and Fu-Sheng Chen. 2020. "Mixed Broadleaved Tree Species Increases Soil Phosphorus Availability but Decreases the Coniferous Tree Nutrient Concentration in Subtropical China" Forests 11, no. 4: 461. https://doi.org/10.3390/f11040461
APA StyleBu, W.-S., Gu, H.-J., Zhang, C.-c., Zhang, Y., Singh, A. N., Fang, X.-M., Fan, J., Wang, H.-M., & Chen, F.-S. (2020). Mixed Broadleaved Tree Species Increases Soil Phosphorus Availability but Decreases the Coniferous Tree Nutrient Concentration in Subtropical China. Forests, 11(4), 461. https://doi.org/10.3390/f11040461






